Abstract
Purpose
To analyze the protective effect of PARP inhibitors on light-damaged retina and explore its possible mechanism from the perspective of ciliopathy.
Methods
A systematic review of the literature was performed to investigate the protection of PARP inhibition on light-damaged cilia. PubMed database was retrieved to find the relevant studies and 119 literatures were involved in the review.
Results
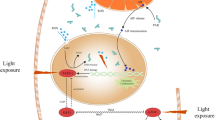
In retina, the outer segment of photoreceptor is regarded as a special type of primary cilium, so various retinal diseases actually belong to a type of ciliopathy. The retina is the only central nervous tissue exposed to light, but poly (ADP-ribose) polymerase (PARP), as a nuclear enzyme repairing DNA breaks, is overactivated during the light-induced DNA damage, and is involved in the cell death cascade. Studies show that both ATR and phosphorylated Akt colocalize with cilium and play an important role in regulating ciliary function. PARP may function at ATR or PI3K/Akt signal to exert protective effect on cilia.
Conclusion
PARP inhibitors may protect the cilia/OS of photoreceptor during light-induced damage, which the possible mechanism may be involved in the activation of ATR and PI3K/Akt signal.


Similar content being viewed by others
References
Lee JE, Gleeson JG (2011) Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol 24(2):98–105
Rao Damerla R, Gabriel GC, Li Y et al (2014) Role of cilia in structural birth defects: insights from ciliopathy mutant mouse models. Birth Defects Res C Embryo Today 102(2):115–125
Otto EA, Schermer B, Obara T et al (2003) Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 34(4):413–420
Zariwala MA, Knowles MR, Omran H (2007) Genetic defects in ciliary structure and function. Annu Rev Physiol 69:423–450
Adams M, Smith UM, Logan CV, Johnson CA (2008) Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J Med Genet 45(5):257–267
Marshall WF (2008) The cell biological basis of ciliary disease. J Cell Biol 180(1):17–21
Gerdes JM, Davis EE, Katsanis N (2009) The vertebrate primary cilium in development, homeostasis, and disease. Cell 137(1):32–45
Hildebrandt F, Benzing T, Katsanis N (2011) Ciliopathies. N Engl J Med 364(16):1533–1543
Hosokawa Y, Miki-Noumura T (1987) Bending motion of Chlamydomonas axonemes after extrusion of central-pair microtubules. J Cell Biol 105(3):1297–1301
Dentler WL (1980) Structures linking the tips of ciliary and flagellar microtubules to the membrane. J Cell Sci 42:207–220
Dentler WL, Pratt MM, Stephens RE (1980) Microtubule-membrane interactions in cilia. II. Photochemical cross-linking of bridge structures and the identification of a membrane-associated dynein-like ATPase. J Cell Biol 84(2):381–403
Brokaw CJ, Kamiya R (1987) Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskelet 8(1):68–75
Rosenbaum JL, Witman GB (2002) Intraflagellar transport. Nat Rev Mol Cell Biol 3(11):813–825
Marshall WF (2010) Cilia self-organize in response to planar cell polarity and flow. Nat Cell Biol 12(4):314–315
Fischer E, Pontoglio M (2009) Planar cell polarity and cilia. Semin Cell Dev Biol 20(8):998–1005
Kim S, Tsiokas L (2011) Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle 10(16):2683–2690
Jackson PK (2011) Do cilia put brakes on the cell cycle? Nat Cell Biol 13(4):340–342
Ko HW, Norman RX, Tran J, Fuller KP, Fukuda M, Eggenschwiler JT (2010) Broad-minded links cell cycle-related kinase to cilia assembly and hedgehog signal transduction. Dev Cell 18(2):237–247
Zhou J (2009) Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol 71:83–113
Ishikawa H, Marshall WF (2011) Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 12(4):222–234
Pazour GJ, Dickert BL, Vucica Y et al (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151(3):709–718
Reiter JF, Blacque OE, Leroux MR (2012) The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep 13(7):608–618
Kim S, Dynlacht BD (2013) Assembling a primary cilium. Curr Opin Cell Biol 25(4):506–511
Graser S, Stierhof YD, Lavoie SB et al (2007) Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol 179(2):321–330
Schmidt KN, Kuhns S, Neuner A, Hub B, Zentgraf H, Pereira G (2012) Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol 199(7):1083–1101
Goetz SC, Liem KF Jr, Anderson KV (2012) The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell 151(4):847–858
Tanos BE, Yang HJ, Soni R et al (2013) Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev 27(2):163–168
Kuhns S, Schmidt KN, Reymann J et al (2013) The microtubule affinity regulating kinase MARK4 promotes axoneme extension during early ciliogenesis. J Cell Biol 200(4):505–522
Veleri S, Manjunath SH, Fariss RN et al (2014) Ciliopathy-associated gene Cc2d2a promotes assembly of subdistal appendages on the mother centriole during cilia biogenesis. Nat Commun 20(5):4207
Inoko A, Matsuyama M, Goto H et al (2012) Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J Cell Biol 197(3):391–405
Bettencourt-Dias M, Carvalho-Santos Z (2008) Double life of centrioles: CP110 in the spotlight. Trends Cell Biol 18(1):8–11
Kobayashi T, Tsang WY, Li J, Lane W, Dynlacht BD (2011) Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell 145(6):914–925
Huang N, Zhang D, Li F et al (2018) M-Phase Phosphoprotein 9 regulates ciliogenesis by modulating CP110-CEP97 complex localization at the mother centriole. Nat Commun. 9(1):4511
Tsang WY, Dynlacht BD (2013) CP110 and its network of partners coordinately regulate cilia assembly. Cilia 2(1):9
Chang B, Khanna H, Hawes N et al (2006) In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet 15(11):1847–1857
Tsang WY, Bossard C, Khanna H et al (2008) CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell 15(2):187–197
Kobayashi T, Kim S, Lin YC, Inoue T, Dynlacht BD (2014) The CP110-interacting proteins Talpid3 and Cep290 play overlapping and distinct roles in cilia assembly. J Cell Biol 204(2):215–229
Pan J (2008) Cilia and ciliopathies: from Chlamydomonas and beyond. Sci China C Life Sci 51(6):479–486
Wang Q, Pan J, Snell WJ (2006) Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell 125(3):549–562
Fliegauf M, Benzing TH (2007) When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 8(11):880–893
Sjöstrand FS (1953) The ultrastructure of the outer segments of rods and cones of the eye as revealed by the electron microscope. J Cell Physiol 42(1):15–44
Ramamurthy V, Cayouette M (2009) Development and disease of the photoreceptor cilium. Clin Genet 76(2):137–145
Lepanto P, Davison C, Casanova G, Badano JL, Zolessi FR (2016) Characterization of primary cilia during the differentiation of retinal ganglion cells in the zebrafish. Neural Dev 11(1):10
Shaban H, Richter C (2002) A2E and blue light in the retina: the paradigm of age-related macular degeneration. Biol Chem. Mar-Apr 383(3–4):537–545
Andrews LD, Cohen AI (1979) Freeze-fracture evidence for the presence of cholesterol in particle-free patches of basal disks and the plasma membrane of retinal rod outer segments of mice and frogs. J Cell Biol 81(1):215–228
Caldwell RB, McLaughlin BJ (1985) Freeze-fracture study of filipin binding in photoreceptor outer segments and pigment epithelium of dystrophic and normal retinas. J Comp Neurol 236(4):523–537
Reme CE, Malnoe A, Jung HH, Wei Q, Munz K (1994) Effect of dietary fish oil on acute light-induced photoreceptor damage in the rat retina. Invest Ophthalmol Vis Sci 35(1):78–90
Wang JY, Saito M (2001) Dietary supplementation of N-3 fatty acids and hydroperoxide levels in rat retinas. Free Radic Res 35(4):367–375
Wiegand RD, Giusto NM, Rapp LM, Anderson RE (1983) Evidence for rod outer segment lipid peroxidation following constant illumination of the rat retina. Invest Ophthalmol Vis Sci 24(10):1433–1435
Richards MJ, Nagel BA, Fliesler SJ (2006) Lipid hydroperoxide formation in the retina: correlation with retinal degeneration and light damage in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res 82(3):538–541
Sanvicens N, Gomez-Vicente V, Masip I, Messeguer A, Cotter TG (2004) Oxidative stress-induced apoptosis in retinal photoreceptor cells is mediated by calpains and caspases and blocked by the oxygen radical scavenger CR-6. J Biol Chem 279(38):39268–39278
Edward DP, Lam TT, Shahinfar S, Li J, Tso MO (1991) Amelioration of light-induced retinal degeneration by a calcium overload blocker. Flunarizine. Arch Ophthalmol 109(4):554–562
Hao W, Wenzel A, Obin MS et al (2002) Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet 32(2):254–260
Yuan J, Lipinski M, Degterev A (2003) Diversity in the mechanisms of neuronal cell death. Neuron 40(2):401–413
Kishimoto N, Ohkuma H, Uyama M (1991) Detection of destruction of anionic sites in the outer blood-retinal barrier and damage caused by iron. Nippon Ganka Gakkai Zasshi 95(2):130–139
Liu B, Hunter DJ, Rooker S et al (2013) Wnt signaling promotes Muller cell proliferation and survival after injury. Invest Ophthalmol Vis Sci 54(1):444–453
Harada T, Harada C, Nakayama N et al (2000) Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron 26(2):533–541
Fontaine V, Kinkl N, Sahel J, Dreyfus H, Hicks D (1998) Survival of purified rat photoreceptors in vitro is stimulated directly by fibroblast growth factor-2. J Neurosci Off J Soc Neurosci 18(23):9662–9672
Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G (1987) Structural characterization and biological functions of fibroblast growth factor. Endocr Rev 8(2):95–114
Noell WK, Walker VS, Kang BS, Berman S (1966) Retinal damage by light in rats. Invest Ophthalmol 5(5):450–473
Kaitz M, Auerbach E (1979) Action spectrum for light-induced retinal degeneration in dystrophic rats. Vision Res 19(9):1041–1044
Williams TP, Howell WL (1983) Action spectrum of retinal light-damage in albino rats. Invest Ophthalmol Vis Sci 24(3):285–287
Peters S, Schraermeyer U (2001) Characteristics and functions of melanin in retinal pigment epithelium. Ophthalmologe 98(12):1181–1185
Sundelin S, Wihlmark U, Nilsson SE, Brunk UT (1998) Lipofuscin accumulation in cultured retinal pigment epithelial cells reduces their phagocytic capacity. Curr Eye Res 17(8):851–857
Powell SR, Wang P, Divald A et al (2005) Aggregates of oxidized proteins (lipofuscin) induce apoptosis through proteasome inhibition and dysregulation of proapoptotic proteins. Free Radic Biol Med 38(8):1093–1101
Szabo C, Ohshima H (1997) DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide 1(5):373–385
Schreiber V, Dantzer F, Ame JC, de Murcia G (2006) Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 7(7):517–528
Hassa PO, Hottiger MO (2008) The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci. 13:3046–3082
Scott GS, Szabo C, Hooper DC (2004) Poly(ADP-ribose) polymerase activity contributes to peroxynitrite-induced spinal cord neuronal cell death in vitro. J Neurotrauma 21(9):1255–1263
Koh DW, Dawson TM, Dawson VL (2005) Mediation of cell death by poly(ADP-ribose) polymerase-1. Pharmacol Res 52(1):5–14
Jagtap P, Szabo C (2005) Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov 4(5):421–440
Li GY, Fan B, Ma TH (2011) Visible light may directly induce nuclear DNA damage triggering the death pathway in RGC-5 cells. Mol Vis. 17:3279–3289
Pacher P, Szabo C (2008) Role of the Peroxynitrite-Poly(ADP-Ribose) Polymerase Pathway in Human Disease. Am J Pathol 173(1):2–13
Virág L, Szabó C (2002) The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 54(54):375–429
Islam BU, Habib S, Ahmad P, Allarakha S, Moinuddin Ali A (2015) Pathophysiological role of peroxynitrite induced DNA damage in human diseases: a special focus on Poly(ADP-ribose) Polymerase (PARP). Indian J Clin Biochem. 30(4):368–385
Aredia F, Scovassi AI (2014) Poly(ADP-ribose): a signaling molecule in different paradigms of cell death. Biochem Pharmacol 92(1):157–163
Burkle A, Virag L (2013) Poly(ADP-ribose): PARadigms and PARadoxes. Mol Aspects Med 34(6):1046–1065
David KK, Andrabi SA, Dawson TM, Dawson VL (2009) Parthanatos, a messenger of death. Front Biosci (Landmark Ed) 14:1116–1128
Wang Y, Dawson VL, Dawson TM (2009) Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol 218(2):193–202
Andrabi SA, Dawson TM, Dawson VL (2008) Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann N Y Acad Sci 1147:233–241
Beneke S (2008) Poly(ADP-ribose) polymerase activity in different pathologies–the link to inflammation and infarction. Exp Gerontol 43(7):605–614
Paquet-Durand F, Silva J, Talukdar T et al (2007) Excessive activation of poly(ADP-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse. J Neurosci 27(38):10311–10319
Arango-Gonzalez B, Trifunovic D, Sahaboglu A et al (2014) Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS ONE 9(11):e112142
Jiao K, Sahaboglu A, Zrenner E, Ueffing M, Ekstrom PA, Paquet-Durand F (2016) Efficacy of PARP inhibition in Pde6a mutant mouse models for retinitis pigmentosa depends on the quality and composition of individual human mutations. Cell Death Discov 2:16040
Sahaboglu A, Tanimoto N, Kaur J et al (2010) PARP1 gene knock-out increases resistance to retinal degeneration without affecting retinal function. PLoS ONE 5(11):e15495
Sahaboglu A, Sharif A, Feng L, Secer E, Zrenner E, Paquet-Durand F (2017) Temporal progression of PARP activity in the Prph2 mutant rd2 mouse: neuroprotective effects of the PARP inhibitor PJ34. PLoS ONE 12(7):e0181374
Vidal-Gil L, Sancho-Pelluz J, Zrenner E, Oltra M, Sahaboglu A (2019) Poly ADP ribosylation and extracellular vesicle activity in rod photoreceptor degeneration. Sci Rep. 9(1):3758
Cimprich KA, Cortez D (2008) ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol 9(8):616–627
Kim H, George E, Ragland R et al (2017) Targeting the ATR/CHK1 axis with PARP inhibition results in tumor regression in BRCA-mutant ovarian cancer models. Clin Cancer Res 23(12):3097–3108
Peasland A, Wang LZ, Rowling E et al (2011) Identification and evaluation of a potent novel ATR inhibitor, NU6027, in breast and ovarian cancer cell lines. Br J Cancer 105(3):372–381
Yazinski SA, Comaills V, Buisson R et al (2017) ATR inhibition disrupts rewired homologous recombination and fork protection pathways in PARP inhibitor-resistant BRCA-deficient cancer cells. Genes Dev 31(3):318–332
Huehls AM, Wagner JM, Huntoon CJ, Karnitz LM (2012) Identification of DNA repair pathways that affect the survival of ovarian cancer cells treated with a Poly(ADP-Ribose) polymerase inhibitor in a novel drug combination. Mol Pharmacol 82(4):767
Ogiwara H, Ui A, Shiotani B, Zou L, Yasui A, Kohno T (2013) Curcumin suppresses multiple DNA damage response pathways and has potency as a sensitizer to PARP inhibitor. Carcinogenesis 34(11):2486
Abu-Sanad A, Wang Y, Hasheminasab F et al (2015) Simultaneous inhibition of ATR and PARP sensitizes colon cancer cell lines to irinotecan. Front Pharmacol 6:147
Yang J, Yu Y, Hamrick HE, Duerksen-Hughes PJ (2003) ATM, ATR and DNA-PK: initiators of the cellular genotoxic stress responses. Carcinogenesis 24(10):1571–1580
Lavin MF, Kozlov S (2007) DNA damage-induced signalling in ataxia-telangiectasia and related syndromes. Radiother Oncol 83(3):231–237
O’Driscoll M (2009) Mouse models for ATR deficiency. DNA Repair (Amst) 8(11):1333–1337
O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA (2003) A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 33(4):497–501
Sivasubramaniam S, Sun X, Pan YR, Wang S, Lee EY (2008) Cep164 is a mediator protein required for the maintenance of genomic stability through modulation of MDC1, RPA, and CHK1. Genes Dev 22(5):587–600
Pan YR, Lee EY (2009) UV-dependent interaction between Cep164 and XPA mediates localization of Cep164 at sites of DNA damage and UV sensitivity. Cell Cycle 8(4):655–664
Walz G (2017) Role of primary cilia in non-dividing and post-mitotic cells. Cell Tissue Res 369(1):11–25
Valdes-Sanchez L, De la Cerda B, Diaz-Corrales FJ et al (2013) ATR localizes to the photoreceptor connecting cilium and deficiency leads to severe photoreceptor degeneration in mice. Hum Mol Genet 22(8):1507–1515
Lempiainen H, Halazonetis TD (2009) Emerging common themes in regulation of PIKKs and PI3Ks. EMBO J 28(20):3067–3073
Stiff T, Walker SA, Cerosaletti K et al (2006) ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J 25(24):5775–5782
Stiff T, Casar Tena T, O’Driscoll M, Jeggo PA, Philipp M (2016) ATR promotes cilia signalling: links to developmental impacts. Hum Mol Genet 25(8):1574–1587
Rundle S, Bradbury A, Drew Y, Curtin NJ (2017) Targeting the ATR-CHK1 axis in cancer therapy. Cancers (Basel). 9(5):41
Jiang X, Li X, Li W, Bai H, Zhang Z (2019) PARP inhibitors in ovarian cancer: sensitivity prediction and resistance mechanisms. J Cell Mol Med 23(4):2303–2313
Huntoon CJ, Flatten KS, Wahner Hendrickson AE et al (2013) ATR inhibition broadly sensitizes ovarian cancer cells to chemotherapy independent of BRCA status. Cancer Res 73(12):3683–3691
Gingras AC, Kennedy SG, O’Leary MA, Sonenberg N, Hay N (1998) 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev 12(4):502–513
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129(7):1261–1274
Noguchi M, Hirata N, Suizu F (2014) The links between AKT and two intracellular proteolytic cascades: ubiquitination and autophagy. Biochim Biophys Acta 1846(2):342–352
Noguchi M, Ropars V, Roumestand C, Suizu F (2007) Proto-oncogene TCL1: more than just a coactivator for Akt. FASEB J 21(10):2273–2284
Balazs V, Ferenc G, Gabor V et al (2003) Decrease of the inflammatory response and induction of the Akt/protein kinase B pathway by poly-(ADP-ribose) polymerase 1 inhibitor in endotoxin-induced septic shock. Biochem Pharmacol 65(8):1373–1382
Kalmar-Nagy K, Degrell P, Szabo A et al (2013) PARP inhibition attenuates acute kidney allograft rejection by suppressing cell death pathways and activating PI-3K-Akt cascade. PLoS ONE 8(12):e81928
Qin WD, Liu GL, Wang J et al (2016) Poly(ADP-ribose) polymerase 1 inhibition protects cardiomyocytes from inflammation and apoptosis in diabetic cardiomyopathy. Oncotarget 7(24):35618–35631
Curtin NJ, Szabo C (2013) Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol Aspects Med 34(6):1217–1256
Onishi K, Higuchi M, Asakura T, Masuyama N, Gotoh Y (2007) The PI3K-Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells 12(4):535–546
Zhu D, Shi S, Wang H, Liao K (2009) Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J Cell Sci 122(Pt 15):2760–2768
Suizu F, Hirata N, Kimura K et al (2016) Phosphorylation-dependent Akt-Inversin interaction at the basal body of primary cilia. EMBO J 35(12):1346–1363
Funding
The study was funded by the National Natural Science Foundation of China (No. 81570864) and the Natural Science Foundation of Jilin Province (Nos. 20160101004JC; 20160414045GH; 2016J041).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Che, L., Song, JY., Lou, Y. et al. Analysis from the perspective of cilia: the protective effect of PARP inhibitors on visual function during light-induced damage. Int Ophthalmol 40, 1017–1027 (2020). https://doi.org/10.1007/s10792-019-01245-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01245-y