Abstract
Background
Previous observational studies have indicated a complex association between gut microbiota (GM) and neuropathic pain (NP). Nonetheless, the precise biological mechanisms underlying this association remain unclear. Therefore, we adopted a Mendelian randomization (MR) approach to investigate the causal relationship between GM and neuropathic pain including post-herpetic neuralgia (PHN), painful diabetic peripheral neuropathy (PDPN), and trigeminal neuralgia (TN), as well as to explore the potential mediation effects of immune cells.
Methods
We performed a two-step, two-sample Mendelian randomization study with an inverse variance-weighted (IVW) approach to investigate the causal role of GM on three major kinds of NP and the mediation effect of immune cells between the association of GM and NP. In addition, we determine the strongest causal associations using Bayesian weighted Mendelian randomization (BWMR) analysis. Furthermore, we will investigate the mediating role of immune cells through a two-step Mendelian randomization design.
Results
We identified 53 taxonomies and pathways of gut microbiota that had significant causal associations with NP. In addition, we also discovered 120 immune cells that exhibited significant causal associations with NP. According to the BWMR and two-step Mendelian randomization analysis, we identified the following results CD4 on CM CD4 + (maturation stages of T cell) mediated 6.7% of the risk reduction for PHN through the pathway of fucose degradation (FUCCAT.PWY). CD28 + DN (CD4-CD8-) AC (Treg) mediated 12.5% of the risk reduction for PHN through the influence on Roseburia inulinivorans. CD45 on lymphocyte (Myeloid cell) mediated 11.9% of the risk increase for TN through the superpathway of acetyl-CoA biosynthesis (PWY.5173). HLA DR + CD8br %T cell (TBNK) mediated 3.2% of the risk reduction for TN through the superpathway of GDP-mannose-derived O-antigen building blocks biosynthesis (PWY.7323). IgD-CD38-AC (B cell) mediated 7.5% of the risk reduction for DPN through the pathway of thiazole biosynthesis I in E. coli (PWY.6892).
Discussion
These findings provided evidence supporting the causal effect of GM with NP, with immune cells playing a mediating role. These findings may inform prevention strategies and interventions directed toward NP. Future studies should explore other plausible biological mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to International Association for the Study of Pain (IASP), neuropathic pain (NP) arises from injury or ailment affecting the somatosensory system (Jensen et al. 2011). NP is categorized into peripherally induced neuropathic pain (pNP) and central neuropathic pain (Dworkin et al. 2003; Colloca et al. 2017), with pNP being more prevalent and typically resulting from peripheral nerve damage. Conditions such as painful diabetic peripheral neuropathy (PDPN), trigeminal neuralgia (TN) and post-herpetic neuralgia (PHN) are major causes of pNP (Bril et al. 2011, 2022, 2021; Yang et al. 2019b; Qing-jun., 2018; Consensus Workgroup on Herpes Zoster 2022). NP not only causes considerable suffering for patients but also places a significant economic burden. Current treatment strategies, which often rely on widespread opioid usage, often fail to provide sufficient pain relief (Schaefer et al. 2014; Yu et al. 2019; Attal et al. 2023).
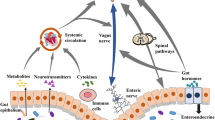
In recent studies, there has been a growing emphasis on the complex interplay between the gut microbiome (GM) and neurological conditions, particularly its involvement in the onset and regulation of NP (Gonzalez-Alvarez et al. 2023; Li et al. 2023; Moloney et al. 2016). Evidence suggests a tight connection between the GM composition and NP, with gut bacteria modulating pain sense through immunomodulation, inflammatory response regulation, and neural pathway activation (Defaye et al. 2020; Guo et al. 2019; Min 2023; Ustianowska et al. 2022). The microbiota–gut–brain axis underscores a two-way communication system, involving immune, neural, endocrine, and metabolic signaling pathways, which play a significant role in disease advancement, particularly in NP (Lin et al. 2020; Nagamine 2023; Magni et al. 2023; Moloney et al. 2016). Despite the importance of these discoveries, precise mechanisms by which gut microbiota interact with neural and immune components in NP remain elusive, with existing studies indicating correlation rather than causation (Gonzalez-Alvarez et al. 2023; Li et al. 2023). Moreover, the diversity in GM investigations and the impact of confounding variables present hurdles for clinical implementation (Lin et al. 2020; Ustianowska et al. 2022; Huang et al. 2019; Yang et al. 2019a). Recent studies also emphasize the crucial involvement of immune cells in NP, with activation of microglia, mast cells, as well as participation of immune responses in early stage inflammation, being associated with NP severity (Rahman-Enyart et al. 2022; Inoue and Tsuda 2018; Barcelon et al. 2019; Colloca et al. 2017; Magni et al. 2023; Bethea and Fischer 2021; Thacker et al. 2007). The modulation of immune cells by gut microbiota, notably through short-chain fatty acids (SCFAs) and pathogen-associated molecular patterns (PAMPs), implies a central involvement in the initiation of NP (Dworsky-Fried et al. 2020; Magni et al. 2023; Barcelon et al. 2019; Lin et al. 2020; Ustianowska et al. 2022; Ramakrishna et al. 2019; Reichenberger et al. 2013; Ding et al. 2021; Fiore et al. 2023).However, intricate interplay underlying GM, immune cells, and NP, compounded by potential confounders, calls for further exploration (Lin et al. 2020; Sun et al. 2023).
Mendelian randomization (MR) leverages genetic variants to emulate the framework of randomized controlled trials, offering strong method for delineating causal relationships between diseases and potential risk factors (Liang and Fan 2023; Skrivankova et al. 2021). The accessibility of Genome-Wide Association Studies (GWAS) data has greatly bolstered the utilization of MR in probing intricate diseases, presenting unparalleled opportunities to examine the causal relationships between GM, immune cells, and NP (Wang et al. 2024a; Lopera-Maya et al. 2022). While progress has been made in comprehending the link between gut microbiota and NP, the precise causal effects mediated by immune cells have yet to be thoroughly investigated (Min 2023; Inoue and Tsuda 2018; Guo et al. 2023; Ding et al. 2021; Chen and Tang 2023). This study employs two-sample Mendelian randomization (TSMR) and Bayesian weighted Mendelian randomization (BWMR) to explore the underlying causal relationships among them. By employing these methods, our objective was to elucidate the intermediary function of immune cells in the correlation between GM and NP, thus providing fresh perspectives for identifying biomarkers and advancing novel treatments for NP.
Method
Study design
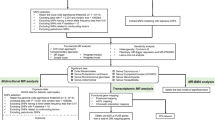
We employed TSMR to investigate causal relationships among GM, immune cells, and three major conditions for NP: PHN, PDPN, and TN. This analysis aimed to establish the causality exerted by GM on immune cells and subsequently on these NP conditions. For supporting causal associations in TSMR, BWMR analyses were conducted to further elucidate significant causal links. (Fig. 1).
GWAS summary data sources
All data utilized were sourced from online databases, with participants of GWAS being of European ancestry. Specifically, GM information was obtained from Netherlands Microbiome Project, where Esteban et al. reported on 412 gut microbial taxa. This research encompassed 7738 participants, conducting a GWAS study on 207 taxa and 205 pathways reflecting microbiome composition and activity (Lopera-Maya et al. 2022). GWAS summary data for Dutch Microbiome Project (DMP) integrated information on 5 phyla, 10 classes, 13 orders, 26 families, 48 genera, and 105 species. Further details are available in Supplementary Material Table S1.
Information regarding immune cells included 731 phenotypes, covering median fluorescence intensity (MFI, n = 389), absolute cell count (AC, n = 118), relative cell count (RC, n = 192), and morphological parameters (MP, n = 32). The first three categories covered data on various cell types, including bone marrow cells, B cells, mature T cells, monocytes, TBNK (T cells, B cells, natural killer cells), and Treg cell populations, while the last category pertained to CDC and TBNK groups. Initial GWAS analyses were centered on immune traits, utilizing samples from 3757 individuals of European ancestry, with no cohort overlap (Orrù et al. 2020). To refine the accuracy of genotype data, approximately 22 million single nucleotide polymorphisms (SNPs) were imputed using a reference panel derived from Sardinian sequences (Sidore et al. 2015). Moreover, potential confounding factors such as gender, age, and the square of age were taken into account in the evaluation of associations.
As there is no dedicated database specifically for PDPN and considering that the majority of cases within the diabetic peripheral neuropathy category are typically associated with painful symptoms, we opted to utilize diabetic polyneuropathy data as a proxy for PDPN. This choice is made on the basis that the available dataset likely encompasses a significant proportion of PDPN cases, thus facilitating meaningful analysis and insights into this condition (Bril et al. 2011). Data on painful diabetic polyneuropathy (PDPN, finn-b-DM_POLYNEURO, n = 375,482, https://storage.googleapis.com/finngen-public-data-r9/summary_stats/finngen_R9_DM_POLYNEURO.gz), post-herpetic neuralgia (PHN, finngen_R9_G6_POSTZOST, n = 330,690, https://storage.googleapis.com/finngen-public-data-r9/summary_stats/finngen_R9_G6_POSTZOST.gz), and trigeminal neuralgia (TN, finngen_R10_G6_TRINEU, n = 362,315, https://storage.googleapis.com/finngen-public-data-r10/summary_stats/finngen_R10_G6_TRINEU.gz) were sourced from the FinnGen consortium’s GWAS summary data, available on FinnGen website (Kurki et al. 2023; Wei et al. 2023; Liang and Fan 2023). As this study relies solely on publicly available aggregated data, no further ethical approval or participant consent was required. Further details are available in Supplementary Material Table S1.
Instrumental variable selection and data harmonization
We selected SNPs across the genome that exhibited significant associations with our traits of interest (P value < 1e-5) to serve as instrumental variables (IVs). These SNPs were clustered based on their linkage disequilibrium (LD) levels, using a window size of 10,000 kb and an r^2 threshold < 0.001, with LD data sourced from the 1000 Genomes Project for European samples (Abecasis et al. 2010). When exposure SNPs were absent in the dataset, proxy SNPs were used. Both palindromic and ambiguous SNPs were excluded to prevent ambiguity in the Mendelian randomization analysis. The validity of IVs was assessed using the F-statistic, calculated as the ratio of the effect size (β) to the square of its standard error. SNPs showing an F-statistic below 10, indicating weak instruments, were excluded from further analysis. (Supplementary Material Table S2, S3, and S4).
Primary statistical analysis
Statistical analyses were performed using R software (version 4.3.2) utilizing the TwoSampleMR, MR-PRESSO, and MendelianRandomization packages (Yavorska and Burgess 2017; Hemani et al. 2018; Verbanck et al. 2018). For the estimation of causal effects, we utilized the inverse variance-weighted (IVW) method to combine Wald ratio for each SNP in a meta-analysis framework. To complement the IVW analysis, we applied various algorithms including MR-Egger, weighted median, simple mode, weighted mode, BWMR, and MR-PRESSO (Bowden et al. 2015, 2016; Verbanck et al. 2018; Zhao et al. 2020). BWMR analysis accounted for uncertainty associated with weak instruments and addressed horizontal pleiotropy estimated in GWAS (Zhao et al. 2020).
To ensure thorough filtering, we employed a range of methods, aiming to establish a causal relationship between exposure and outcome based on the presence of consistent positive results from both the IVW method and Bayesian weighted analysis (Hemani et al. 2018; Chen et al. 2020). Sensitivity analyses were conducted to evaluate horizontal pleiotropy and heterogeneity using three approaches: the MR-Egger Intercept Test, Mendelian Randomization Pleiotropy RESidual Sum and leave-one-out analysis, and Cochran’s Q test, thereby bolstering the robustness of our findings (Bowden et al. 2015; Long et al. 2023). MR-PRESSO allows for detecting overall horizontal pleiotropy among IVs and also identifies outliers causing pleiotropy (Verbanck et al. 2018). P values > 0.05 in both MR-Egger Intercept Test and MR-PRESSO indicated pleiotropy absence. Cochran’s Q test indicated non-heterogeneity with P values > 0.05. Microbial communities showing signs of horizontal pleiotropy or heterogeneity were omitted from the analysis. Leave-one-out analysis was performed to investigate the effects of potential outlier genetic variants, thereby mitigating influences from heterogeneity and horizontal pleiotropy.
Mediation analysis
To delve deeper into the potential of immune cells within the causal pathway linking GM to NP outcomes, we implemented TSMR framework for mediation analysis. An advanced analytical approach enabled the disentanglement of the overall effect into indirect (i.e., mediated through the intermediary of immune cells) and direct (i.e., independent of the mediator) components (Carter et al. 2021), as shown in Fig. 2.
Mediation analysis. A The total effect between Gut microbiota (GM) and Neuropathic Pain (NP), where c is the total effect using genetically predicted GM as exposure and NP as outcome and d is the total effect using genetically predicted NP as exposure and GM as outcome. B The total effect was decomposed into: (i) indirect effect using a two-step approach (where a is the total effect of GM on immune cell, and b is the effect of immune cell on NP) and the product method (a × b) and (ii) direct effect (c′ = c – a × b). Proportion mediated was the indirect effect divided by the total effect
More precisely, the aggregate influence of GM on NP was dissected into (1) a direct effect of GM on NP, represented as path c in Fig. 2A, and (2) an indirect effect mediated through immune cells, denoted as a × b path in Fig. 2B. This methodology facilitated the estimation of the mediation effect as a percentage of the total effect by comparing the magnitude of the indirect effect against the total effect. Moreover, the 95% confidence intervals (CI) for the mediation effect were accurately computed using the delta method, ensuring the accuracy and robustness of our findings (Michael Lynch 2001).
Result
Causal effects of GM on NP
Significant IVW results for the taxonomic units of the gut microbiome are displayed in a forest plot. Utilizing a threshold of P < 1e-5, MR analysis was conducted on 412 GM taxa, identifying 53 microbial taxa and pathways associated with PHN, PDPN, or TN as identified through the IVW method. (Detailed data can be found in the supplementary materials table S5-S9 and Fig. 3).
MR analysis showed the causality of GM on NP was significant. A: MR analysis of GM and PDPN (IVW and BWMR). B: MR analysis of GM and PHN (IVW and BWMR). A: MR analysis of GM and TN (IVW and BWMR). CI confidence interval, GM gut microbiota, MR Mendelian randomization, OR odds ratio, nsnp single nucleotide polymorphism, IVW inverse variance-weighted, BWMR Bayesian weighted Mendelian randomization
Causal effects of GM on PDPN
In our study, we identified six bacterial groups and metabolic pathways significantly associated with an increased risk of PDPN. The most significant were Bacteroides finegoldii (OR_IVW: 1.339, 95% CI: 1.012 to 1.773, P_IVW = 0.041, P_BW = 0.039) and the glyoxylate bypass pathway (PWY_GLYOXYLATE.BYPASS) (OR_IVW: 1.845, 95% CI: 1.114 to 3.056, P_IVW = 0.017, P_BW = 0.019). In addition, we found ten bacterial groups and metabolic pathways significantly associated with a decreased risk of PDPN. The most notable among these were Bifidobacterium longum (OR_IVW: 0.433, 95% CI: 0.246 to 0.759, P_IVW = 0.004, P_BW = 0.019) and the biosynthesis pathway of pantothenate and coenzyme A III (PWY.4242) (OR_IVW: 0.498, 95% CI: 0.298 to 0.832, P_IVW = 0.008, P_BW = 0.010). (Fig. 3A).
Causal effects of GM on PHN
In our MR analysis of GM communities associated with PHN, we identified 11 bacterial groups and metabolic pathways significantly associated with an increased risk of PHN. Rothia mucilaginosa from the Rothia genus (OR_IVW: 1.960, 95% CI: 1.181 to 3.252, P_IVW = 0.009, P_BW = 0.014) was found to have the most significant association with increased PHN risk. In terms of metabolic pathways, the biosynthesis of uric acid, particularly through the degradation of inosine 5'-phosphate (PWY.5695), (OR_IVW: 3.398, 95% CI: 1.661 to 6.950, P_IVW = 0.001, P_BW = 0.001), showed the strongest association with increased risk of PHN. However, we also identified six bacterial groups and metabolic pathways significantly related to a decreased risk of PHN. The most notable among these were Eubacterium hallii (OR_IVW: 0.541, 95% CI: 0.355 to 0.826, P_IVW = 0.004, P_BW = 0.005) and the degradation process of fucoidan (FUCCAT.PWY) (OR_IVW: 0.381, 95% CI: 0.203 to 0.714, P_IVW = 0.003, P_BW = 0.003). (Fig. 3B).
Causal effects of GM on TN
In our MR analysis of gut microbial communities associated with TN, we identified nine bacterial groups and metabolic pathways significantly associated with an increased risk of TN. The most significant among these were an unclassified strain of the Roseburia genus (OR_IVW: 1.213, 95% CI: 1.021 to 1.440, P_IVW = 0.028, P_BW = 0.022) and the superpathway of acetyl-CoA biosynthesis (PWY.5173) (OR_IVW: 1.328, 95% CI: 1.080 to 1.631, P_IVW = 0.007, P_BW = 0.009). We also identified 12 bacterial groups and metabolic pathways significantly associated with a decreased risk of TN. Among these, the most notable were bacteria from the order Desulfovibrionales (OR_IVW: 0.637, 95% CI: 0.439 to 0.925, P_IVW = 0.018, P_BW = 0.023) and the biosynthetic pathway for the common antigen in Enterobacteriaceae (ECA) (ECASYN.PWY) (OR_IVW: 0.844, 95% CI: 0.745 to 0.956, P_IVW = 0.008, P_BW = 0.008 (Fig. 3C).
Causal effects of immune cells on PDPN
In our MR analysis of immune cells associated with PDPN, we identified 27 immune cells significantly associated with an increased risk of PDPN. These included five types in the maturation stages of T cell group, three in the Myeloid cell group, three in the TBNK group, three in the B cell group, five in the cDC group, five in the Treg group, and three in the Monocyte group. The strongest significant association was noted with HLA DR on DC (from the cDC panel) (OR_IVW: 1.468, 95% CI: 1.271 to 1.696, P_IVW = 1.77E-07, P_BW = 0.006). In addition, we identified 30 immune cells associated with a decreased risk of PDPN, including 8 in the B cell group, 6 in the cDC group, 5 in the Treg group, 4 in the TBNK group, 3 in the Myeloid cell group, and 3 in the Maturation stages of T cell group. The most significant association was with CD39 on monocyte (from the Treg panel) (OR_IVW: 0.848, 95% CI: 0.756 to 0.951, P_IVW = 0.012, P_BW = 0.013) (Fig. 4A).
MR analysis showed the causality of immune cell on NP was significant. A: MR analysis of immune cell and PDPN (IVW and BWMR). B: MR analysis of immune cell and PHN (IVW and BWMR). A: MR analysis of immune cell and TN (IVW and BWMR). CI confidence interval, MR Mendelian randomization, OR odds ratio, nsnp single nucleotide polymorphism, IVW inverse variance-weighted, BWMR Bayesian weighted Mendelian randomization
Causal effects of immune cells on PHN
In our MR analysis of immune cells associated with PHN, we identified 16 immune cells significantly associated with an increased risk of PHN. These include eight in the B cell group, three in the TBNK group, two in the Maturation stages of T cell group, two in the cDC group, and one in the Monocyte group. Notably, PB/PC AC (B cell panel) exhibited the strongest significant association (OR_IVW: 1.160, 95% CI: 1.034 to 1.301, P_IVW = 0.012, P_BW = 0.035). In addition, we identified 21 immune cells related to a decreased risk of PHN, with 6 in the myeloid cell group, 3 in the TBNK group, 5 in the Treg group, 3 in the B cell group, 2 in the Maturation stages of T cell group, 1 in the Monocyte group, and 1 in the cDC group. Among these, the most significant was CD4 on HLA DR + CD4 + (TBNK panel) (OR_IVW: 0.689, 95% CI: 0.539 to 0.881, P_IVW = 0.003, P_BW = 0.004) (Fig. 4B).
Causal effects of immune cells on TN
In our MR analysis of immune cells related to TN, we identified 15 immune cells significantly associated with an increased risk of TN. These included six in the B cell group, four in the Treg group, two in the TBNK group, and one each in the Monocyte, Myeloid cell, and cDC groups. The strongest significant association was observed with CD28- CD8dim AC (Treg panel) (OR_IVW: 1.099, 95% CI: 1.032 to 1.172, P_IVW = 0.003, P_BW = 0.006). In addition, we identified 11 immune cells associated with a decreased risk of TN, including 3 in the Maturation stages of T cell group, 2 in the B cell group, 2 in the TBNK group, 2 in the Treg group, and 1 each in the cDC and Myeloid cell groups. The most significant association was found with CD25hi %T cell (Treg panel) (OR_IVW: 0.893, 95% CI: 0.813 to 0.980, P_IVW = 0.017, P_BW = 0.007) (Fig. 4C).
Reverse MR analysis
In reverse MR analysis, we discovered a reverse causal relationship between PHN and 28 types of gut microbiota, with PHN leading to a reduction in the abundance of 19 of these microbes. When TN is considered as the exposure, 19 types of GM are affected in reverse by TN. For PDPN, 14 types of GM are influenced by PDPN. Notably, only two types of GM were significant in both the forward and reverse MR analyses for PDPN: Proteobacteria (OR_rIVW: 1.020, 95% CI: 1.005 to 1.037, P_IVW = 0.012) and Parabacteroides merdae (OR_rIVW: 0.981, 95% CI: 0.965 to 0.998, P_IVW = 0.027). (Fig. 5).
Mediation analysis
To ensure the rigor of our findings, we meticulously screened data used in our mediation analyses. For the MR analysis between gut microbiota and study outcomes, we selected data that showed positive associations in at least three statistical methods, including IVW and BWMR, with at least one other method confirming these results. This selection ensured the consistency and reliability of the data, which also had to demonstrate no reverse causality and be free from heterogeneity and pleiotropy issues. Similarly, for the MR analysis between immune cells and study outcomes, we chose immune cells data that showed positive results in both the IVW method and Bayesian weighted analysis, and was free from heterogeneity and pleiotropy, to act as potential mediating variables. This careful selection process guaranteed that the mediation analysis data were consistent, reliable, and representative.
Based on the above data selection process, our analysis results are as follows: we analyzed immune cells as mediators in the pathway from gut microbiota to PDPN, PHN, and TN. Our study indicates that the degradation pathway of fucose mediated by CD4 on CM CD4 + (Maturation stages of T cell) accounts for a 6.7% reduction in PHN risk (total effect beta: −0.966, direct effect GM to immune cells: 0.239, direct effect immune cells to outcome: −0.272, mediation effect: −0.065). CD28 + DN (CD4-CD8-) AC (Treg) associated with Roseburia inulinivorans of the Roseburia genus accounts for a 12.6% reduction in PHN risk (total effect beta: −1.356, direct effect GM to immune cells: 0.387, direct effect immune cells to outcome: −0.441, mediation effect: −0.171). CD45 on lymphocyte (Myeloid cell) associated with the superpathway of acetyl-CoA biosynthesis increases the risk of TN by 12% (total effect beta: 0.283, direct effect GM to immune cells: 0.249, direct effect immune cells to outcome: 0.135, mediation effect: 0.034). HLA DR + CD8br %T cell (TBNK) associated with the biosynthesis superpathway of GDP-mannose into O-antigen building blocks reduces the risk of TN by 3.3% (total effect beta: −0.366, direct effect GM to immune cells: 0.217, direct effect immune cells to outcome: −0.056, mediation effect: −0.012). IgD-CD38-AC (B cell) associated with thiazole biosynthesis I pathway reduces the risk of PDPN by 7.5% (total effect beta: −0.759, direct effect GM to immune cells: 0.226, direct effect immune cells to outcome: −0.252, mediation effect: −0.057) (Table 1).
Sensitive analysis
To deepen our understanding of the potential pleiotropy issues identified in our causal estimations, we conducted several sensitivity analyses. Initially, we applied Cochran’s Q test and MR-Egger regression to explore heterogeneity and pleiotropy among different SNPs regarding their causal relationships. The results revealed that in the MR analysis of the gut microbiota and NP, only the preQ₀ biosynthesis pathway (PWY.6703) exhibited horizontal pleiotropy (P_pleiotropy < 0.05) in the MR analysis involving GM and TN. In the MR analysis of immune cells and NP, horizontal pleiotropy was observed in PHN with IMM CD25 on activated and secreting Treg.
Other results from MR-PRESSO and MR-Egger regression did not indicate horizontal pleiotropy, and Cochran’s Q test also did not show significant heterogeneity. The influence of each SNP on the overall causal estimation was verified through leave-one-out analysis. After removing each SNP, we systematically re-conducted MR analysis on the remaining SNPs. The results demonstrated consistency, with no single SNP significantly violating the overall effect between the GM and NP or between immune cells and NP. Detailed data can be found in the supplementary materials, Tables S10–S12.
Discussion
Taking advantage of the wealth of public genetic data, our research delved into the causal links among 731 features of immune cells, 412 taxa of GM, and NP. This constitutes the inaugural systematic inquiry utilizing MR analysis to investigate how immune cells play a mediating role in the causal associations between GM (including their metabolic pathways) and three varieties of NP. Unlike earlier studies using 16S rRNA gene sequencing, which poorly identified microbial taxa at finer taxonomic levels, our use of metagenomic sequencing enabled precise species-level identification. This significantly advanced our comprehension of microbiota diversity and its specific roles in the gut ecosystem.
Previous research often neglected potential confounders such as socioeconomic status and dietary habits, and failed to thoroughly explore direct links between specific microbes, immune cell types, and NP. Our study addresses this gap. Our findings reveal causal impacts of 53 GM taxa and pathways out of 207 taxa and 205 pathways reflecting microbial composition and activity, as well as 84 immune phenotypes spanning four immune features (MFI, RC, AC, and MP) on PHN, TN, and PDPN. Moreover, we discovered the mediating effects of five immune cells, enhancing our understanding of the complex interactions among GM, the immune system, and NP.
Gut microbiota and neuropathy pain
Equilibrium between GM and the host is vital for upholding intestinal barrier integrity, defending against pathogenic intrusions, fostering brain development, and ensuring the normal operation of the immune system. Disruption of this delicate equilibrium can precipitate a range of health complications, including metabolic diseases, cardiovascular diseases, and neurological disorders. A growing body of research suggests that the alteration of diseases such as Alzheimer’s, Parkinson’s, traumatic brain injury, depression, and NP may be linked to gut microbiota dysbiosis (Guo et al. 2019).
The microbiota–gut–brain axis highlights the bidirectional communication among the brain, endocrine system, gut, immune system, and GM, maintaining homeostasis within the organism. GMs are directly associated with pain sensation through peripheral and central systems, affecting the neuroexcitability of primary sensory neurons. They may indirectly regulate inflammatory responses by activating non-neuronal cells, including immune cells (Guo et al. 2019; Min 2023; Lin et al. 2020).
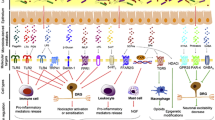
Within peripheral nervous system (PNS), metabolites from GM can directly alter the neuroexcitability of primary sensory neurons in the dorsal root ganglia (DRGs). This modulation is mediated through the activation or sensitization of pain-related receptors or ion channels, such as Toll-like receptors (TLRs), transient receptor potential (TRP) channels, γ-aminobutyric acid (GABA) receptors, and acid-sensing ion channels (Lin et al. 2020). In addition, microbial mediators also indirectly influence the activity of primary sensory neurons within DRGs by prompting immune cells like macrophages to release inflammatory mediators (e.g., TNF-α, IL-1β, IL-6), chemokines (e.g., CCL2, CXCL-1), anti-inflammatory cytokines (e.g., IL-4), or neuropeptides (e.g., endogenous opioids) (Guo et al. 2019).
TLRs serve as crucial transmembrane pattern recognition receptors in the innate immune system, capable of identifying exogenous ligands, PAMPs, and damage-associated molecular pattern molecules (DAMPs) (Min 2023). TLRs are expressed not only on immune system cells like leukocytes but also on nervous system cells including neurons, astrocytes, and microglia. Post-tissue injury, the release of DAMPs recognized by receptors such as TLRs triggers an immune response. PAMPs produced by gut microbiota, such as lipopolysaccharides (LPS), lipoteichoic acid (LTA), peptidoglycan (PGN), and β-glucans, serve pivotally in peripheral sensitization during chronic pain conditions. These molecules, by binding to pattern recognition receptors like TLRs on immune cells and sensory neurons in the DRGs, directly influence neuronal excitability or indirectly trigger inflammatory responses through immune cell activation (Lin et al. 2020; Guo et al. 2019; Ustianowska et al. 2022).
Gut microbiota, immune system, and pain
When discussing the correlation between GM and pain, the involvement of the immune system emerges as a pivotal factor. Association between immune cells and pain sense, particularly crucial involvement of TLR4 in NP progression, has been extensively studied (Bethea and Fischer 2021; Inoue and Tsuda 2018). In vivo experiments in mouse harboring TLR4 gene mutations demonstrated remarkable reductions in pain hypersensitivity and abnormal behaviors in chemotherapy and nerve injury-induced NP (Su et al. 2022; Bethea and Fischer 2021). This underscores the distinct ability of various TLRs to identify PAMPs, with TLR4 specifically recognizing LPS. This recognition triggers a series of signaling pathways that activate microglia, resulting in the secretion of pro-inflammatory mediators such as CCL2, CXCL-1, and IL-1β, thereby driving the progression of NP.
Within the immune system, T cells, particularly Th2-type T cells, play a pivotal role in regulating chronic neuropathic pain (CNP), characterized by elevated levels of anti-inflammatory cytokines IL-10 and IL-4 (Bethea and Fischer 2021; Uçeyler et al. 2007). While CD4 + Th1 cells are conventionally associated with promoting pain, studies also suggest a potential pain-alleviating role for CD8 + T cells (Krukowski et al. 2016; Liu et al. 2014). Mouse models lacking T cells exhibit prolonged chemotherapy induced pain hypersensitivity, whereas the transfer of CD8 + T cells facilitates pain relief. Regulatory T cells (Tregs), as an important T cell subgroup within pain recovery, function by regulating the actions of innate and adaptive immune cells (Bethea and Fischer 2021; Fischer et al. 2019, 2018).
Studies show significant differences in the diversity and abundance of immune cells and microbial composition between individuals with different pain states, such as visceral pain, chronic pelvic pain, fibromyalgia, and knee pain associated with osteoarthritis, and healthy populations (Gonzalez-Alvarez et al. 2023; Dworsky-Fried et al. 2020; Lin et al. 2020). In the context of complex regional pain syndrome (CRPS), patients’ enduring pain is closely associated with heightened activation of microglia in both the spinal cord and brain, alongside a decline in gut microbiota diversity (Dworsky-Fried et al. 2020). Microglia assume a central role in instigating and perpetuating these adaptive alterations within the central nervous system.
While injured or degenerating sensory neurons incite an inflammatory response to trauma, culminating in NP onset, recent investigations suggest that GM may also contribute to the inflammatory processes linked to chronic pain. Notably, metabolic byproducts of GM, such as SCFAs, exert significant regulatory effects on microglia maturation and function. (Dworsky-Fried et al. 2020; Magni et al. 2023).
MR analysis of immune cells and gut microbiota in neuropathic pain
In this study, we identified Rothia mucilaginosa from the Rothia genus as a significant factor increasing the risk of PHN. Commonly found in the human oral cavity and upper respiratory tract as part of normal flora, this bacterium is mostly harmless but can cause infections in immunocompromised individuals (Getzenberg et al. 2021; Daoub et al. 2021). It is associated with a variety of clinical conditions, including periodontal disease, respiratory infections, and even bloodstream infections (Daoub et al. 2021; Getzenberg et al. 2021; Shaeer et al. 2017). In addition, we studied the urate biosynthesis and inosine 5'-phosphate degradation pathway, which converts inosine 5'-monophosphate to uric acid (UA) through a series of enzymatic reactions. Elevated UA levels are linked to gout, cardiovascular risk, hypertension, diabetes, and metabolic syndrome (Zhang et al. 2022; Novak et al. 2007; Heda et al. 2021; Gherghina et al. 2022). Soluble urates promote the production of interleukin-1 beta (IL-1β), triggering acute gouty inflammation mediated by the NLRP3 inflammasome activation, which leads to the release of IL-1β by macrophages and neutrophils, resulting in severe pain and joint swelling (Crișan et al. 2016; Zhang et al. 2022). Furthermore, uric acid-induced oxidative stress, a balance disruption between free radicals and antioxidants, exacerbates inflammation and pain (Gherghina et al. 2022). Interestingly, our findings suggest that this pathway may also increase the risk of PHN, a connection not previously demonstrated between this microbial pathway and neuropathic pain.
In our study, an unclassified strain of the genus Roseburia emerged as the factors most markedly associated with an elevated risk of TN. Members of the Lachnospiraceae family, to which Roseburia belongs, are typically anaerobic, fermentative, chemoheterotrophic bacteria associated with health due to their role as major producers of SCFAs. SCFAs are crucial for modulating the local microbial environment and interacting with the host’s immune system, playing a key role in regulating intestinal inflammation and immune maturation. Various strains of Roseburia have diverse effects on metabolism and inflammation and are linked to conditions such as inflammatory bowel disease, type 2 diabetes, and chronic pain (Dekker Nitert et al. 2020; Kulkarni et al. 2021; Goudman et al. 2024; Machiels et al. 2014; Ruan et al. 2022).
It highlights the risk association between Bacteroides finegoldii and PDPN. B. finegoldii is a significant intestinal microbe typically found in human feces. While generally symbiotic and beneficial in maintaining a healthy gut microbiota, it can cause infections if it escapes the gut. B. finegoldii may play a positive role in managing digestive diseases such as inflammatory bowel disease and Clostridium difficile infections, potentially serving as a probiotic to aid in recovery (Wang et al. 2024b). However, B. finegoldii was the only strain identified in our study as potentially increasing the risk of PDPN. Conversely, an increased abundance of Bifidobacterium longum was associated with a decreased risk of developing PDPN. As a commensal bacterium in the gut, Bifidobacterium longum has demonstrated potential in ameliorating colitis in mouse models. Moreover, it has shown beneficial effects in patients experiencing delayed recovery from hepatocellular carcinoma (HCC) by enhancing liver function and facilitating repair (Yu et al. 2024). Clinical trials have demonstrated that oral administration of a probiotic cocktail containing B. longum notably diminishes the proportion of HCC patients experiencing delayed recovery. This intervention also leads to shortened hospital stays and enhances 1-year survival rates (Yu et al. 2024). Moreover, B. longum has been shown to lower depression scores in patients with irritable bowel syndrome, enhance quality of life, and reduce reactivity in the limbic system by modulating brain activation patterns (Pinto-Sanchez et al. 2017). In research focused on osteoarthritis (OA), administering B. longum orally was found to mitigate pain perception in rats with OA, safeguard cartilage from damage, and diminish the expression of inflammatory cytokines and catabolic markers(Oh et al. 2023).
In reverse MR analysis, PHN, TN, and PDPN are found to decrease the abundance of 19, 14, and 8 types of gut microbiota respectively. Notably, the reductions are primarily in the phyla Bacteroidetes and Firmicutes, which constitute over 90% of the gastrointestinal microbiota. Changes in the ratio of these bacterial groups significantly impact health, influencing conditions like obesity and inflammatory diseases. These bacteria are key in breaking down complex polysaccharides and cellulose, producing SCFAs such as butyrate and acetate, which are crucial energy sources for intestinal cells and support gut health (Jandhyala et al. 2015; Valdes et al. 2018). They also interact with the gut immune system to regulate immune responses and the mucosal barrier, impacting autoimmune and inflammatory responses (Round and Mazmanian 2009). Variations in Bacteroidetes proportions are linked to diseases like obesity and inflammatory bowel disease, while Firmicutes influence the gut’s pH and oxygen levels, affecting the growth of bacterial communities and maintaining microbial balance (Round and Mazmanian 2009; Bäckhed et al. 2004). An imbalance in the Firmicutes/Bacteroidetes ratio is associated with obesity and metabolic syndrome, with higher Firmicutes abundance potentially increasing energy intake efficiency, thus impacting energy balance (Turnbaugh et al. 2006). Despite odds ratios around 0.9, monitoring gut microbiota balance in these patients remains clinically important.
Our research suggests that an elevation in CD4 levels on HLA DR + CD4 + cells within the immune system correlates with a decreased PHN risk. The effector functions of CD4 T cells exhibit a high degree of heterogeneity, primarily exerting their influence through the production of a variety of cytokines and chemokines, as well as the expression of different cell surface proteins on other immune cells, infected cells, or pathogens. CD4 T cells can also exhibit cytotoxic effects, directly eliminating pathogens or infected cells. Given their effector cell diversity, CD4 T cells play a crucial role in controlling infections by a wide range of pathogens, including bacteria, viruses, parasites, and fungi (Tippalagama et al. 2021; Ahmed et al. 2018; Jung et al. 2017; Fiszer et al. 1994).Our research findings are consistent with other literature reports, indicating that CD4 can effectively reduce the occurrence of pain and inflammation (Krukowski et al. 2016; Liu et al. 2014).
CD25hi %T cells are identified as a protective factor against TN. Tregs, typically characterized by CD4 + CD25 + Foxp3 + markers, exert their immunomodulatory effects by directly inhibiting the activation of target cells and secreting anti-inflammatory cytokines such as TGF-β and IL-10. Tregs play pivotal roles in various diseases, including immune tolerance, autoimmune disorders, infectious diseases, organ transplantation, and cancer (Cohen and Boyer 2006). Treg cells’ deficiency can precipitate the development of a spectrum of autoimmune and lymphoproliferative disorders, including autoimmune diabetes. Sustaining FOXP3 expression in CD25-high expressing Tregs is critical for inflammation control. Our study reveals that an augmentation in CD25hi %T cell count correlates with a diminished TN risk.
In our MR analysis of PDPN-related immune cells, we discovered that CD39 on monocyte exerts a significant protective effect against PDPN. Monocytes not only act as effector cells, but also serve as precursors to myeloid dendritic cells and macrophages, with their differentiation rate increasing during periods of inflammation activation (Gu et al. 2023; Boyette et al. 2017). CD39 regulates the conversion process from ATP to adenosine, thereby modulating Treg cells activation and impacting cancer growth (Park et al. 2021). In the context of NP, there is ample evidence indicating the involvement of microglia and blood-derived infiltrating macrophages in pain sensing (Austin and Moalem-Taylor 2010). Particularly in the scenario of PDPN, monocytes and M1 polarized macrophages become the primary producers of TNF-α in the peripheral blood under CNP conditions. Notably, individuals with type 1 diabetes exhibited augmented CD39 expression exclusively in those with PDPN, suggesting the engagement of specific inflammatory cascades (O’Brien et al. 2021). Furthermore, we observed that an elevation in HLA-DR expression on DCs exacerbates the risk of PDPN.
The primary objective of our study is to unravel the causal relationship between GM and NP, shedding light on the intermediary role of immune cells in these intricate connections. Through mediation analysis, we revealed that the pathway of fucose degradation significantly reduces the risk of developing PHN, with 6.7% of this effect mediated by the immune cell CD4 on CM CD4 + . Similarly, the Roseburia inulinivorans, belonging to the genus Roseburia, also reduces the risk of PHN, with 12.5% of the protective effect mediated by the immune cell CD28 + DN (CD4-CD8-) AC. In TN, we observed that the GDP-mannose derived O-antigen building blocks biosynthesis pathway reduces the risk of TN, with 3.3% of this effect mediated by HLA DR + CD8br %T cell. Conversely, the superpathway of acetyl-CoA biosynthesis increases the risk of TN, with 12% mediated by CD45 on lymphocyte cells. Regarding PDPN, our analysis revealed that the pathway of thiazole biosynthesis I in E. coli diminishes PDPN risk, with 7.5% of the effect mediated by IgD-CD38-AC. These findings not only deepen our understanding of the intricate connections between the gut microbiota and neuropathic pain but also underscore the pivotal role of specific immune cells in mediating these connections. They enhance our comprehension of the gut–brain axis interactions and provide valuable insights into potential biomarkers and intervention targets for the development of novel therapeutic approaches, particularly in the realm of NP prevention and treatment.
There are several limitations to this study. First, the analysis relied on data exclusively from European populations, potentially constraining the applicability of the results to other ethnic groups. Second, the GWAS data for NP analyzed had a relatively small number of cases, and larger GWAS datasets are needed for future validation. Third, our study utilized aggregated data rather than individual-level data, restricting our ability to explore subgroup causal relationships, such as gender differences. Fourth, the study results indicated that the genetic predisposition to TN mediated through HLA DR + CD8br %T cells was only 3.3%, a relatively low proportion, suggesting the role of other mediators needs further research to be quantified. Fifth, since data for PDPN were substituted with that of PDPN, the interpretation of results necessitates extra caution. Furthermore, the descriptive nature of the pain data constrained our ability to pinpoint specific pain etiologies, potentially influencing the study outcomes.
Although our study has its limitations, we believe it offers valuable insights into the potential relationships between GM and NP, serving as a foundational piece for future investigations. While generalizing our findings across diverse populations and contexts requires caution, we deem this study significant in unraveling the intricate interplay between GM and NP. Moreover, it provides informative cues for the development of novel therapeutic approaches.
Conclusion
Our study investigates the causal connections linking the gut microbiota, immune cells, and NP ailments. Employing the BWMR approach, we pinpointed immune cells and gut microbiota exhibiting robust causal associations with NP, while also uncovering the mediating roles of five immune cells in bridging the gap between gut microbiota and NP ailments. These findings provide valuable insights for identifying NP biomarkers and developing therapeutic targets.
Data availability
The datasets analyzed during the present study are available as GWAS summary data. The immune cell traits (731 in total) can be accessed at GWAS MRC IEU, with the accession numbers Ebi-a-GCST0001391 to Ebi-a-GCST0002121. The summary statistics of 412 gut microbial features (Study accession numbers: GCST90027446 to GCST90027857) used in this article can be downloaded from the NHGRI-EBI GWAS Catalog. The datasets for painful diabetic polyneuropathy (finn-b-DM_POLYNEURO) can be accessed here. The datasets for post-herpetic neuralgia (finngen_R9_G6_POSTZOST) are available here. The datasets for trigeminal neuralgia (finngen_R10_G6_TRINEU) can be accessed here. These datasets were sourced from the FinnGen consortium’s GWAS summary data, accessible at FinnGen.
Abbreviations
- BWMR:
-
Bayesian weighted Mendelian randomization
- CI:
-
Confidence intervals
- CNP:
-
Chronic neuropathic pain
- CRPS:
-
Complex regional pain syndrome
- DAMPs:
-
Damage-associated molecular pattern molecules
- DMP:
-
Dutch Microbiome Project
- DRGs:
-
Dorsal root ganglia
- GM:
-
Gut microbiome
- GWAS:
-
Genome-Wide Association Studies
- HCC:
-
Hepatocellular carcinoma
- IV:
-
Instrumental variables
- IVW:
-
Inverse variance-weighted
- LD:
-
Linkage disequilibrium
- LPS:
-
Lipopolysaccharides
- LTA:
-
Lipoteichoic acid
- MR:
-
Mendelian randomization
- NP:
-
Neuropathic pain
- PAMPs:
-
Pathogen-associated molecular patterns
- PDPN:
-
Painful diabetic peripheral neuropathy
- PGN:
-
Peptidoglycan
- PHN:
-
Post-herpetic neuralgia
- PNS:
-
Peripheral nervous system
- SCFAs:
-
Short-chain fatty acids
- SNP:
-
Single nucleotide polymorphism
- TLRs:
-
Toll-like receptors
- TN:
-
Trigeminal neuralgia
- TRP:
-
Transient receptor potential
- TSMR:
-
Two-sample Mendelian randomization
- UA:
-
Uric acid
References
Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA (2010) A map of human genome variation from population-scale sequencing. Nature 467:1061–1073
Ahmed A, Adiga V, Nayak S, Uday Kumar JAJ, Dhar C, Sahoo PN, Sundararaj BK, Souza GD, Vyakarnam A (2018) Circulating HLA-DR+CD4+ effector memory T cells resistant to CCR5 and PD-L1 mediated suppression compromise regulatory T cell function in tuberculosis. PLoS Pathog 14:e1007289
Attal N, Bouhassira D, Colvin L (2023) Advances and challenges in neuropathic pain: a narrative review and future directions. Br J Anaesth 131:79–92
Austin PJ, Moalem-Taylor G (2010) The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol 229:26–50
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101:15718–15723
Barcelon EE, Cho WH, Jun SB, Lee SJ (2019) Brain microglial activation in chronic pain-associated affective disorder. Front Neurosci 13:213
Bethea JR, Fischer R (2021) Role of peripheral immune cells for development and recovery of chronic pain. Front Immunol 12:641588
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–525
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40:304–314
Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM (2017) Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 12:e0176460
Bril V, England J, Franklin GM, Backonja M, Cohen J, del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D (2011) Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American academy of neurology, the American association of neuromuscular and electrodiagnostic medicine, and the American academy of physical medicine and rehabilitation. Neurology 76:1758–1765
Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, Taylor AE, Davies NM, Howe LD (2021) Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol 36:465–478
Chen Y, Tang S (2023) Gut microbiota and immune mediation: a Mendelian randomization study on granulomatosis with polyangiitis. Front Immunol 14:1296016
Chen X, Kong J, Diao X, Cai J, Zheng J, Xie W, Qin H, Huang J, Lin T (2020) Depression and prostate cancer risk: a Mendelian randomization study. Cancer Med 9:9160–9167
Cohen JL, Boyer O (2006) The role of CD4+CD25hi regulatory T cells in the physiopathogeny of graft-versus-host disease. Curr Opin Immunol 18:580–585
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN (2017) Neuropathic pain. Nat Rev Dis Primers 3:17002
Consensus workgroup on herpes zoster, C. D. A., National Clinical Research Center For Skin And Immune Diseases (2022) Chinese consensus on the diagnosis and management of herpes zoster (2022). Chin J Dermatol 55:1033–1040
Crișan TO, Cleophas MC, Oosting M, Lemmers H, Toenhake-Dijkstra H, Netea MG, Jansen TL, Joosten LA (2016) Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann Rheum Dis 75:755–762
Daoub A, Ansari H, Orfanos G, Barnett A (2021) Rothia mucilaginosa: a case of septic arthritis in a native knee and review of the literature. BMJ Case Rep 14:e237015
Defaye M, Gervason S, Altier C, Berthon JY, Ardid D, Filaire E, Carvalho FA (2020) Microbiota: a novel regulator of pain. J Neural Transm (vienna) 127:445–465
Dekker Nitert M, Mousa A, Barrett HL, Naderpoor N, De Courten B (2020) Altered gut microbiota composition is associated with back pain in overweight and obese individuals. Front Endocrinol (lausanne) 11:605
Ding W, You Z, Chen Q, Yang L, Doheny J, Zhou X, Li N, Wang S, Hu K, Chen L, Xia S, Wu X, Wang C, Zhang C, Chen L, Ritchie C, Huang P, Mao J, Shen S (2021) Gut microbiota influences neuropathic pain through modulating proinflammatory and anti-inflammatory T Cells. Anesth Analg 132:1146–1155
Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM (2003) Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol 60:1524–1534
Dworsky-Fried Z, Kerr BJ, Taylor AMW (2020) Microbes, microglia, and pain. Neurobiol Pain 7:100045
Fiore NT, Debs SR, Hayes JP, Duffy SS, Moalem-Taylor G (2023) Pain-resolving immune mechanisms in neuropathic pain. Nat Rev Neurol 19:199–220
Fischer R, Proske M, Duffey M, Stangl H, Martinez GF, Peters N, Kraske A, Straub RH, Bethea JR, Kontermann RE, Pfizenmaier K (2018) Selective activation of tumor necrosis factor receptor II Induces antiinflammatory responses and alleviates experimental arthritis. Arthritis Rheumatol 70:722–735
Fischer R, Sendetski M, del Rivero T, Martinez GF, Bracchi-Ricard V, Swanson KA, Pruzinsky EK, Delguercio N, Rosalino MJ, Padutsch T, Kontermann RE, Pfizenmaier K, Bethea JR (2019) TNFR2 promotes Treg-mediated recovery from neuropathic pain across sexes. Proc Natl Acad Sci USA 116:17045–17050
Fiszer U, Mix E, Fredrikson S, Kostulas V, Link H (1994) Parkinson’s disease and immunological abnormalities: increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol Scand 90:160–166
Getzenberg RB, Hijano DR, Hakim H, Dallas RH, Ferrolino JA, Van De Cardenas JB, Garner CD, Tang L, Su Y, Wolf J, Hayden RT, Maron G (2021) Rothia mucilaginosa infections in pediatric cancer patients. J Pediatric Infect Dis Soc 10:341–344
Gherghina ME, Peride I, Tiglis M, Neagu TP, Niculae A, Checherita IA (2022) Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int J Mol Sci 23:3188
Gonzalez-Alvarez ME, Sanchez-Romero EA, Turroni S, Fernandez-Carnero J, Villafañe JH (2023) Correlation between the altered gut microbiome and lifestyle interventions in chronic widespread pain patients: a systematic review. Medicina (kaunas) 59:256
Goudman L, Demuyser T, Pilitsis JG, Billot M, Roulaud M, Rigoard P, Moens M (2024) Gut dysbiosis in patients with chronic pain: a systematic review and meta-analysis. Front Immunol 15:1342833
Gu J, Yan GM, Kong XL, Zhang YY, Huang LH, Lu HM (2023) Assessing the causal relationship between immune traits and systemic lupus erythematosus by bi-directional Mendelian randomization analysis. Mol Genet Genom 298:1493–1503
Chinese Diabetes Society (2021) Guideline for the prevention and treatment of type 2 diabetes mellitus in China. Chinese J Pract Int Med 41:668–695
Guo R, Chen LH, Xing C, Liu T (2019) Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth 123:637–654
Guo D, Chen Q, Wang G, Li C (2023) Causal relationship between gut microbiota and immune thrombocytopenia: a Mendelian randomization study of two samples. Front Microbiol 14:1190866
Heda R, Yazawa M, Shi M, Bhaskaran M, Aloor FZ, Thuluvath PJ, Satapathy SK (2021) Non-alcoholic fatty liver and chronic kidney disease: retrospect, introspect, and prospect. World J Gastroenterol 27:1864–1882
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Smith GD, Gaunt TR, Haycock PC (2018) The MR-Base platform supports systematic causal inference across the human phenome. Elife 7:e34408
Huang J, Zhang C, Wang J, Guo Q, Zou W (2019) Oral Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement do not produce analgesic effects on neuropathic and inflammatory pain in rats. Brain Behav 9:e01260
Inoue K, Tsuda M (2018) Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 19:138–152
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN (2015) Role of the normal gut microbiota. World J Gastroenterol 21:8787–8803
Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice ASC, Treede RD (2011) A new definition of neuropathic pain. Pain 152:2204–2205
Jung HY, Kim YJ, Choi JY, Cho JH, Park SH, Kim YL, Kim HK, Huh S, Won DI, Kim CD (2017) Increased circulating T lymphocytes expressing HLA-DR in kidney transplant recipients with microcirculation inflammation. J Korean Med Sci 32:908–918
Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A (2016) CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci 36:11074–11083
Kulkarni P, Devkumar P, Chattopadhyay I (2021) Could dysbiosis of inflammatory and anti-inflammatory gut bacteria have an implications in the development of type 2 diabetes? Pilot Investig BMC Res Notes 14:52
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, Reeve MP, Laivuori H, Aavikko M, Kaunisto MA, Loukola A, Lahtela E, Mattsson H, Laiho P, Della Briotta Parolo P, Lehisto AA, Kanai M, Mars N, Rämö J, Kiiskinen T, Heyne HO, Veerapen K, Rüeger S, Lemmelä S, Zhou W, Ruotsalainen S, Pärn K, Hiekkalinna T, Koskelainen S, Paajanen T, Llorens V, Gracia-Tabuenca J, Siirtola H, Reis K, Elnahas AG, Sun B, Foley CN, Aalto-Setälä K, Alasoo K, Arvas M, Auro K, Biswas S, Bizaki-Vallaskangas A, Carpen O, Chen CY, Dada OA, Ding Z, Ehm MG, Eklund K, Färkkilä M, Finucane H, Ganna A, Ghazal A, Graham RR, Green EM, Hakanen A, Hautalahti M, Hedman ÅK, Hiltunen M, Hinttala R, Hovatta I, Hu X, Huertas-Vazquez A, Huilaja L, Hunkapiller J, Jacob H, Jensen JN, Joensuu H, John S, Julkunen V, Jung M, Junttila J, Kaarniranta K, Kähönen M, Kajanne R, Kallio L, Kälviäinen R, Kaprio J, Kerimov N, Kettunen J, Kilpeläinen E, Kilpi T, Klinger K, Kosma VM, Kuopio T, Kurra V, Laisk T, Laukkanen J, Lawless N, Liu A, Longerich S, Mägi R, Mäkelä J, Mäkitie A, Malarstig A, Mannermaa A, Maranville J, Matakidou A, Meretoja T, Mozaffari SV et al (2023) FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613:508–518
Li W, Tu J, Zheng J, Das A, Yan Q, Jiang X, Ding W, Bai X, Lai K, Yang S (2023) Disease-associated gut microbiome and metabolome changes in chronic low back pain patients with bone marrow lesions. bioRxiv. https://doi.org/10.1101/2023.07.26.550629
Liang X, Fan Y (2023) Bidirectional two-sample Mendelian randomization analysis reveals a causal effect of interleukin-18 levels on postherpetic neuralgia risk. Front Immunol 14:1183378
Lin B, Wang Y, Zhang P, Yuan Y, Zhang Y, Chen G (2020) Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J Headache Pain 21:103
Liu XJ, Zhang Y, Liu T, Xu ZZ, Park CK, Berta T, Jiang D, Ji RR (2014) Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res 24:1374–1377
Long Y, Tang L, Zhou Y, Zhao S, Zhu H (2023) Causal relationship between gut microbiota and cancers: a two-sample Mendelian randomisation study. BMC Med 21:66
Lopera-Maya EA, Kurilshikov A, Van Der Graaf A, Hu S, Andreu-Sánchez S, Chen L, Vila AV, Gacesa R, Sinha T, Collij V, Klaassen MAY, Bolte LA, Gois MFB, Neerincx PBT, Swertz MA, Harmsen HJM, Wijmenga C, Fu J, Weersma RK, Zhernakova A, Sanna S (2022) Effect of host genetics on the gut microbiome in 7738 participants of the Dutch microbiome project. Nat Genet 54:143–151
Lynch BMW (2001) Genetics and analysis of quantitative traits. Am J Hum Genet 68(2):548–549
Machiels K, Joossens M, Sabino J, Preter VD, Arijs I, Eeckhaut V, Ballet V, Claes K, Immerseel FV, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S (2014) A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63:1275–1283
Magni G, Riboldi B, Ceruti S (2023) Modulation of glial cell functions by the gut-brain axis: a role in neurodegenerative disorders and pain transmission. Cells 12:1612
Min LYYZL (2023) Research progress of gut microbiota in neuropathic pain. Basic Clin Med 43:188–191
Moloney RD, Johnson AC, O’Mahony SM, Dinan TG, Greenwood-Van Meerveld B, Cryan JF (2016) Stress and the microbiota-gut-brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther 22:102–117
Nagamine T (2023) Burning mouth syndrome needs to consider the gut-brain axis from three types of pain: nociceptive, neuropathic, and nociplastic pain. J Gastrointestin Liver Dis 32:558–559
Novak S, Melkonian AK, Patel PA, Kleinman NL, Joseph-Ridge N, Brook RA (2007) Metabolic syndrome-related conditions among people with and without gout: prevalence and resource use. Curr Med Res Opin 23:623–630
O’Brien JA, McGuire HM, Shinko D, De St F, Groth B, Russo MA, Bailey D, Santarelli DM, Wynne K, Austin PJ (2021) T lymphocyte and monocyte subsets are dysregulated in type 1 diabetes patients with peripheral neuropathic pain. Brain Behav Immun Health 15:100283
Oh DK, Na HS, Jhun JY, Lee JS, Um IG, Lee SY, Park MS, Cho ML, Park SH (2023) Bifidobacterium longum BORI inhibits pain behavior and chondrocyte death, and attenuates osteoarthritis progression. PLoS ONE 18:e0286456
Orrù V, Steri M, Sidore C, Marongiu M, Serra V, Olla S, Sole G, Lai S, Dei M, Mulas A, Virdis F, Piras MG, Lobina M, Marongiu M, Pitzalis M, Deidda F, Loizedda A, Onano S, Zoledziewska M, Sawcer S, Devoto M, Gorospe M, Abecasis GR, Floris M, Pala M, Schlessinger D, Fiorillo E, Cucca F (2020) Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet 52:1036–1045
Park HJ, Seo EH, Piao L, Park ST, Lee MK, Koh SE, Lee SH, Kim SH (2021) The preventive effect of the phenotype of tumour-associated macrophages, regulated by CD39, on colon cancer in mice. Int J Mol Sci 22:7478
Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O, Welsh C, Rieder A, Traynor J, Gregory C, de Palma G, Pigrau M, Ford AC, Macri J, Berger B, Bergonzelli G, Surette MG, Collins SM, Moayyedi P, Bercik P (2017) Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153:448–459
Prevention, Diabetes and Treatment of Clinical Guidelines Writing Group and others (2022) Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China. Zhonghua Nei Ke Za Zhi 61:12–50
Qing-Jun L (2018) Interpretation of “Chinese expert consensus on diagnosis and treatment of trigeminal neuralgia.” Chin J Contemp Neurol Neurosurg 18:643–646
Rahman-Enyart A, Yaggie RE, Bollinger JL, Arvanitis C, Winter DR, Schaeffer AJ, Klumpp DJ (2022) Acyloxyacyl hydrolase regulates microglia-mediated pelvic pain. PLoS ONE 17:e0269140
Ramakrishna C, Corleto J, Ruegger PM, Logan GD, Peacock BB, Mendonca S, Yamaki S, Adamson T, Ermel R, McKemy D, Borneman J, Cantin EM (2019) Dominant role of the gut microbiota in chemotherapy induced neuropathic pain. Sci Rep 9:20324
Reichenberger ER, Alexander GM, Perreault MJ, Russell JA, Schwartzman RJ, Hershberg U, Rosen G (2013) Establishing a relationship between bacteria in the human gut and complex regional pain syndrome. Brain Behav Immun 29:62–69
Round JL, Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9:313–323
Ruan G, Chen M, Chen L, Xu F, Xiao Z, Yi A, Tian Y, Ping Y, Lv L, Cheng Y, Wei Y (2022) Roseburia intestinalis and Its metabolite butyrate inhibit colitis and upregulate TLR5 through the SP3 signaling pathway. Nutrients 14:3041
Schaefer C, Sadosky A, Mann R, Daniel S, Parsons B, Tuchman M, Anschel A, Stacey BR, Nalamachu S, Nieshoff E (2014) Pain severity and the economic burden of neuropathic pain in the United States: BEAT neuropathic pain observational study. Clini Outcomes Res 6:483–496
Shaeer K, Addisu A, Nanjappa S, Greene J (2017) Epidemiologic evaluation of Rothia Bacteremia-a single cancer center 3 year experience. Open Forum Infect Dis 4:S555–S555
Sidore C, Busonero F, Maschio A, Porcu E, Naitza S, Zoledziewska M, Mulas A, Pistis G, Steri M, Danjou F, Kwong A, Vecchyo VDOD, ChiangBragg-Gresham CWKJ, Pitzalis M, Nagaraja R, Tarrier B, Brennan C, Uzzau S, Fuchsberger C, Reinier F, Berutti R, Huang J, Timpson NJ, Toniolo D, Gasparini P, Malerba G, Dedoussis G, Zeggini E, Soranzo N, Jones C, Lyons R, Angius A, Kang HM, Novembre J, Sanna S, Schlessinger D, Cucca F, Abecasis GR (2015) Genome sequencing elucidates Sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers. Nat Genet 47:1272–1281
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, Vanderweele TJ, Higgins JPT, Timpson NJ, Dimou N, Langenberg C, Golub RM, Loder EW, Gallo V, Tybjaerg-Hansen A, Smith DG, EggerRichards MJB (2021) Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA 326:1614–1621
Su PP, Zhang L, He L, Zhao N, Guan Z (2022) The role of neuro-immune interactions in chronic pain: implications for clinical practice. J Pain Res 15:2223–2248
Sun J, Ince MN, Abraham C, Barrett T, Brenner LA, Cong Y, Dashti R, Dudeja PK, Elliott D, Griffith TS, Heeger PS, Hoisington A, Irani K, Kim TK, Kapur N, Leventhal J, Mohamadzadeh M, Mutlu E, Newberry R, Peled JU, Rubinstein I, Sengsayadeth S, Tan CS, Tan XD, Tkaczyk E, Wertheim J, Zhang ZJ (2023) Modulating microbiome-immune axis in the deployment-related chronic diseases of veterans: report of an expert meeting. Gut Microbes 15:2267180
Thacker MA, Clark AK, Marchand F, McMahon SB (2007) Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg 105:838–847
Tippalagama R, Singhania A, Dubelko P, ArlehamnCrinklawPomaznoy LCSAM, Seumois G, Desilva AD, Premawansa S, Vidanagama D, Gunasena B, Goonawardhana NDS, Ariyaratne D, Scriba TJ, Gilman RH, Saito M, Taplitz R, Vijayanand P, Sette A, Peters B, Burel JG (2021) HLA-DR marks recently divided antigen-specific effector CD4 T cells in active tuberculosis patients. J Immunol 207:523–533
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031
UçEYLER N, RogauschTOYKASOMMER JPKVC (2007) Differential expression of cytokines in painful and painless neuropathies. Neurology 69:42–49
Ustianowska K, Ustianowski Ł, Machaj F, Gorący A, Rosik J, Szostak B, Szostak J, Pawlik A (2022) The role of the human microbiome in the pathogenesis of pain. Int J Mol Sci 23:13267
Valdes AM, Walter J, Segal E, Spector TD (2018) Role of the gut microbiota in nutrition and health. BMJ 361:k2179
Verbanck M, Chen CY, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50:693–698
Wang X, Gao H, Zeng Y, Chen J (2024a) A Mendelian analysis of the relationships between immune cells and breast cancer. Front Oncol 14:1341292
Wang Y, Ma M, Dai W, Shang Q, Yu G (2024b) Bacteroides salyersiae is a potent chondroitin sulfate-degrading species in the human gut microbiota. Microbiome 12:41
Wei X, Zhou H, Zhang S, Hu X, Wei Z, Li Y (2023) A comprehensive two-sample Mendelian randomization analysis of trigeminal neuralgia and modifiable risk factors. Front Neurol 14:1292958
Yang C, Fang X, Zhan G, Huang N, Li S, Bi J, Jiang R, Yang L, Miao L, Zhu B, Luo A, Hashimoto K (2019a) Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl Psychiatry 9:57
Yang F, Yu S, Fan B, Liu Y, Chen YX, Kudel I, Concialdi K, Dibonaventura M, Hopps M, Hlavacek P, Cappelleri JC, Sadosky A, Parsons B, Udall M (2019b) The epidemiology of herpes zoster and postherpetic neuralgia in China: results from a cross-sectional study. Pain Ther 8:249–259
Yavorska OO, Burgess S (2017) Mendelian randomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 46:1734–1739
Yu SY, Fan BF, Yang F, Dibonaventura M, Chen YX, Li RY, King-Concialdi K, Kudel I, Hlavacek P, Hopps M, Udall M, Sadosky A, Cappelleri JC (2019) Patient and economic burdens of postherpetic neuralgia in China. Clin Outcomes Res 11:539–550
Yu J, Zhu P, Shi L, Gao N, Li Y, Shu C, Xu Y, Yu Y, He J, Guo D, Zhang X, Wang X, Shao S, Dong W, Wang Y, Zhang W, Zhang W, Chen WH, Chen X, Liu Z, Yang X, Zhang B (2024) Bifidobacterium longum promotes postoperative liver function recovery in patients with hepatocellular carcinoma. Cell Host Microbe 32:131–144
Zhang Y, Chen S, Yuan M, Xu Y, Xu H (2022) Gout and diet: a comprehensive review of mechanisms and management. Nutrients 14:3525
Zhao J, Ming J, Hu X, Chen G, Liu J, Yang C (2020) Bayesian weighted Mendelian randomization for causal inference based on summary statistics. Bioinformatics 36:1501–1508
Acknowledgements
The GWAS summary data were obtained from the online public platform (https://gwas.mrcieu.ac.uk/). We want to acknowledge the participants and investigators of the FinnGen study.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Zhixuan Lan, Yi Wei, Ruilin He, and Zongbin Jiang. Zhixuan Lan was responsible for data harmonization and SNP selection. The first draft of the manuscript was written by Zhixuan Lan, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lan, Z., Wei, Y., Yue, K. et al. Genetically predicted immune cells mediate the association between gut microbiota and neuropathy pain. Inflammopharmacol 32, 3357–3373 (2024). https://doi.org/10.1007/s10787-024-01514-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-024-01514-y