Abstract
Background
This study aimed to investigate the effect of intracameral human cord blood stem cells on lasered rabbit trabecular meshwork.
Methods
Immediately following diode laser application to the trabecular meshwork, human cord blood stem cells were injected intracamerally, in one eye of 12 albino rabbits. The other eye of ten rabbits was lasered controls and two eyes were normal controls. Rabbits were killed after 4, 8 and 12 weeks.
Results
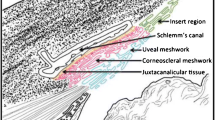
Lasered control rabbit eyes showed significant disruption of trabecular architecture, loss and pleomorphism of trabecular endothelial cells and progressive narrowing of trabecular spaces till 12 weeks. In contrast, lasered eyes, concurrently injected with human cord blood stem cells, showed relatively preserved endothelial cellularity and structure of the trabecular meshwork, at all time points. Human CD34- and CD44-positive cells were identified in 7/8 eyes treated with stem cells, at 4 and 8 weeks, and 2 of 3 at 12 weeks. Many PKH26-labeled human cord blood cells were visible throughout the trabecular area at 4 weeks. They gradually decreased in number by 8 weeks, and at 12 weeks, they appeared to be oriented along trabecular beams.
Conclusions
There was a relative preservation of cellularity and architecture of the trabecular meshwork in eyes injected with human cord blood stem cells, as compared to lasered control eyes up to 12 weeks, without significant inflammation. This suggests a probable role for such stem cells in eyes with glaucoma, having trabecular dysfunction.




Similar content being viewed by others
References
Kingman S (2004) Glaucoma is second leading cause of blindness globally. Bull World Health Organ 82(11):887–888
Coleman AL, Miglior S (2008) Risk factors for glaucoma onset and progression. Surv Ophthalmol 53(Suppl 1):S3–S10
Tektas OY, Lütjen-Drecoll E (2009) Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res 88:769–775
Sihota R, Lakshmaiah NC, Walia KB, Sharma S, Pailoor J, Agarwal HC (2001) The trabecular meshwork in acute and chronic angle closure glaucoma. Indian J Ophthalmol 49:255–259
Tripathi RC (1978) Aqueous outflow pathway in normal and glaucomatous eyes. Br J Ophthalmol 56:157–174
Spencer WH (1985) Glaucoma. In: Spencer WH (ed) Ocular Pathology, An Atlas and Textbook. WB Saunders, Philadelphia, pp 480–547
Alvarado JA, Murphy CG (1992) Outflow obstruction in pigmentary and open angle glaucoma. Arch Ophthalmol 110:1769–1772
Abu-Hassan DW, Li X, Ryan EI, Acott TS, Kelley MJ (2015) Induced pluripotent stem cells restore function in a human cell loss model of open-angle glaucoma. Stem Cells (Dayton, Ohio) 33(3):751–761. https://doi.org/10.1002/stem.1885
Manuguerra-Gagné R, Boulos PR, Ammar A, Leblond FA, Krosl G, Pichette V, Lesk MR, Roy DC (2013) Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem Cells 31:1136–1148
Ding QJ, Zhu W, Cook AC, Anfinson KR, Tucker BA, Kuehn MH (2014) Induction of trabecular meshwork cells from induced pluripotent stem cells. Invest Ophthalmol Vis Sci 55:7065–7072
Zhu W et al (2016) Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc Natl Acad Sci USA 113:E3492–E3500
Roubeix C et al (2015) Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res Therapy 6:177
Snider EJ, Kubelick KP, Tweed K, Kim RK, Li Y, Gao K, Read AT, Emelianov S, Ethier CR (2018) Improving stem cell delivery to the trabecular meshwork using magnetic nanoparticles. Sci Rep 8(1):12251
Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME (2002) Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci 43:402–410
Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR (1998) Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. Int Soc Hematother Graft Eng Cytom 34:61–70
Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR (2010) Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci 51:2051–2059
Otani A, Dorrell MI, Kinder K, Moreno SK (2004) Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest 114:765–774
Du Y, Yun H, Yang E, Schuman JS (2013) Stem cells from trabecular meshwork home to TM tissue in vivo. Invest Ophthalmol Vis Sci 54:1450–1459
Grewal SS, Barker JN, Davies SM, Wagner JE (2003) Unrelated donor hematopoietic cell transplantation: marrow or umbilical cord blood? Blood 101:4233–4244
Zigova T, Song S, Willing AE, Hudson JE, Newman MB, Saporta S, Sanchez-Ramos J, Sanberg PR (2002) Human umbilical cord blood cells express neural antigens after transplantation into the developing rat brain. Cell Transplant 11:265–274
Hao L, Zhang C, Chen XH, Zou ZM, Zhang X, Kong PY, Liang X, Gao L, Peng XG, Sun AH, Wang QY (2009) Human umbilical cord blood-derived stromal cells suppress xenogeneic immune cell response in vitro. Croat Med J. 50:351–360
Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, Xu J (2009) The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology 126:220–232
Laughlin MJ (2001) Umbilical cord blood for allogeneic transplantation in children and adults. Bone Marrow Transplant 27:1–6
Jablonska A, Kozlowska H, Markiewicz I, Domanska-Janik K, Lukomska B (2010) Transplantation of neural stem cells derived from human cord blood to the brain of adult and neonatal rats. Acta Neurobiol Exp (Wars) 70:337–350
Gherezghiher T, March WF, Nordquist RE, Koss MC (1986) Laser Induced glaucoma in rabbits. Exp Eye Res 43:885–894
Wang RF, Schumer RA, Serle JB, Podos SM (1998) A comparison of Argon laser and Diode laser photocoagulation of trabecular meshwork to produce the Glaucoma Monkey Model. J Glaucoma 7:45–49
Johnson DH (2007) Histologic findings after argon laser trabeculoplasty in glaucomatous eyes. Exp Eye Res 85:557–562
Koller T, Stürmer J, Remé C, Gloor B (2000) Membrane formation in the chamber angle after failure of argon laser trabeculoplasty: analysis of risk factors. Br J Ophthalmol 84:48–53
Leow SN, Luu CD, Nizam MHH, Mok PL, Ruhaslizan R, Wong HS, Halim WHWA, Ng MH, Ruszymah BH, Chowdhury SR, Bastion ML, Then KY (2015) Safety and efficacy of human Wharton’s Jelly-derived mesenchymal stem cells therapy for retinal degeneration. PLoS ONE 10:e0128973
Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NK, Davies SM (2002) Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 100:1611–1618
Lin CS, Ning H, Lin G, Lue TF (2012) Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 14:1159–1163
Zhang YJ, Yang YX, Hu MY, Ni QK, Li HG, Miao ZC (2015) Importance of CD44 on umbilical cord mesenchymal stem cells for expansion of hematopoietic cells. Cell Mol Biol (Noisy-le-grand) 61:18–25
Siqueira RC, Messias A, Messias K, Arcieri RS, Ruiz MA, Souza NF, Martins LC, Jorge R (2015) Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell -clinical trial). Stem Cell Res Ther 6:29
Park SS, Bauer G, Abedi M, Pontow S, Panorgias A, Jonnal R, Zawadzki RJ, Werner JS, Nolta J (2014) Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci 56:81–89
Srivastava A, Bapat M, Ranade S, Srinivasan V, Murugan P, Manjunath S, Thamaraikannan P, Abraham S (2010) Autologous multiple injections of in vitro expanded autologous bone marrow stem cells for cervical level spinal cord injury—a case report. J Stem Cells Regen Med 6:175–176
Subrammaniyan R, Amalorpavanathan J, Shankar R, Rajkumar M, Baskar S, Manjunath SR, Senthilkumar R, Murugan P, Srinivasan VR, Abraham S (2011) Application of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experience. Cytotherapy 13:993–999
Roncarolo MG, Bigler M, Martino S, Ciuti E, Tovo PA, Wagner J (1996) Immune functions of cord blood cells before and after transplantation. J Hematother 5:157–160
Coulson-Thomas VJ, Gesteira TF, Hascall V, Kao W (2014) Umbilical cord mesenchymal stem cells suppress host rejection: the role of the glycocalyx. J Biol Chem 289:23465–23481
Rieck B (2003) Unexpected durability of PKH 26 staining on rat adipocytes. Cell Biol Int 27:445–447
Ishikawa F, Shimazu H, Shultz LD, Fukata M, Nakamura R, Lyons B, Shimoda K, Shimoda S, Kanemaru T, Nakamura K, Ito H, Kaji Y, Perry AC, Harada M (2006) Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FASEB J 20:950–952
Lund RD, Wang S, Lu B et al (2007) Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 25:602–611
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving animals
The study was carried out after clearance from our Institutional Animal Ethics and Stem Cell Ethics Committees. The animals were housed in pathogen-free conditions and treated as per the ARVO statement on the use of animals in ophthalmic and vision research. All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Statement on human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all stem cell donors included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sihota, R., Sen, S., Mohanty, S. et al. Effect of intracameral human cord blood-derived stem cells on lasered rabbit trabecular meshwork. Int Ophthalmol 39, 2757–2766 (2019). https://doi.org/10.1007/s10792-019-01120-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01120-w