Abstract
Purpose
To describe and evaluate the main direct health costs, in routine clinical practice, of age-related macular degeneration (AMD) patients, from hospital perspective, in Spain.
Methods
Retrospective, multicenter, and observational study conducted on five third-level Spanish hospitals, between December 2018 and December 2019. The study included patients who were diagnosed of AMD before December 2018. Direct healthcare costs were obtained from a Spanish database. Study variables included demographic and clinical variables, and resources, such as treatment, diagnostic tests, medical examination, and surgery. Among the 1414 screened AMD patients, 1164 patients were included. In the overall study patients, the total cost was €5,386,511.0, with a mean cost per patient of €4627.6 ± 2383.9. The largest cost items were diagnostic examinations (€2.832.902,0) and vascular endothelial growth factor inhibitors (anti-VEGF) treatment (€2.038.257,2). Bevacizumab was administered to 325 (27.9%) patients, ranibizumab to 328 (28.2%), and aflibercept to 626 (53.8%); 115 (10.7%) patients received two anti-VEGF treatments, while 90 (7.7%) did not receive any. Over the course of the study, a total of 6,057 anti-VEGF injections were administered, with a mean (95% confidence interval) of 4.8 (4.4–5.2) injections per patient. Regarding safety, 29 patients experience injection-related adverse events, among them 12 patients had cataract and 11 ones elevated intraocular pressure (IOP). The incidence of endophthalmitis was 0.5% (6/1164).
Conclusions
AMD was associated with considerable healthcare costs for regional healthcare systems. Diagnostic examinations, particularly OCT examinations, and anti-VEGF treatment represented the largest cost items.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is a prevalent, chronic, and progressive retinal degenerative disease of the macula [1, 2].
AMD constitutes one of the leading causes of severe and irreversible visual impairment globally, but most notably in developed countries, among the elderly [3,4,5,6,7,8,9]. Its overall prevalence is approximately 8.7%, although variation among different populations is substantial [3,4,5,6,7,8,9]. The results of a metaanalysis that included 129,664 subjects showed that the prevalence of AMD ranged from 7.3% in Asian population to 12.3% in European ancestry population [5].
Additionally, as the life expectancy is rising up, the importance of AMD increases [10]. It was estimated that the number of people with the disease would be around 196 million in 2020, increasing to 288 million in 2040 [5].
Generally speaking, AMD can be classified as early, intermediate, or late stage [11]. Compared with early AMD, late AMD is far less frequent but most damaging to the sight [11]. According to the latest global estimate of AMD, the prevalence of late AMD in populations of European ancestry was 0.5% (95% confidence interval, CI: 0.26–1.08%) [5].
The information about the prevalence of AMD in Spain is very limited. Based on the currently available evidence, the estimated prevalence of late AMD (either geographic atrophy or macular neovascularization) ranges between 1.1% (95% CI: 1.0–1.2%) [7] and 1.9% [12].
Despite the fact that the introduction of vascular endothelial growth factor inhibitors (anti-VEGF) has supposed a significant advance in therapeutic management of neovascular AMD (NVAMD), none of them cures the disease or reverses its course [13,14,15,16]. Additionally, the main drawback of anti-VEGF is their high cost, which suppose a significant burden for health systems, often making such a regimen unaffordable in clinical practice.
According to the results of a metaanalysis, intravitreal aflibercept was associated with a higher overall treatment cost than ranibizumab (18,187 € vs. 17,168€, respectively) [17].
Although bevacizumab has been identified as the most cost-effective treatment, its use is "off-label" and not considered the standard of care for NVAMD in Europe (nor it is approved for treating NVAMD by US or European regulatory agencies) [18].
The Spanish Health Systems are public, universal, and mostly free of charge for the patients except for the share of out-of-pocket expenditure, such as transportation-associated costs, meals, glasses and contact lenses prescription, or medicine co-payment, among others [19].
Because AMD treatment entails a significant impact on the Health System budget, it is extremely important to accurately know the cost of these therapeutic strategies.
This study aimed to describe and evaluate the main direct health costs (monetary value), in routine clinical practice, of AMD patients, from hospital perspective, in Spain. Additionally, this study also assessed different clinical and demographic characteristics of the study population.
Methods
Retrospective, multicenter, and observational study conducted on patients diagnosed of AMD, who were treated in 5 third-level Spanish hospitals, between December 2018 and December 2019.
The study was conducted in accordance with the tenets of the Declaration of Helsinki. The protocol was approved by the ethics committee of Puerta de Hierro-Majadahonda University Hospital, which waived the need for informed consent for study participation.
Inclusion/exclusion criteria
This study included patients with a clinical diagnosis of AMD, who were treated in the Ophthalmology department, between December 2018 and December 2019, in one of the five third-level university hospitals that participated in the study. Patients must have been diagnosed of AMD before December 2018.
Those patients with a clinical diagnosis of AMD who did not require medical interventions, either treatments or visits, during the study follow-up were excluded.
Study centers
Five third-level hospitals (Listed in the Annex I), representing five Spanish Autonomous Communities (in alphabetical order: Castilla y León; Cataluña; Galicia; Madrid; and Valencian Community), were selected to participate in the study.
Each center was represented by a principal investigator and two sub-investigators from the ophthalmology department.
The Research Group was constituted under the name of Real-World Evidence study of patients with Age-Related Macular Degeneration to evaluate the Economic Burden in Spain (RAMDEBURS).
Costs
Direct healthcare costs were obtained from a Spanish database [20].
The cost of sanitary and consumable supplies, as well as that of antiangiogenic treatments, was provided and averaged by the study centers.
Costs are expressed in euros (€) and have been updated for the year 2020 [20]. An overview of the unit costs is shown in Annex II.
The overall direct healthcare costs were calculated, as well as the mean cost per patient.
Total costs were estimated considering the unit cost of the different resources and the number of resources consumed by each patient.
Study variables
The information, collected from the medical record, was introduced in an electronic case report form (CRF). For each study participant, the following information was registered:
-
Demographic variables: Age, sex, and smoking habit.
-
Clinical variables: Type of AMD (exudative, dry, or both); affected area; year of diagnosis; topical prophylactic treatment (before and after intravitreal injections); injection site; type of anesthesia; and injection-related adverse events (cataract, retinal detachment, endophthalmitis, elevated intraocular pressure).
-
Resources:
-
Treatment: Anti-VEGF administered (type and number of injections), and sanitary and consumable supplies.
-
Diagnostic examinations: visual acuity (VA), tonometry, optical coherence tomography (OCT), fluorescein angiography (FA), indocyanine green (ICG), autofluorescence, retinography, fundus (indirect ophthalmoscopy), fundus (biomicroscopy), ultrasonography, and genetic tests.
-
Medical examinations: ophthalmology and emergency.
-
Surgery: vitrectomy.
-
Statistical analysis
A standard statistical analysis was performed using the MedCalc® Statistical Software version 19.5.3 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020).
Descriptive statistics number (percentage), mean [standard deviation (SD)], mean [95% confidence interval (95% CI)], or median (95% CI) were used, as appropriate.
Results
Among the 1414 screened AMD patients, 1164 patients fulfilled the respective demands of the inclusion and exclusion criteria. Figure 1 shows the study flowchart.
Mean (95% CI) age of study sample was 79.8 (79.3–80.2) years, and 689 (59.2%) were women. Table 1 shows the main demographic and clinical characteristics of the study population.
Regarding AMD treatment, bevacizumab was administered to 325 (27.9%) patients, ranibizumab to 331 (28.4%), and aflibercept to 626 (53.8%); 115 patients received two anti-VEGF treatments, while 90 did not receive any. Similar proportion of patients received treatment with bevacizumab or ranibizumab (p = 0.8302). However, a significant proportion of patients received treatment with aflibercept than with bevacizumab (mean difference 25.9%, 95% confidence interval: 21.9–29.6%, p < 0.0001) or ranibizumab (mean difference 25.4%, 95% confidence interval: 21.5–29.2%, p < 0.0001).
Over the course of the study, a total of 6057 anti-VEGF injections were administered, with a mean (95% CI) of 4.8 (4.4–5.2) injections per patient. Figure 2 shows the distribution of the anti-VEGF treatments administered during the study.
In the overall study patients, the total cost was €5,386,511.0, with a mean cost per patient of €4627.6. The largest cost items were diagnostic examinations (€2.832.902,0) and anti-VEGF treatment (€2.038.257,2) (Table 2).
The costs of anti-VEGF treatment supposed 37.8% of total direct health costs (Fig. 3).
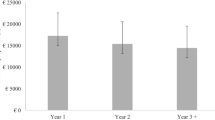
Among the 1,074 patients who received anti-VEGF treatment, the mean (SD) cost/per patient was €257.8 (162.2), €3525.8 (2242.4), and €1274.8 (718.1) for patients treated with bevacizumab, ranibizumab, and aflibercept, respectively.
In these group of patients, the mean cost per patient was 4727.4 ± 2281.8 €, which was significantly greater than that observed in the 90 patients who did not receive anti-VEGF therapy (2042.6 ± 1307.8 €); mean difference: 2684.8 €; 95% CI: 2206.3–3163.3 €, p < 0.0001.
Table 3 summarizes the direct costs of the different items. Among diagnostic examinations, OCT (€1,125,541.5) and retinography (€775,951.7) represented the largest cost items. About examinations costs, ophthalmology examination represented 96.6% of the total amount.
Throughout the study follow-up, 29 patients experience injection-related adverse events (AEs), among them 12 patients had cataract and 11 ones elevated IOP. The incidence of endophthalmitis was 0.099% (95% confidence interval: 0.036–0.215%) per intravitreal injection.
Three patients had more than one AE. An overview of the different treatment-related AEs is shown in Table 4.
Discussion
This study was designed to assess the economic burden, in terms of direct health costs, of AMD in a patient population where the predominant treatment was anti-VEGF therapy.
Late-stage AMD may be divided into two different forms, namely the nonvascular subtype or dry AMD (geographic atrophy) and the neovascular subtype (NVAMD) or wet AMD, which is less frequent but responsible of approximately the 90% of blindness related to AMD [1, 2].
To our knowledge, information evaluating the economic burden of AMD in Spain, since the advent of anti-VEGF therapy as the standard of care, is very limited.
According to the results of this study, diagnostic tests and intravitreal anti-VEGF injections represented the items with the largest direct health costs.
Preserving population health requires work and money. Achieving it implies that National Health Systems should face unlimited demand with limited resources. That is why, health economics is exerting an influence on decision making at all levels of health care [24].
Demographic aging is leading to a substantial increase in the prevalence of age-related sight-impairing conditions and associated increases in their costs [10, 25, 26]. However, despite the relevance of this issue, to date, there has been little work evaluating the economic impact of AMD in a Spanish setting.
The average annual societal cost per bilateral NVAMD patient treated was estimated to be euro 5732 in Spain, direct vision-related medical costs accounted for 23–63% of the total cost [27]. When we update the prices by using the cost price index (CPI), it results in an increase of the 14.8% between January 2008 and July 2020 [28]. With this rate of variation, the updated costs of Cruess et al. [27] are €6,534.5. In our study, mean annual cost/per patient was slightly lower (€4,627.6), but we did not consider direct non-medical-related costs, like, for example, home healthcare and social services costs.
According to data of National Statistics Institute, there are about 9.27 million people ≥ 65 years in Spain [29]. The prevalence of NVAMD in Europe, among subjects ≥ 65 years, has been estimated to be 2.29% [27]. Based on this assumption, there are approximately 212,280 patients (≥ 65 years) with NVAMD in Spain [29, 30]. Based on this estimation, the main direct health costs associated with NVAMD might suppose €982.4 million. On the other hand, the Spanish Eyes Epidemiological (SEE) Study estimated an overall prevalence of AMD of 3.4% among subjects ≥ 65 years [12]. Assuming these figures, approximately 315,180 patients (≥ 65 years) would have NVAMD in Spain, which suppose €1458.5 million. In summary, it is possible to estimate that the total direct health burden associated with NVAMD (main direct health costs per patient x estimated number of Spanish patients with NVAMD) would range between €982.4 million and €1458.6 million, which represents an 1.37–2.10% of Spanish total public health spending [31].
Although medical treatment of NVAMD experienced a significant advance due to the introduction of anti-VEGF agents, they have several drawbacks, including their high cost and the lack of efficiency.
Among patients included in the current study, 27.9% received treatment with bevacizumab, 28.2% with ranibizumab, and 53.8% with aflibercept. Although, when compared to Italy, the proportion of patients treated with bevacizumab was similar, the proportion of patients treated with ranibizumab and aflibercept was totally different [32]. While in the current study 53.8% of patients were treated with aflibercept, in Italy only 25.3% of patients received treatment with it. Similarly, 28.2% of patients received treatment with ranibizumab, while that proportion was 55.3% in Italy [32].
The cost differences between ranibizumab and aflibercept may be due to the fact that costing approach assumes vial splitting for aflibercept, but not ranibizumab. Although preloaded ranibizumab is usually the first option, in those cases that the ophthalmologist decides to use a vial, vial splitting, as performed with aflibercept, would be used for ranibizumab as well.
Despite anti-VEGF therapy has become the current standard of care for NVAMD [33], many patients do not respond adequately to this therapy or experience a slow loss of efficacy of anti-VEGF agents after repeated administration over time [34, 35].
Although new approaches for treating NVAMD have been proposed, as far as we know, there is no evidence about their cost or cost-effectiveness.
Regarding complications, the high incidence of endophthalmitis reported in the current study is noteworthy. On average, the incidence of endophthalmitis after intravitreal injections of anti-VEGF or corticosteroids is low [36,37,38,39,40,41]. It ranges between 0.00% [41] and 0.021% [36]. In our study, the incidence of endophthalmitis was 0.099% (95% CI: 0.036–0.215%). Although the incidence of endophthalmitis was slightly greater than that reported in other studies, there is no any objective reason, with the exception of the small sample size, that might justify this finding.
It is important to acknowledge some limitations when interpreting these findings. Our study did not evaluate direct non-medical-related costs (e.g., home healthcare and social services), patient transportation, or other incidentals, to establish economic parameters. The study sample was limited to five Spanish Autonomous Communities, which may only reflect the reality of these regions. Nevertheless, the methodology could be easily replicated in other regions. Finally, it should be mentioned that mean cost per patient in the overall study sample was affected by the fact that 90 patients did not receive anti-VEGF therapy. Nevertheless, the mean cost per patient in eyes who underwent anti-VEGF therapy and those who did not was calculated, which solves this limitation.
Conclusions
NVAMD was associated with considerable healthcare costs. Diagnostic examinations, particularly OCT examinations, represented the largest cost item.
Additionally, anti-VEGF treatment represented a relevant burden for healthcare systems, due mainly to its high price, needs for repetitive administration, and frequent outpatient visits.
Further studies are needed to determine the role of future therapies, which may reduce the burden of current therapies but maintain high efficacy/safety profile, on the human and economic burden of AMD in Spain.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cheung LK, Eaton A (2013) Age-related macular degeneration. Pharmacotherapy 33(8):838–855. https://doi.org/10.1002/phar.1264
Gheorghe A, Mahdi L, Musat O (2015) Age-related macular degeneration. Rom J Ophthalmol 59(2):74–77
Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CF et al (1995) The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology 102:205–1011. https://doi.org/10.1016/s0161-6420(95)31034-2
Pascolini D, Mariotti SP (2012) Global estimates of visual impairment: 2010. Br J Ophthalmol 96(5):614–618. https://doi.org/10.1136/bjophthalmol-2011-300539
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY et al (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2(2):e106-116. https://doi.org/10.1016/S2214-109X(13)70145-1
Korb CA, Kottler UB, Wolfram C, Hoehn R, Schulz A, Zwiener I et al (2014) Prevalence of age-related macular degeneration in a large European cohort: results from the population-based Gutenberg Health Study. Graefes Arch Clin Exp Ophthalmol 252(9):1403–1411. https://doi.org/10.1007/s00417-014-2591-9
Zapata MA, Arcos G, Fonollosa A, Abraldes M, Oleñik A, Gutierrez E et al (2017) Telemedicine for a general screening of retinal disease using nonmydriatic fundus cameras in optometry centers: three-year results. Telemed J E Health 23(1):30–36. https://doi.org/10.1089/tmj.2016.0020
García-Layana A, Cabrera-López F, García-Arumí J, Arias-Barquet L, Ruiz-Moreno JM (2017) Early and intermediate age-related macular degeneration: update and clinical review. Clin Interv Aging 12:1579–1587. https://doi.org/10.2147/CIA.S142685
Klein R, Klein BEK, Linton KLP (2020) Prevalence of age-related maculopathy: the beaver dam eye study. Ophthalmology 127(4S):S122–S132. https://doi.org/10.1016/j.ophtha.2020.01.033
Pennington KL, DeAngelis MM (2016) Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond) 3:34. https://doi.org/10.1186/s40662-016-0063-5
Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Beckman Initiative for Macular Research Classification Committee et al (2013) Clinical classification of age-related macular degeneration. Ophthalmology 120(4):844–851. https://doi.org/10.1016/j.ophtha.2012.10.036
Spanish Eyes Epidemiological (SEE) Study Group (2011) Prevalence of age-related macular degeneration in Spain. Br J Ophthalmol 95(7):931–936. https://doi.org/10.1136/bjo.2010.187773
Jager RD, Mieler WF, Miller JW (2008) Age-related macular degeneration. N Engl J Med 358(24):2606–2617
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al (2006) ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355(14):1432–1444. https://doi.org/10.1056/NEJMoa062655
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al (2006) MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355(14):1419–1431. https://doi.org/10.1056/NEJMoa054481
Kaiser PK, Blodi BA, Shapiro H, Acharya NR, MARINA Study Group (2007) Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 114(10):1868–1875. https://doi.org/10.1016/j.ophtha.2007.04.030
Carrasco J, Pietsch GA, Nicolas MP, Koerber C, Bennison C, Yoon J (2020) Real-world effectiveness and real-world cost-effectiveness of intravitreal aflibercept and intravitreal ranibizumab in neovascular age-related macular degeneration: systematic review and meta-analysis of real-world studies. Adv Ther 37(1):300–315. https://doi.org/10.1007/s12325-019-01147-6
van Asten F, Michels CTJ, Hoyng CB, van der Wilt GJ, Klevering BJ, Rovers MM et al (2018) The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration-a cost-effectiveness analysis from a societal perspective. PLoS ONE 13(5):e0197670. https://doi.org/10.1371/journal.pone.0197670
OECD/European Observatory on Health Systems sand Policies (2017), Spain: Country Health Profile 2017, State of Health in the EU, OECD Publishing, Paris/European Observatory on Health Systems and Policies, Brussels. Available in https://doi.org/10.1787/9789264283565-en. Last accessed March 6, 2021
Authors no listed. Spanish database of healthcare costs: eSalud [Internet]. Barcelona: Oblikue Consulting, S.L. 2007. Available in: http://www.oblikue.com/bddcostes/ Last accessed March 6, 2021
Authors no listed. Bevacizumab. Summary of product characteristics. Available in: https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf Last accessed March 6, 2021
Authors no listed. Ranibizumab. Summary of product characteristics. Available in: https://www.ema.europa.eu/en/documents/product-information/lucentis-epar-product-information_en.pdf Last accessed March 6, 2021
Authors no listed. Aflibercept. Summary of product characteristics. Available in: https://www.ema.europa.eu/en/documents/product-information/eylea-epar-product-information_en.pdf Last accessed March 6, 2021
Kernick DP (2003) Introduction to health economics for the medical practitioner. Postgrad Med J 79(929):147–150. https://doi.org/10.1136/pmj.79.929.147
Taylor HR, Pezzullo ML, Keeffe JE (2006) The economic impact and cost of visual impairment in Australia. Br J Ophthalmol 90(3):272–275. https://doi.org/10.1136/bjo.2005.080986
Pezzullo L, Streatfeild J, Simkiss P, Shickle D (2018) The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv Res 18(1):63. https://doi.org/10.1186/s12913-018-2836-0
Cruess AF, Zlateva G, Xu X, Soubrane G, Pauleikhoff D, Lotery A et al (2008) Economic burden of bilateral neovascular age-related macular degeneration: multi-country observational study. Pharmacoeconomics 26(1):57–73. https://doi.org/10.2165/00019053-200826010-00006
ESCPI2013 [Spain - Instituto Nacional de Estadistica (INE)]. Available in https://www.ine.es/calcula/calcula.do;jsessionid=78F41704406F099CA1055CDF51947999.calcula01 Last accessed March 7, 2021
Autores no listados. Instituto nacional de Estadística. Data of Spanish Population. Available in: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176951&menu=ultiDatos&idp=1254735572981 Last accessed March 7, 2021
Augood CA, Vingerling JR, de Jong PT, Chakravarthy U, Seland J, Soubrane G et al (2006) Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE). Arch Ophthalmol 124(4):529–535. https://doi.org/10.1001/archopht.124.4.529
Rodríguez-Blas MC. Estadística de Gasto Sanitario Público 2018. Available in: https://www.mscbs.gob.es/estadEstudios/estadisticas/docs/EGSP2008/egspPrincipalesResultados.pdf Last accessed March 7, 2021
Authors not listed. L'uso dei farmaci in Italia-Rapporto OsMed 2018. Available in: https://www.aifa.gov.it/web/guest/-/rapporto-osmed-20-1 Last accessed March 7, 2021
Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R et al (2014) European Society of Retina Specialists. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol 98(9):1144–1167. https://doi.org/10.1136/bjophthalmol-2014-305702
Broadhead GK, Hong T, Chang AA (2014) Treating the untreatable patient: current options for the management of treatment-resistant neovascular age-related macular degeneration. Acta Ophthalmol 92(8):713–723. https://doi.org/10.1111/aos.12463
Yang S, Zhao J, Sun X (2016) Resistance to anti-VEGF therapy in neovascular age- related macular degeneration: a comprehensive review. Drug Des Devel Ther 10:1857–1867. https://doi.org/10.2147/DDDT.S97653
Dossarps D, Bron AM, Koehrer P, Aho-Glélé LS, Creuzot-Garcher C, FRCR net (FRenCh Retina specialists net) (2015) Endophthalmitis after intravitreal injections: incidence, presentation, management, and visual outcome. Am J Ophthalmol 160(1):17-25.e1. https://doi.org/10.1016/j.ajo.2015.04.013
Stem MS, Todorich B, Yonekawa Y, Capone A Jr, Williams GA, Ruby AJ (2017) Incidence and visual outcomes of culture-proven endophthalmitis following dexamethasone intravitreal implant. JAMA Ophthalmol 135(4):379–382. https://doi.org/10.1001/jamaophthalmol.2016.5883
Rajesh B, Zarranz-Ventura J, Fung AT, Busch C, Sahoo NK, Rodriguez-Valdes PJ, International Ozurdex Study Group et al (2020) Safety of 6000 intravitreal dexamethasone implants. Br J Ophthalmol 104(1):39–46. https://doi.org/10.1136/bjophthalmol-2019-313991
VanderBeek BL, Bonaffini SG, Ma L (2015) The association between intravitreal steroids and post-injection endophthalmitis rates. Ophthalmology 122(11):2311-2315.e1. https://doi.org/10.1016/j.ophtha.2015.07.005
Freiberg FJ, Brynskov T, Munk MR, Sørensen TL, Wolf S, Wirth MA et al (2017) Low endophthalmitis rates after intravitreal anti-vascular endothelial growth factor injections in an operation room: a retrospective multicenter study. Retina 37(12):2341–2346. https://doi.org/10.1097/IAE.0000000000001488
Cidad-Betegón MDP, Armadá-Maresca F, Amorena-Santesteban G, Coca-Robinot J, D’Anna-Mardero O, de la Rosa-Pérez I et al (2020) Can the dexamethasone intravitreal implant Ozurdex be safely administered in an out-of-operating room setting? J Drug Assess 9(1):66–71. https://doi.org/10.1080/21556660.2020.1742723
Acknowledgements
Medical writing and Editorial assistant services have been provided by Ciencia y Deporte S.L. and covered by a Grant from Allergan. Support for this assistance was funded by Allergan, an AbbVie company.
Funding
The authors wish to acknowledge Allergan for their support with the medical writing. It should be noted that Allergan was not involved in the preparation of the manuscript nor did the company influence in any way the scientific conclusions reached. Editorial assistance in the preparation of this manuscript was provided by Antonio Martinez MD (Ciencia y Deporte S.L.). Support for this assistance was funded by Allergan, an AbbVie company.
Author information
Authors and Affiliations
Consortia
Contributions
All authors met the ICMJE authorship criteria. All authors made substantial contributions to conception, design, analysis and interpretation of data, contributed to writing the article, provided critical revision of the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
Dr Ruiz-Moreno has received a grant from Allergan during the conduct of the study. None of the co-authors have any conflict of interest to declare.
Consent to participate
The local ethics committee waived the need for written informed consent of the participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruiz-Moreno, J.M., Arias, L., Abraldes, M.J. et al. Economic burden of age-related macular degeneration in routine clinical practice: the RAMDEBURS study. Int Ophthalmol 41, 3427–3436 (2021). https://doi.org/10.1007/s10792-021-01906-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01906-x