Abstract
Purpose
Due to the inimitable anatomical structure of the eyeball and various physiological barriers, conventional ocular local administration is often complicated by apparent shortcomings, such as limited bioavailability and short drug retention. Thus, developing methods for sustainable, safe and efficient drug delivery to ocular target sites has long been an urgent need. This study briefly summarizes the barriers to ocular drug administration and various ocular drug delivery routes and highlights recent progress in ocular implantable sustained-release drug delivery systems (DDSs) to provide literature evidence for developing novel ocular implants for sustained drug delivery.
Methods
We conducted a comprehensive search of studies on ocular implantable sustained-release DDSs in PubMed and Web of Science using the following keywords: ocular, implantable and drug delivery system. More than 400 papers were extracted. Publications focused on sustained and controlled drug release were primarily considered. Experimental articles involving DDSs that cannot be implanted into the eye through surgeries and cannot be inserted into ocular tissues in solid form were excluded. Approximately 143 publications were reviewed to summarize the most current information on the subject.
Results
In recent years, numerous ocular sustained-release DDSs using lipids, nanoparticles and hydrogels as carriers have emerged. With unique properties and systematic design, ocular implantable sustained-release DDSs are able to continuously maintain drug release, effectively sustain the therapeutic concentration in target tissues, and substantially enhance the therapeutic efficacy. Nevertheless, few ocular implantable sustained-release DDSs have been available in clinical use.
Conclusions
Ocular implantable sustained-release DDSs have become a new focus in the field of ocular drug development through unique designs and improvements in the materials of drug carriers, administration methods and dosage forms. With more ocular implantable sustained-release DDSs being commercialized, ocular therapeutics may be revolutionized.
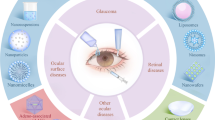
Graphical Abstract



Similar content being viewed by others
Abbreviations
- AMD:
-
Age-related macular degeneration
- BRB:
-
Blood retinal barrier
- CNV:
-
Choroidal neovascularization
- DDS:
-
Drug delivery system
- Dex:
-
Dexamethasone
- DR:
-
Diabetic retinopathy
- FA:
-
Fluocinolone acetonide
- HA:
-
Hyaluronic acid
- ILM:
-
Inner limiting membrane
- MN:
-
Microneedle
- MXF:
-
Oxifloxacin
- PDMS:
-
Polydimethylsiloxane
- PEGDM:
-
Poly (ethylene glycol) dimethacrylate
- pEOEMA:
-
Poly(2-ethoxyethyl methacrylate)
- P-gp:
-
P-glycoprotein
- pHEMA:
-
Poly(2-hydroxyethyl methacrylate)
- PLC:
-
Poly(D, L-lactide-co-ε-caprolactone)
- PLGA:
-
Polylactic-co-glycolic acid
- PVA:
-
Poly (vinyl alcohol)
- Rb:
-
Retinoblastoma
- RPE:
-
Retinal pigment epithelium
- TA:
-
Triamcinolone acetonide
- TEGDM:
-
Tri (ethylene glycol) dimethacrylate
- TPT:
-
Topotecan
- UNO:
-
Unoprostone
- URD:
-
UNO release device
References
Nayak K, Misra M (2018) A review on recent drug delivery systems for posterior segment of eye. Biomed Pharmacother 107:1564–1582. https://doi.org/10.1016/j.biopha.2018.08.138
Rafiei F, Tabesh H, Farzad F (2020) Sustained subconjunctival drug delivery systems: current trends and future perspectives. Int Ophthalmol 40(9):2385–2401. https://doi.org/10.1007/s10792-020-01391-8
Joseph RR, Venkatraman SS (2017) Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine Lond 12(6):683–702. https://doi.org/10.2217/nnm-2016-0379
Rodrigues GA, Lutz D, Shen J, Yuan X, Shen H, Cunningham J, Rivers HM (2018) Topical drug delivery to the posterior segment of the eye: addressing the challenge of preclinical to clinical translation. Pharm Res 35(12):245. https://doi.org/10.1007/s11095-018-2519-x
Burhan AM, Klahan B, Cummins W, Andrés-Guerrero V, Byrne ME, O’Reilly NJ, Chauhan A, Fitzhenry L, Hughes H (2021) Posterior Segment ophthalmic drug delivery: role of Muco-adhesion with a special focus on chitosan. Pharmaceutics 13(10):1685. https://doi.org/10.3390/pharmaceutics13101685
Awwad S, Mohamed Ahmed AHA, Sharma G, Heng JS, Khaw PT, Brocchini S, Lockwood A (2017) Principles of pharmacology in the eye. Br J Pharmacol 174(23):4205–4223. https://doi.org/10.1111/bph.14024
Kim HM, Woo SJ (2021) Ocular drug delivery to the retina: current innovations and future perspectives. Pharmaceutics 13(1):108. https://doi.org/10.3390/pharmaceutics13010108
Gote V, Sikder S, Sicotte J, Pal D (2019) Ocular drug delivery: present innovations and future challenges. J Pharmacol Exp Ther 370(3):602–624. https://doi.org/10.1124/jpet.119.256933
Löscher M, Seiz C, Hurst J, Schnichels S (2022) Topical drug delivery to the posterior segment of the eye. Pharmaceutics 14(1):134. https://doi.org/10.3390/pharmaceutics14010134
Mittal S, Miranda O (2018) Recent advancemnts in biodegradable ocular implants. Curr Drug Deliv 15(2):144–154. https://doi.org/10.2174/1567201814666170508104254
Mofidfar M, Abdi B, Ahadian S, Mostafavi E, Desai TA, Abbasi F, Sun Y, Manche EE, Ta CN, Flowers CW (2021) Drug delivery to the anterior segment of the eye: a review of current and future treatment strategies. Int J Pharm 607:120924. https://doi.org/10.1016/j.ijpharm.2021.120924
Gote V, Ansong M, Pal D (2020) Prodrugs and nanomicelles to overcome ocular barriers for drug penetration. Expert Opin Drug Metab Toxicol 16(10):885–906. https://doi.org/10.1080/17425255.2020.1803278
Sripetch S, Loftsson T (2021) Topical drug delivery to the posterior segment of the eye: thermodynamic considerations. Int J Pharm 597:120332. https://doi.org/10.1016/j.ijpharm.2021.120332
Huang D, Chen YS, Rupenthal ID (2018) Overcoming ocular drug delivery barriers through the use of physical forces. Adv Drug Deliv Rev 126:96–112. https://doi.org/10.1016/j.addr.2017.09.008
Li SK, Hao J (2018) Transscleral passive and iontophoretic transport: theory and analysis. Expert Opin Drug Deliv 15(3):283–299. https://doi.org/10.1080/17425247.2018.1406918
Kim YH, Oh J (2021) Choroidal thickness profile in chorioretinal diseases: beyond the macula. Front Med Lausanne 8:797428. https://doi.org/10.3389/fmed.2021.797428
Peynshaert K, Devoldere J, De Smedt SC, Remaut K (2018) In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv Drug Deliv Rev 126:44–57. https://doi.org/10.1016/j.addr.2017.09.007
Del Amo EM, Rimpelä AK, Heikkinen E, Kari OK, Ramsay E, Lajunen T, Schmitt M, Pelkonen L, Bhattacharya M, Richardson D, Subrizi A, Turunen T, Reinisalo M, Itkonen J, Toropainen E, Casteleijn M, Kidron H, Antopolsky M, Vellonen KS, Ruponen M, Urtti A (2017) Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res 57:134–185. https://doi.org/10.1016/j.preteyeres.2016.12.001
Loftsson T (2022) Topical drug delivery to the retina: obstacles and routes to success. Expert Opin Drug Deliv 19(1):9–21. https://doi.org/10.1080/17425247.2022.2017878
Varela-Fernández R, Díaz-Tomé V, Luaces-Rodríguez A, Conde-Penedo A, García-Otero X, Luzardo-Álvarez A, Fernández-Ferreiro A, Otero-Espinar FJ (2020) Drug delivery to the posterior segment of the eye: biopharmaceutic and pharmacokinetic considerations. Pharmaceutics 12(3):269. https://doi.org/10.3390/pharmaceutics12030269
Pavan B, Dalpiaz A (2018) Retinal pigment epithelial cells as a therapeutic tool and target against retinopathies. Drug Discov Today 23(9):1672–1679. https://doi.org/10.1016/j.drudis.2018.06.009
Madni A, Rahem MA, Tahir N, Sarfraz M, Jabar A, Rehman M, Kashif PM, Badshah SF, Khan KU, Santos HA (2017) Non-invasive strategies for targeting the posterior segment of eye. Int J Pharm 530(1–2):326–345. https://doi.org/10.1016/j.ijpharm.2017.07.065
Akhter MH, Ahmad I, Alshahrani MY, Al-Harbi AI, Khalilullah H, Afzal O, Altamimi ASA, Najib Ullah SNM, Ojha A, Karim S (2022) Drug delivery challenges and current progress in nanocarrier-based ocular therapeutic system. Gels 8(2):82. https://doi.org/10.3390/gels8020082
Yellepeddi VK, Palakurthi S (2016) Recent advances in topical ocular drug delivery. J Ocul Pharmacol Ther 32(2):67–82. https://doi.org/10.1089/jop.2015.0047
Vellonen KS, Hellinen L, Mannermaa E, Ruponen M, Urtti A, Kidron H (2018) Expression, activity and pharmacokinetic impact of ocular transporters. Adv Drug Deliv Rev 126:3–22. https://doi.org/10.1016/j.addr.2017.12.009
Agban Y, Thakur SS, Mugisho OO, Rupenthal ID (2019) Depot formulations to sustain periocular drug delivery to the posterior eye segment. Drug Discov Today 24(8):1458–1469. https://doi.org/10.1016/j.drudis.2019.03.023
Rimpelä AK, Reinisalo M, Hellinen L, Grazhdankin E, Kidron H, Urtti A, Del Amo EM (2018) Implications of melanin binding in ocular drug delivery. Adv Drug Deliv Rev 126:23–43. https://doi.org/10.1016/j.addr.2017.12.008
Teng YN, Chen LH, Chen Kui Vavulengan YH (2022) Repositioning application of polyoxyethylene (20) sorbitan monooleate on ocular drug resistance and cancer multi-drug resistance by inhibiting the ATPase activity of human multidrug resistance protein 1 and P-glycoprotein. Eur J Pharm Biopharm 170:77–90. https://doi.org/10.1016/j.ejpb.2021.12.002
Janga KY, Tatke A, Shukla S, Lamichhane SP, Avula B, Wang X, Jablonski MM, Khan IA, Majumdar S (2018) Retina compatible interactions and effective modulation of blood ocular barrier P-gp activity by third-generation inhibitors improve the ocular penetration of loperamide. J Pharm Sci 107(8):2128–2135. https://doi.org/10.1177/1747493018778713
Djebli N, Khier S, Griguer F, Coutant AL, Tavernier A, Fabre G, Leriche C, Fabre D (2017) Ocular drug distribution after topical administration: population pharmacokinetic model in rabbits. Eur J Drug Metab Pharmacokinet 42(1):59–68. https://doi.org/10.1007/s13318-016-0319-4
Bachu RD, Chowdhury P, Al-Saedi ZHF, Karla PK, Boddu SHS (2018) Ocular drug delivery barriers-role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 10(1):28. https://doi.org/10.3390/pharmaceutics10010028
Durgun ME, Güngör S, Özsoy Y (2020) Micelles: promising ocular drug carriers for anterior and posterior segment diseases. J Ocul Pharmacol Ther 36(6):323–341. https://doi.org/10.1089/jop.2019.0109
Alami-Milani M, Zakeri-Milani P, Valizadeh H, Fathi M, Salatin S, Salehi R, Jelvehgari M (2020) PLA-PCL-PEG-PCL-PLA based micelles for improving the ocular permeability of dexamethasone: development, characterization, and in vitro evaluation. Pharm Dev Technol 25(6):704–719. https://doi.org/10.1080/10837450.2020.1733606
Alami-Milani M, Zakeri-Milani P, Valizadeh H, Salehi R, Jelvehgari M (2018) Preparation and evaluation of PCL-PEG-PCL micelles as potential nanocarriers for ocular delivery of dexamethasone. Iran J Basic Med Sci 21(2):153–164. https://doi.org/10.22038/IJBMS.2017.26590.6513
Barbosa-Alfaro D, Andrés-Guerrero V, Fernandez-Bueno I, García-Gutiérrez MT, Gil-Alegre E, Molina-Martínez IT, Pastor-Jimeno JC, Herrero-Vanrell R, Bravo-Osuna I (2021) Dexamethasone PLGA microspheres for sub-tenon administration: influence of sterilization and tolerance studies. Pharmaceutics 13(2):228. https://doi.org/10.3390/pharmaceutics13020228
Lindholm JM, Taipale C, Ylinen P, Tuuminen R (2020) Perioperative subconjunctival triamcinolone acetonide injection for prevention of inflammation and macular oedema after cataract surgery. ACTA Ophthalmol 98(1):36–42. https://doi.org/10.1111/aos.14175
Yavuz B, Bozdağ Pehlivan S, Kaffashi A, Çalamak S, Ulubayram K, Palaska E, Çakmak HB, Ünlü N (2016) In vivo tissue distribution and efficacy studies for cyclosporin a loaded nano-decorated subconjunctival implants. Drug Deliv 23(9):3279–3284. https://doi.org/10.3109/10717544.2016.1172368
Wang R, Gao Y, Liu A, Zhai G (2021) A review of nanocarrier-mediated drug delivery systems for posterior segment eye disease: challenges analysis and recent advances. J Drug Target 29(7):687–702. https://doi.org/10.1080/1061186X.2021.1878366
Huang Z, Yang W, Zong Y, Qiu S, Chen X, Sun X, Zhou Y, Xie Z, Gao Q (2016) A study of the dexamethasone sodium phosphate release properties from a periocular capsular drug delivery system. Drug Deliv 23(3):849–857. https://doi.org/10.3109/10717544.2014.919543
Huang X, Peng M, Yang Y, Duan Y, Li K, Liu S, Ye C, Lin D (2017) Dexamethasone distribution characteristic following controllable continuous sub-tenon drug delivery in rabbit. Drug Deliv 24(1):818–824. https://doi.org/10.1080/10717544.2017.1324531
Yang YH, Hsu WC, Hsieh YT (2021) Anterior migration of triamcinolone acetonide after posterior subtenon injection for macular edema predisposes to intraocular pressure elevation. Curr Eye Res 46(5):689–693. https://doi.org/10.1080/02713683.2020.1826979
Chiang B, Jung JH, Prausnitz MR (2018) The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev 126:58–66. https://doi.org/10.1016/j.addr.2018.03.001
Sher I, Goldberg Z, Bubis E, Barak Y, Rotenstreich Y (2021) Suprachoroidal delivery of bevacizumab in rabbit in vivo eyes: Rapid distribution throughout the posterior segment. Eur J Pharm Biopharm 169:200–210. https://doi.org/10.1016/j.ejpb.2021.10.003
Hanif J, Iqbal K, Perveen F, Arif A, Iqbal RN, Jameel F, Hanif K, Seemab A, Khan AY, Ahmed M (2021) Safety and efficacy of suprachoroidal injection of triamcinolone in treating macular edema secondary to noninfectious uveitis. Cureus 13(11):e20038. https://doi.org/10.7759/cureus.20038
Iovino C, Mastropasqua R, Lupidi M, Bacherini D, Pellegrini M, Bernabei F, Borrelli E, Sacconi R, Carnevali A, D’Aloisio R, Cerquaglia A, Finocchio L, Govetto A, Erba S, Triolo G, Di Zazzo A, Forlini M, Vagge A, Giannaccare G (2020) Intravitreal dexamethasone implant as a sustained release drug delivery device for the treatment of ocular diseases: a comprehensive review of the literature. Pharmaceutics 12(8):703. https://doi.org/10.3390/pharmaceutics12080703
Mezu-Ndubuisi OJ, Wang Y, Schoephoerster J, Falero-Perez J, Zaitoun IS, Sheibani N, Gong S (2019) Intravitreal delivery of VEGF-A165-loaded PLGA microparticles reduces retinal vaso-obliteration in an in vivo mouse model of retinopathy of prematurity. Curr Eye Res 44(3):275–286. https://doi.org/10.1080/02713683.2018.1542736
Bisht R, Jaiswal JK, Chen YS, Jin J, Rupenthal ID (2016) Light-responsive in situ forming injectable implants for effective drug delivery to the posterior segment of the eye. Expert Opin Drug Deliv 13(7):953–962. https://doi.org/10.1517/17425247.2016.1163334
Naguib S, Bernardo-Colón A, Rex TS (2021) Intravitreal injection worsens outcomes in a mouse model of indirect traumatic optic neuropathy from closed globe injury. Exp Eye Res 202:108369. https://doi.org/10.1016/j.exer.2020.108369
Urban B, Szwabowicz M, Bakunowicz-Łazarczyk A (2020) Effect of repeated intravitreal ranibizumab and aflibercept injections on the cornea in patients with age-related macular degeneration. J Ophthalmol 2020:4928905. https://doi.org/10.1155/2020/4928905
Qamar Z, Qizilbash FF, Iqubal MK, Ali A, Narang JK, Ali J, Baboota S (2019) Nano-based drug delivery system: recent strategies for the treatment of ocular disease and future perspective. Recent Pat Drug Deliv Formul 13(4):246–254. https://doi.org/10.2174/1872211314666191224115211
Nirbhavane P, Sharma G, Singh B, Begum G, Jones MC, Rauz S, Vincent R, Denniston AK, Hill LJ, Katare OP (2020) Triamcinolone acetonide loaded-cationic nano-lipoidal formulation for uveitis: Evidences of improved biopharmaceutical performance and anti-inflammatory activity. Colloids Surf B Biointerfaces 190:110902. https://doi.org/10.1016/j.colsurfb.2020.110902
Srinivasarao DA, Lohiya G, Katti DS (2019) Fundamentals, challenges, and nanomedicine-based solutions for ocular diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol 11(4):e1548. https://doi.org/10.1002/wnan.1548
Vaneev A, Tikhomirova V, Chesnokova N, Popova E, Beznos O, Kost O, Klyachko N (2021) Nanotechnology for topical drug delivery to the anterior segment of the eye. Int J Mol Sci 22(22):12368. https://doi.org/10.3390/ijms222212368
Yu A, Shi H, Liu H, Bao Z, Dai M, Lin D, Lin D, Xu X, Li X, Wang Y (2020) Mucoadhesive dexamethasone-glycol chitosan nanoparticles for ophthalmic drug delivery. Int J Pharm 575:118943. https://doi.org/10.1016/j.ijpharm.2019.118943
Cooper RC, Yang H (2019) Hydrogel-based ocular drug delivery systems: emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J Control Release 306:29–39. https://doi.org/10.1016/j.jconrel.2019.05.034
Egbu R, Brocchini S, Khaw PT, Awwad S (2018) Antibody loaded collapsible hyaluronic acid hydrogels for intraocular delivery. Eur J Pharm Biopharm 124:95–103. https://doi.org/10.1016/j.ejpb.2017.12.019
Kim DJ, Jung MY, Park JH, Pak HJ, Kim M, Chuck RS, Park CY (2021) Moxifloxacin releasing intraocular implant based on a cross-linked hyaluronic acid membrane. Sci Rep 11(1):24115. https://doi.org/10.1038/s41598-021-03605-0
Su Y, Zhang B, Sun R, Liu W, Zhu Q, Zhang X, Wang R, Chen C (2021) PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Deliv 28(1):1397–1418. https://doi.org/10.1080/10717544.2021.1938756
Lee K, Song HB, Cho W, Kim JH, Kim JH, Ryu W (2018) Intracorneal injection of a detachable hybrid microneedle for sustained drug delivery. ACTA Biomater 80:48–57. https://doi.org/10.1016/j.actbio.2018.09.039
Than A, Liu C, Chang H, Duong PK, Cheung CMG, Xu C, Wang X, Chen P (2018) Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat Commun 9(1):4433. https://doi.org/10.1038/s41467-018-06981-w
Liu YC, Ng XW, Teo EPW, Ang HP, Lwin NC, Chan NSW, Venkatraman SS, Wong TT, Mehta JS (2018) A biodegradable, sustained-released, tacrolimus microfilm drug delivery system for the management of allergic conjunctivitis in a mouse model. Invest Ophthalmol Vis Sci 59(2):675–684. https://doi.org/10.1167/iovs.17-23066
Liu YC, Ng AHC, Ng XW, Yan P, Venkatraman SS, Mehta JS, Wong TT (2017) Evaluation of a sustained-release prednisolone acetate biodegradable subconjunctival implant in a non-human primate model. Transl Vis Sci Technol 6(5):9. https://doi.org/10.1167/tvst.6.5.9
Zhou C, Robert MC, Kapoulea V, Lei F, Stagner AM, Jakobiec FA, Dohlman CH, Paschalis EI (2017) Sustained subconjunctival delivery of infliximab protects the cornea and retina following alkali burn to the eye. Invest Ophthalmol Vis Sci 58(1):96–105. https://doi.org/10.1167/iovs.16-20339
Nagai N, Saijo S, Song Y, Kaji H, Abe T (2019) A drug refillable device for transscleral sustained drug delivery to the retina. Eur J Pharm Biopharm 136:184–191. https://doi.org/10.1016/j.ejpb.2019.01.024
Zhou C, Singh A, Qian G, Wolkow N, Dohlman CH, Vavvas DG, Chodosh J, Paschalis EI (2020) Microporous drug delivery system for sustained anti-VEGF delivery to the eye. Transl Vis Sci Technol 9(8):5. https://doi.org/10.1167/tvst.9.8.5
Tsuruma K, Tanaka Y, Shimazawa M, Mashima Y, Hara H (2011) Unoprostone reduces oxidative stress- and light-induced retinal cell death, and phagocytotic dysfunction, by activating BK channels. Mol Vis 17:3556–3565
Nagai N, Koyanagi E, Izumida Y, Liu J, Katsuyama A, Kaji H, Nishizawa M, Osumi N, Kondo M, Terasaki H, Mashima Y, Nakazawa T, Abe T (2016) Long-term protection of genetically ablated rabbit retinal degeneration by sustained transscleral unoprostone delivery. Invest Ophthalmol Vis Sci 57(15):6527–6538. https://doi.org/10.1167/iovs.16-20453
Nagai N, Yamada S, Kawasaki J, Koyanagi E, Saijo S, Kaji H, Nishizawa M, Nakazawa T, Abe T (2018) Pharmacokinetic and safety evaluation of a transscleral sustained unoprostone release device in monkey eyes. Invest Ophthalmol Vis Sci 59(2):644–652
Nagai N, Nezhad ZK, Daigaku R, Saijo S, Song Y, Terata K, Hoshi A, Nishizawa M, Nakazawa T, Kaji H, Abe T (2019) Transscleral sustained ranibizumab delivery using an episcleral implantable device: Suppression of laser-induced choroidal neovascularization in rats. Int J Pharm 567:118458. https://doi.org/10.1016/j.ijpharm.2019.118458
Sato Y, Nagai N, Abe T, Kaji H (2019) A multilayered sheet-type device capable of sustained drug release and deployment control. Biomed Microdevices 21(3):60. https://doi.org/10.1007/s10544-019-0411-z
Kojima H, Raut B, Chen LJ, Nagai N, Abe T, Kaji H (2020) A 3D printed self-sustainable cell-encapsulation drug delivery device for periocular transplant-based treatment of retinal degenerative diseases. Micromachines Basel 11(4):436. https://doi.org/10.3390/mi11040436
Munier FL, Beck-Popovic M, Chantada GL, Cobrinik D, Kivelä TT, Lohmann D, Maeder P, Moll AC, Carcaboso AM, Moulin A, Schaiquevich P, Bergin C, Dyson PJ, Houghton S, Puccinelli F, Vial Y, Gaillard MC, Stathopoulos C (2019) Conservative management of retinoblastoma: challenging orthodoxy without compromising the state of metastatic grace Alive, with good vision and no comorbidity. Prog Retin Eye Res 73:100764. https://doi.org/10.1016/j.preteyeres.2019.05.005
Fabian ID, Onadim Z, Karaa E, Duncan C, Chowdhury T, Scheimberg I, Ohnuma SI, Reddy MA, Sagoo MS (2018) The management of retinoblastoma. Oncogene 37(12):1551–1560. https://doi.org/10.1038/s41388-017-0050-x
Cocarta AI, Hobzova R, Sirc J, Cerna T, Hrabeta J, Svojgr K, Pochop P, Kodetova M, Jedelska J, Bakowsky U, Uhlik J (2019) Hydrogel implants for transscleral drug delivery for retinoblastoma treatment. Mater Sci Eng C Mater Biol Appl 103:109799. https://doi.org/10.1016/j.msec.2019.109799
Hobzova R, Kodetova M, Pochop P, Uhlik J, Dunovska K, Svojgr K, Hrabeta J, Feriancikova B, Cocarta AI, Sirc J (2021) Hydrogel implants for transscleral diffusion delivery of topotecan: In vivo proof of concept in a rabbit eye model. Int J Pharm 606:120832. https://doi.org/10.1016/j.ijpharm.2021.120832
Covert JC, Thomasy SM, Kado-Fong H, Kon LN, Kass PH, Reilly CM, Lappin MR, Margulies BJ, Maggs DJ (2019) Pilot study of the safety and tolerability of a subconjunctival penciclovir implant in cats experimentally infected with herpesvirus. J Ocul Pharmacol Ther 35(1):38–49. https://doi.org/10.1089/jop.2018.0043
Solano AGR, de Fátima PA, de Faria LGA, Fialho SL, de Oliveira Patricio PS, da Silva-Cunha A, Fulgêncio GO, da Silva GR, Pianetti GA (2018) Etoposide-loaded poly(lactic-co-glycolic acid) intravitreal implants in vitro and in vivo evaluation. AAPS Pharm Sci Tech 19(4):1652–1661. https://doi.org/10.1208/s12249-018-0978-3
Wang C, Seo SJ, Kim JS, Lee SH, Jeon JK, Kim JW, Kim KH, Kim JK, Park J (2018) Intravitreal implantable magnetic micropump for on-demand VEGFR-targeted drug delivery. J Control Release 283:105–112. https://doi.org/10.1016/j.jconrel.2018.05.030
Iyer S, Radwan AE, Hafezi-Moghadam A, Malyala P, Amiji M (2019) Long-acting intraocular delivery strategies for biological therapy of age-related macular degeneration. J Control Release 296:140–149. https://doi.org/10.1016/j.jconrel.2019.01.007
Leinonen S, Immonen I, Kotaniemi K (2018) Fluocinolone acetonide intravitreal implant (Retisert® ) in the treatment of sight threatening macular oedema of juvenile idiopathic arthritis-related uveitis. Acta Ophthalmol 96(6):648–651. https://doi.org/10.1111/aos.13744
Behar-Cohen F (2019) Recent advances in slow and sustained drug release for retina drug delivery. Expert Opin Drug Deliv 16(7):679–686. https://doi.org/10.1080/17425247.2019.1618829
Miguel-Escuder L, Olate-Pérez Á, Sala-Puigdoners A, Moll-Udina A, Figueras-Roca M, Navarro-Angulo MJ, Adán A, Pelegrín L (2021) Intravitreal fluocinolone acetonide implant for the treatment of persistent post-surgical cystoid macular edema in vitrectomized eyes. Eur J Ophthalmol. https://doi.org/10.1177/11206721211046718
Cao Y, Samy KE, Bernards DA, Desai TA (2019) Recent advances in intraocular sustained-release drug delivery devices. Drug Discov Today 24(8):1694–1700. https://doi.org/10.1016/j.drudis.2019.05.031
Lee A, Blair HA (2020) Dexamethasone intracanalicular insert: a review in treating post-surgical ocular pain and inflammation. Drugs 80(11):1101–1108. https://doi.org/10.1007/s40265-020-01344-6
Trivedi RH, Wilson ME (2021) A sustained-release intracanalicular dexamethasone insert (Dextenza) for pediatric cataract surgery. J AAPOS 25(1):43–45. https://doi.org/10.1016/j.jaapos.2020.10.001
Shirley M (2020) Bimatoprost implant: first approval. Drugs Aging 37(6):457–462. https://doi.org/10.1007/s40266-020-00769-8
Kesav NP, Young CEC, Ertel MK, Seibold LK, Kahook MY (2021) Sustained-release drug delivery systems for the treatment of glaucoma. Int J Ophthalmol 14(1):148–159. https://doi.org/10.18240/ijo.2021.01.21
Funding
This work was supported by the National Natural Science Foundation of China (No. 82171053, 81570864), and the Natural Science Foundation of Jilin Province (No. 20200801043GH; No. 20190201083JC).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Yun-Yi Cong and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
Yun-Yi Cong, Bin Fan, Zi-Yuan Zhang and Guang-Yu Li declare they have no financial interests.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cong, YY., Fan, B., Zhang, ZY. et al. Implantable sustained-release drug delivery systems: a revolution for ocular therapeutics. Int Ophthalmol 43, 2575–2588 (2023). https://doi.org/10.1007/s10792-023-02637-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02637-x