Abstract
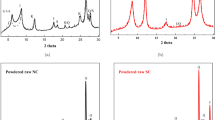
The first synthesis of CaO—CaCO3 pillared clay is described. Ion exchange between acid montmorillonite K 10 and a Ca(OH)2 solution ensures the incorporation of Ca ions into the gaps between the layers of the clay, leading to the formation of metal oxide (hydroxide) clusters, which increase the interlayer spacing in the clay. Air drying is accompanied by partial carbonation of the calcium oxide. The synthesized pillared clay is stable up to 300°C and has an interlayer spacing of ≃ 16 Å and a specific surface of ≃ 100 m2/g.
Similar content being viewed by others
REFERENCES
Kloprogge, J.T., Synthesis of Smectites and Porous Pillared Clay Catalysts: A Review, J. Porous Mater., 1998, vol. 5, no. 1, pp. 5–41.
Vicente, M.A., Banaresmunoz, M.A., Gandia, L.M., and Gil, A., On the Structural Changes of a Saponite Intercalated with Various Polycations upon Thermal Treatments, Appl. Catal., A, 2001, vol. 217, no. 1/2, pp. 191–204.
Issaadi, R., Garin, F., Chitour, C.E., and Maire, G., Catalytic Behavior of Combined Palladium—Acid Catalysts—Use of Al and Zr-Pillared Montmorillonite as Supports: Part I. Reactivity of Linear, Branched, and Cyclic Hexane Hydrocarbons, Appl. Catal., A,2001, vol. 207, nos. 1/2, pp. 323–332.
Gandia, L.M., Vicente, M.A., and Gil, A., Preparation and Characterization of Manganese Oxide Catalysts Supported on Alumina and Zirconia-Pillared Clays, Appl. Catal., A, 2000, vol. 196, no. 2, pp. 281–292.
Trombetta, M., Busca, G., Lenarda, M., et al., Solid Acid Catalysts from Clays—Evaluation of Surface Acidity of Mono-pillared and Bi-pillared Smectites by FT-LR Spectroscopy Measurements, NH3-TPD, and Catalytic Tests, Appl. Catal., A 2000, vol. 193, no. 1/2. pp. 55–69.
Salerno, P., Asenjo, M.B., and Mendioroz, S., Influence of Preparation Method on Thermal Stability and Acidity of Al-Pilcs, Thermochim. Acta, 2001, vol. 379, no. 1/2, pp. 101–109.
Louloudi, A. and Papayannakos, N., Hydrogenation of Benzene on La-Ni and Clay Supported La—Ni Catalysts, Appl. Catal., A, 1999, vol. 175, no. 1/2, pp. 21–31.
Benito, I., del Riego, A., Martinez, M., et al., Toluene Methylation on Al13- and GaAl12-Pillared Clay Catalysts, Appl. Catal., A, 1999, vol. 180, no. 1/2, pp. 175–182.
Choudary, B.M., Kantam, M.L., Sateesh, M., et al., Iron Pillared Clays—Efficient Catalysts for Friedel—Crafts Reactions, Appl. Catal., A, 1997, vol. 149, no. 1, pp. 257–264.
Kantam, M.L., Lakshmi Santhi, P., Ram Prasad, K.V., and Figueras, F., Iron Pillared Clay—An Efficient Catalyst for Ring Opening of Oxiranes, J. Mol. Catal. A: Chem., 2000, vol. 156, no. 1/2, pp. 289–292.
Shimizu, K.I., Kaneko, T., Fujishima, T., et al., Selective Oxidation of Liquid Hydrocarbons over Photoirradiated TiO2 Pillared Clays, Appl. Catal., A, 2002, vol. 225, no. 1/2, pp. 185–191
Issaadi, R. and Garin, F., Catalytic Behaviour of Acid Catalysts Supported Palladium: Use of Al and Zr-Pillared Montmorillonite as Supports: Part II. Kinetic Study, Appl. Catal., A, 2003, vol. 243, no. 2, pp. 367–377.
Long, R.Q. and Yang, R.T., Acid-and Base-Treated Fe3+-TiO2-Pillared Clays for Selective Catalytic Reduction of NO by NH3, Catal. Lett., 1999, vol. 59, no. 1, pp. 39–44.
Long, R.Q. and Yang, R.T., Catalytic Performance and Characterization of VO2+-Exchanged Titania-Pillared Clays for Selective Catalytic Reduction of Nitric Oxide with Ammonia, J. Catal., 2000, vol. 196, no. 1, pp. 73–85.
Sirilumpen, M., Yang, R.T., and Tharapiwattananon, N., Selective Catalytic Reduction of NO with Hydrocarbon on Cu2+-Exchanged Pillared Clay: An IR Study of the NO Decomposition Mechanism, J. Mol. Catal. A: Chem., 1999, vol. 137, nos. 1–3, pp. 273–286.
Konin, G.A., Il’ichev, A.N., Matyshak, V.A., et al., Cu, Co, Ag-Containing Pillared Clays as Catalysts for the Selective Reduction of NOx by Hydrocarbons in an Excess of Oxygen, Topics Catal., 2001, vol. 16/17, nos. 1–4, p. 193.
Sadykov, V.A., Kuznetsova, T.G., Doronin, V.R., et al., Zirconia Pillared Clays: Synthesis, Characterization, and Catalytic Properties in the NOx Selective Reduction by Hydrocarbons in the Oxygen Excess, Khim. Interesah Ustoich. Razvit., 2003, vol. 11, no. 1, pp. 249–262.
Sadykov, V.A., Lunin, V.V., and Ross, J.R.H., Mechanisms of Selective Hydrocarbon Reduction of Nitrogen Oxides in Excess Oxygen: Intermediates, Their Reactivity, and Transformation Paths, VI Rossiiskaya konferentsiya po mekhanizmam kataliticheskikh reaktsii(VI Russ. Conf. on the Mechanisms of Catalytic Reactions, Moscow, 2002, pp. 13–14.
Ding, Z., Kloprogge, J.T., Frost, R.L., et al., Porous Clays and Pillared Clays-Based Catalysts: Part 2. A Review of the Catalytic and Molecular Sieve Applications, J. Porous Mater., 2001, vol. 8, no. 4, pp. 273–293.
Nefedov, V.N., Rentgenoelektronnaya spektroskopiya khimicheskikh soedinenii (X-ray Photoelectron Spectroscopy of Chemical Compounds), Moscow: Khimiya, 1984.
Author information
Authors and Affiliations
Additional information
Translated from Neorganicheskie Materialy, Vol. 41, No. 2, 2005, pp. 254–256.
Original Russian Text Copyright © 2005 by Gorobinskii, Efimova, Fattakhova, Korchak.
Rights and permissions
About this article
Cite this article
Gorobinskii, L.V., Efimova, N.N., Fattakhova, Z.T. et al. Preparation of CaO—CaCO3 pillared clay. Inorg Mater 41, 203–205 (2005). https://doi.org/10.1007/s10789-005-0045-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10789-005-0045-9