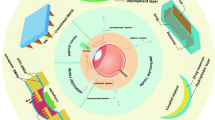
Abstract
Purpose
One of the challenges of ocular drug delivery systems is sustainable delivery of the intended drug to the posterior segment of the eye. Periocular routes of drug delivery are a promising method to bridge this challenge. The purpose of this study is to investigate the recent advances and potentials of subconjunctival route and its pros and cons in comparison with other ocular drug delivery routes.
Methods
In this literature review, the comprehensive search of publications was performed in MEDLINE/PubMed indicating investigations on subconjunctival drug delivery systems using relevant keywords.
Results
Based on the resultant detailed criteria to choose an appropriate ocular drug delivery route, it is revealed that most of these routes are either highly invasive and/or provide low bioavailability of drug to the target tissue. Nevertheless, subconjunctival drug delivery could be considered as one of the less invasive and easily accessible routes for delivering various drugs to both anterior and posterior segments of the eye. However, most of such researches are at the stage of animal study in their pipelines.
Conclusion
Periocular route of drug delivery is one of the most efficient routes for delivering the drugs to both anterior and posterior segments of the eye. Subconjunctival sustained drug delivery is highly effective and less invasive compared to other periocular routes. This makes subconjunctival implants and injections one of the most proper ways of treating various ranges of ocular diseases and disorders, e.g., diabetic retinopathy, dry eye syndrome, glaucoma, etc.

Similar content being viewed by others
Abbreviations
- BRB:
-
Blood–retinal barrier
- BAB:
-
Blood–aqueous barrier
- ODD:
-
Ocular drug delivery
- AMD:
-
Age-related macular degeneration
- DR:
-
Diabetic retinopathy
- RPE:
-
Retinal pigment epithelium
- CNV:
-
C Neovascularization
- DES:
-
Dry eye syndrome
References
Le Bourlais C, Acar L, Zia H, Sado PA, Needham T, Leverge R (1998) Ophthalmic drug delivery systems—recent advances. Prog Retinal Eye Res 17(1):33–58
Gulsen D, Chauhan A (2004) Ophthalmic drug delivery through contact lenses. Invest Ophthalmol Vis Sci 45(7):2342–2347
Patel A, Cholkar K, Agrahari V, Mitra AK (2013) Ocular drug delivery systems: an overview. World J Pharmacol 2(2):47
Gaudana R, Ananthula HK, Parenky A, Mitra AK (2010) Ocular drug delivery. The AAPS journal 12(3):348–360
Gaudana R, Jwala J, Boddu SH, Mitra AK (2009) Recent perspectives in ocular drug delivery. Pharm Res 26(5):1197
Djebli N, Khier S, Griguer F et al (2017) Ocular drug distribution after topical administration: population pharmacokinetic model in rabbits. Eur J Drug Metab Pharmacokinet 42(1):59–68
Nayak K, Misra M (2018) A review on recent drug delivery systems for posterior segment of eye. Biomed Pharmacother 107:1564–1582
Rodrigues GA, Lutz D, Shen J et al (2018) Topical drug delivery to the posterior segment of the eye: addressing the challenge of preclinical to clinical translation. Pharm Res 35(12):245
Bochot A, Fattal E (2012) Liposomes for intravitreal drug delivery: a state of the art. J Control Release 161(2):628–634
Zafar A, Ahmad J, Addo RT, Akhter S (2016) Progress of controlled drug delivery systems in topical ophthalmology: focus on nano and micro drug carriers. Challenges Appl Ocular Drug Deliv Adv pp 131–163. Springer, cham
zafar A, Ali J, Fazil M, Qumbar M, Khan N, Ali A (2016) Colloidal drug delivery system amplify the ocular delivery. Drug Delivery. 23(3):700–716
Morishita M, Park K (2016) Biodrug delivery systems: fundamentals, applications and clinical development. CRC Press, Boca Raton
Edelhauser HF, Rowe-Rendleman CL, Robinson MR et al (2010) Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci 51(11):5403–5420
Kadam RS, Williams J, Tyagi P, Edelhauser HF, Kompella UB (2013) Suprachoroidal delivery in a rabbit ex vivo eye model: influence of drug properties, regional differences in delivery, and comparison with intravitreal and intracameral routes. Molecular vision 19:1198
Shen HH, Lin TW, Dusting GJ, Liu GS (2015) Nanocarriers for treatment of ocular neovascularization in the back of the eye. Nanomedicine 10(13):2093–2107
Ananthula HK, Vaishya R, Barot M, Mitra A (2009) Duane's ophthalmology. In: Bioavailability. Lippincott Williams & Wilkins, Philadelphia
Kiran Vaka SR, Sammeta SM, Day LB, Murthy SN (2008) Transcorneal iontophoresis for delivery of ciprofloxacin hydrochloride. Curr Eye Res 33(8):661–667
Tirucherai GS, Dias C, Mitra AK (2002) Corneal permeation of ganciclovir: mechanism of ganciclovir permeation enhancement by acyl ester prodrug design. J Ocul Pharmacol Ther 18(6):535–548
Gunda S, Hariharan S, Mitra AK (2006) Corneal absorption and anterior chamber pharmacokinetics of dipeptide monoester prodrugs of ganciclovir (GCV): in vivo comparative evaluation of these prodrugs with Val-GCV and GCV in rabbits. J Ocul Pharmacol Ther 22(6):465–476
Gallarate M, Chirio D, Bussano R et al (2013) Development of O/W nanoemulsions for ophthalmic administration of timolol. Int J Pharm 440(2):126–134
Tirucherai GS, Mitra AK (2003) Effect of hydroxypropyl beta cyclodextrin complexation on aqueous solubility, stability, and corneal permeation of acyl ester prodrugs of ganciclovir. AAPS PharmSciTech 4(3):124–135
Lang J, Roehrs R, Jani R (2009) Remington: the science and practice of pharmacy. 21. philadelphia: Lippincott Williams and Wilkins
Hughes PM, Olejnik O, Chang-Lin J-E, Wilson CG (2005) Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev 57(14):2010–2032
Shen H-H, Chan EC, Lee JH et al (2015) Nanocarriers for treatment of ocular neovascularization in the back of the eye: new vehicles for ophthalmic drug delivery. Nanomedicine 10(13):2093–2107
Agrahari V, Mandal A, Agrahari V et al (2016) A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res 6(6):735–754
Durairaj C (2016) Ocular pharmacokinetics. Pharmacol Ther Ocular Dis. pp 31-55.Springer, Berlin
Maurice DM (2002) Drug delivery to the posterior segment from drops. Surv Ophthalmol 47:S41–S52
Lee VH, Robinson JR (1986) Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol Ther 2(1):67–108
Zhang C, Wang H, Nie J, Wang F (2014) Protective factors in diabetic retinopathy: focus on blood-retinal barrier. Discov Med 18(98):105–112
Cunha-Vaz J, Bernardes R, Lobo C (2011) Blood-retinal barrier. Eur J Ophthalmol. 21(6-suppl):3–9
Barar J, Javadzadeh AR, Omidi Y (2008) Ocular novel drug delivery: impacts of membranes and barriers. Expert Opinion Drug Deliv 5(5):567–581
Suzuki T, Uno T, Chen G, Ohashi Y (2008) Ocular distribution of intravenously administered micafungin in rabbits. J Infect Chemother 14(3):204–207
Regnier A, Schneider M, Concordet D, Toutain P-L (2008) Intraocular pharmacokinetics of intravenously administered marbofloxacin in rabbits with experimentally induced acute endophthalmitis. Am J Vet Res 69(3):410–415
Goldblum D, Rohrer K, Frueh BE, Theurillat R, Thormann W, Zimmerli S (2002) Ocular distribution of intravenously administered lipid formulations of amphotericin B in a rabbit model. Antimicrob Agents Chemother 46(12):3719–3723
Shirasaki Y (2008) Molecular design for enhancement of ocular penetration. J Pharm Sci 97(7):2462–2496
Kaur IP, Smitha R, Aggarwal D, Kapil M (2002) Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm 248(1):1–14
Kampougeris G, Antoniadou A, Kavouklis E, Chryssouli Z, Giamarellou H (2005) Penetration of moxifloxacin into the human aqueous humour after oral administration. Br J Ophthalmol 89(5):628–631
Samtani S, Amaral J, Campos MM, Fariss RN, Becerra SP (2009) Doxycycline-mediated inhibition of choroidal neovascularization. Invest Ophthalmol Vis Sci 50(11):5098–5106
Srinivas A, Azad RV, Sharma YR, Kumar A, Satpathy G, Velpandian T (2009) Evaluation of vitreous levels of gatifloxacin after systemic administration in inflamed and non-inflamed eyes. Acta Ophthalmol 87(6):648–652
Smith VA, Khan-Lim D, Anderson L, Cook SD, Dick AD (2008) Does orally administered doxycycline reach the tear film? Br J Ophthalmol 92(6):856–859
Chong DY, Johnson MW, Huynh TH, Hall EF, Comer GM, Fish DN (2009) Vitreous penetration of orally administered famciclovir. Am J Ophthalmol. 148(1):38–42 e31
Takahashi K, Saishin Y, Saishin Y, King AG, Levin R, Campochiaro PA (2009) Suppression and regression of choroidal neovascularization by the multitargeted kinase inhibitor pazopanib. Arch Ophthalmol 127(4):494–499
Young S, Larkin G, Branley M, Lightman S (2001) Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Exp Ophthalmol 29(1):2–6
Ladas ID, Karagiannis DA, Rouvas AA, Kotsolis AI, Liotsou A, Vergados I (2009) Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: our experience after 2,000 injections. Retina 29(3):313–318
Swita Raghava MH, Uday B Kompella. Periocular routes for retinal drug delivery
Zahl K (1992) Selection of techniques for regional blockade of the eye and adnexa. McGoldrick KE Anesthesia for opthalmic and otolaryngologic surgery Philadelphia: WB Saunders Co. p 240
Jaffe NS, Jaffe MS, Jaffe GF (1997) Cataract surgery and its complications, 6th ed. Mosby, St. Louis
Lee TW-Y, Robinson JR (2004) Drug delivery to the posterior segment of the eye III: the effect of parallel elimination pathway on the vitreous drug level after subconjunctival injection. J Ocul Pharmacol Ther 20(1):55–64
Amrite AC, Kompella UB (2005) Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol 57(12):1555–1563
Kim JH, Kim MH, Jo DH, Yu YS, Lee TG, Kim JH (2011) The inhibition of retinal neovascularization by gold nanoparticles via suppression of VEGFR-2 activation. Biomaterials 32(7):1865–1871
Cho W-K, Kang S, Choi H, Rho CR (2015) Topically administered gold nanoparticles inhibit experimental corneal neovascularization in mice. Cornea 34(4):456–459
Kalishwaralal K, Banumathi E, Pandian SRK et al (2009) Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf, B 73(1):51–57
Kalishwaralal K, BarathManiKanth S, Pandian SRK, Deepak V, Gurunathan S (2010) Silver nano—a trove for retinal therapies. J Control Release 145(2):76–90
Gurunathan S, Lee K-J, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R, Eom SH (2009) Antiangiogenic properties of silver nanoparticles. Biomaterials 30(31):6341–6350
Jo DH, Kim JH, Yu YS, Lee TG, Kim JH (2012) Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomed Nanotechnol Biol Med. 8(5):784–791
Gross N, Ranjbar M, Evers C et al (2013) Choroidal neovascularization reduced by targeted drug delivery with cationic liposome-encapsulated paclitaxel or targeted photodynamic therapy with verteporfin encapsulated in cationic liposomes. Mol Vis 19:54
Jiang S, Franco YL, Zhou Y, Chen J (2018) Nanotechnology in retinal drug delivery. Int J Ophthalmol 11(6):1038
Kim YC, Chiang B, Wu X, Prausnitz MR (2014) Ocular delivery of macromolecules. J Control Release 190:172–181
Xu Q, Kambhampati SP, Kannan RM (2013) Nanotechnology approaches for ocular drug delivery. Middle East Afr J Ophthalmol 20(1):26
Del Amo EM, Urtti A (2008) Current and future ophthalmic drug delivery systems: a shift to the posterior segment. Drug Discov Today 13(3–4):135–143
Agban Y, Thakur SS, Mugisho OO, Rupenthal ID (2019) Depot formulations to sustain periocular drug delivery to the posterior eye segment. Drug Discov Today
Mitra AK, Anand BS, Duvvuri S (2005) Drug delivery to the eye. Adv Organ Biol 10:307–351
Urtti A (2006) Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev 58(11):1131–1135
Hosseini K, Matsushima D, Johnson J et al (2008) Pharmacokinetic study of dexamethasone disodium phosphate using intravitreal, subconjunctival, and intravenous delivery routes in rabbits. J Ocul Pharmacol Ther 24(3):301–308
Weijtens O, Feron EJ, Schoemaker RC et al (1999) High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am J Ophthalmol 128(2):192–197
Kim SH, Csaky KG, Wang NS, Lutz RJ (2008) Drug elimination kinetics following subconjunctival injection using dynamic contrast-enhanced magnetic resonance imaging. Pharm Res 25(3):512–520
Ranta V-P, Mannermaa E, Lummepuro K et al (2010) Barrier analysis of periocular drug delivery to the posterior segment. J Control Release 148(1):42–48
Gutiérrez-Hernández J-C, Caffey S, Abdallah W et al (2014) One-year feasibility study of replenish micropump for intravitreal drug delivery: a pilot study. Transl Vis Sci Technol 3(4):1
Eljarrat-Binstock E, Pe’er J, Domb AJ (2010) New techniques for drug delivery to the posterior eye segment. Pharm Res. 27(4):530–543
Srirangam R, Majumdar S (2012) Transscleral drug delivery to the posterior segment of the eye: particulate and colloidal formulations and biopharmaceutical considerations. In: Advances in ocular drug delivery. Research Signpost, Kerala, India
Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR (2012) Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci 53(8):4433–4441
Patel SR, Lin AS, Edelhauser HF, Prausnitz MR (2011) Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res 28(1):166–176
Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD (2011) Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci 52(7):4749–4756
Lallemand F, Felt-Baeyens O, Besseghir K, Behar-Cohen F, Gurny R (2003) Cyclosporine A delivery to the eye: a pharmaceutical challenge. Eur J Pharm Biopharm 56(3):307–318
Hosoya K-I, Lee VH, Kim K-J (2005) Roles of the conjunctiva in ocular drug delivery: a review of conjunctival transport mechanisms and their regulation. Eur J Pharm Biopharm 60(2):227–240
Yasin MN, Svirskis D, Seyfoddin A, Rupenthal ID (2014) Implants for drug delivery to the posterior segment of the eye: A focus on stimuli-responsive and tunable release systems. J Control Release 196:208–221
Apel A, Oh C, Chiu R, Saville B, Cheng Y-L, Rootman D (1995) A subconjunctival degradable implant for cyclosporine delivery in corneal transplant therapy. Curr Eye Res 14(8):659–667
Misra GP, Singh RS, Aleman TS, Jacobson SG, Gardner TW, Lowe TL (2009) Subconjunctivally implantable hydrogels with degradable and thermoresponsive properties for sustained release of insulin to the retina. Biomaterials 30(33):6541–6547
Huang X, Lowe TL (2005) Biodegradable thermoresponsive hydrogels for aqueous encapsulation and controlled release of hydrophilic model drugs. Biomacromol 6(4):2131–2139
Peng Y, Ang M, Foo S et al (2011) Biocompatibility and biodegradation studies of subconjunctival implants in rabbit eyes. PLoS ONE 6(7):e22507
Paula JS, Ribeiro VRC, Chahud F et al (2013) Bevacizumab-loaded polyurethane subconjunctival implants: effects on experimental glaucoma filtration surgery. J Ocul Pharmacol Ther 29(6):566–573
Nagai N, Kaji H, Onami H et al (2014) A polymeric device for controlled transscleral multi-drug delivery to the posterior segment of the eye. Acta Biomater 10(2):680–687
Imai H, Misra GP, Wu L, Janagam DR, Gardner TW, Lowe TL (2015) Subconjunctivally Implanted Hydrogels for Sustained Insulin Release to Reduce Retinal Cell Apoptosis in Diabetic RatsHydrogels for Sustained Retinal Delivery of Insulin. Invest Ophthalmol Vis Sci 56(13):7839–7846
Chang D, Park K, Famili A (2019) Hydrogels for sustained delivery of biologics to the back of the eye. Drug Discov Today
Pehlivan SB, Yavuz B, Çalamak S et al (2015) Preparation and In Vitro/In Vivo Evaluation of Cyclosporin A-Loaded Nanodecorated Ocular Implants for Subconjunctival Application. J Pharm Sci 104(5):1709–1720
Zhou C, Robert M-C, Kapoulea V et al (2017) Sustained Subconjunctival Delivery of Infliximab Protects the Cornea and Retina Following Alkali Burn to the EyeOcular Protection With Sustained Anti-TNF-α Delivery. Invest Ophthalmol Vis Sci 58(1):96–105
Covert JC, Thomasy SM, Kado-Fong H et al (2019) Pilot Study of the Safety and Tolerability of a Subconjunctival Penciclovir Implant in Cats Experimentally Infected with Herpesvirus. J Ocul Pharmacol Ther 35(1):38–49
Alghadyan AA, Peyman GA, Khoobehi B, Milner S, Liu K-R (1988) Liposome-bound cyclosporine: aqueous and vitreous level after subconjunctival injection. Int Ophthalmol 12(2):101–104
Hoshino M, Nakamura Y, Hamid QA (2001) Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol 107(6):1034–1038
Beck PL, Podolsky DK (1999) Growth factors in inflammatory bowel disease. Inflamm Bowel Dis 5(1):44–60
Miotla J, Maciewicz R, Kendrew J, Feldmann M, Paleolog E (2000) Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab Invest 80(8):1195
Kompella UB, Bandi N, Ayalasomayajula SP (2003) Subconjunctival nano-and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest Ophthalmol Vis Sci 44(3):1192–1201
Van Quill KR, Dioguardi PK, Tong CT et al (2005) Subconjunctival carboplatin in fibrin sealant in the treatment of transgenic murine retinoblastoma. Ophthalmology 112(6):1151–1158
Ayalasomayajula SP, Kompella UB (2005) Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur J Pharmacol 511(2):191–198
Kompella U, Bandi N, Ayalasomayajula S (2001) Poly (lactic acid) nanoparticles for sustained release of budesonide. Drug Deliv Technol 1(1):7
Kang SJ, Durairaj C, Kompella UB, O’Brien JM, Grossniklaus HE (2009) Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Arch Ophthalmol 127(8):1043–1047
Rieke ER, Amaral J, Becerra SP, Lutz RJ (2010) Sustained subconjunctival protein delivery using a thermosetting gel delivery system. J Ocul Pharmacol Ther 26(1):55–64
Wong CW, Czarny B, Metselaar JM et al (2018) Evaluation of subconjunctival liposomal steroids for the treatment of experimental uveitis. Sci Rep 8(1):6604
Liu D, Wu Q, Zhu Y et al (2019) Co-delivery of metformin and levofloxacin hydrochloride using biodegradable thermosensitive hydrogel for the treatment of corneal neovascularization. Drug Deliv 26(1):522–531
Bartlett JD, Jaanus SD (2007) Clinical ocular pharmacology: elsevier health sciences
Atchison EA, Gilca M, Civantos JM, Pollack JS (2019) Elimination of Steroid Drops After Vitreoretinal Surgery. J VitreoRetinal Dis 3(5):324–327
Sundelin KC, Dafgård Kopp EM (2015) Complications associated with secondary orbital implantations. Acta Ophthalmol 93(7):679–683
Rubinfeld RS, Pfister RR, Stein RM et al (1992) Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology 99(11):1647–1654
Davari MH, Gheytasi H, Davari E (2016) Subconjunctival mitomycin C injection into pterygium decreases its size and reduces associated complications. Adv Eye Surg 67
Chu H-S, Hu F-R, Yang C-M et al (2011) Subconjunctival injection of bevacizumab in the treatment of corneal neovascularization associated with lipid deposition. Cornea 30(1):60–66
Kwon HS, Nah YS, Seo KY, Kim EK (2002) Necrotizing Conjunctival Ulceration following Subconjunctival Atropine Depot Injection. J Korean Ophthalmol Soc 43(9):1806
Weijtens O, Schoemaker RC, Lentjes EG, Romijn FP, Cohen AF, van Meurs JC (2000) Dexamethasone concentration in the subretinal fluid after a subconjunctival injection, a peribulbar injection, or an oral dose. Ophthalmology 107(10):1932–1938
Yuan F, Li L, Chen X, Yan X, Wang L (2015) Biodegradable 3D-porous collagen matrix (ologen) compared with mitomycin C for treatment of primary open-angle glaucoma: results at 5 years. J Ophthalmol. P 2015
Angmo D, Wadhwani M, Upadhyay AD, Temkar S, Dada T (2017) Outcomes of trabeculectomy augmented with subconjunctival and subscleral ologen implantation in primary advanced glaucoma. J Glaucoma 26(1):8–14
Singh K, Bhattacharyya M, Mutreja A, Dangda S (2018) Trabeculectomy with subconjunctival collagen implant in Indian eyes: Long-term results. Indian J Ophthalmol 66(10):1429
Chaudhary A, Salinas L, Guidotti J, Mermoud A, Mansouri K (2018) XEN Gel Implant: a new surgical approach in glaucoma. Expert Rev Med Devices 15(1):47–59
Rajoria G, Gupta A (2012) In-situ gelling system: a novel approach for ocular drug delivery. AJPTR 2:24–53
Gupta H, Aqil M (2012) Contact lenses in ocular therapeutics. Drug Discov Today 17(9–10):522–527
Alipour F, Khaheshi S, Soleimanzadeh M, Heidarzadeh S, Heydarzadeh S (2017) Contact lens-related complications: A review. J Ophthalmic Vis Res 12(2):193
Kim J, Chauhan A (2008) Dexamethasone transport and ocular delivery from poly (hydroxyethyl methacrylate) gels. Int J Pharm 353(1–2):205–222
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Fojan Rafiei, Hadi Tabesh, and Farrokh Farzad declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rafiei, F., Tabesh, H. & Farzad, F. Sustained subconjunctival drug delivery systems: current trends and future perspectives. Int Ophthalmol 40, 2385–2401 (2020). https://doi.org/10.1007/s10792-020-01391-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01391-8