Abstract
Introduction
Inflammatory bowel disease (IBD), consists of two primary types: Ulcerative Colitis (UC) and Crohn’s Disease (CD). Infliximab (IFX) and Adalimumab (ADA) are frequently utilized in the management of moderate to severe cases of IBD.
Aim
This study aimed to assess the efficacy and safety of IFX and ADA in individuals diagnosed with moderate to severe IBD.
Method
This study is a prospective open-labeled randomized parallel study that included moderate to severe IBD patients treated with either IFX or ADA. A total of 56 patients participated, with 34 patients received IFX and 22 patients received ADA. Various measures, including Crohn’s Disease Activity Index (CDAI), Mayo Score/ Disease Activity Index (DAI), and C-reactive protein (CRP) levels, were taken at baseline and week 14 to assess the efficacy of the treatments. In addition, the levels of drugs and sTREM-1 were measured at 14 weeks. Patient safety was monitored throughout the study period.
Results
In the group received IFX, there was a notable decrease in CDAI (P = 0.045), DAI (P = 0.026), and CRP (P = 0.023 for CD, and P = 0.021 for UC) levels. In addition, the group received ADA experienced a significant reduction in CDAI (P = 0.001), DAI (P = 0.032), and CRP (P < 0.018 for CD and P = 0.003 for UC) levels. Responders had higher drug concentrations than non-responders, notably IFX concentration was higher in responders with CD (P = 0.001) and UC (P < 0.001). ADA concentration was higher in UC (P <= 0.001) and all CD patients responded to the treatment. The same trend was observed for sTREM-1 levels in CD and UC patients (P = 0.042, and P = 0.015, respectively) in the IFX group. In UC patients treated with ADA, the level of sTREM-1 was significantly low (P = 0.002).
Conclusion
Both IFX and ADA have a good safety profile and deliver a beneficial clinical and laboratory response in moderate-severe IBD patients.
Clinical Trial Registration
This study is registered on ClinicalTrials.gov under the identifier NCT05291039. (You can access the study at https://clinicaltrials.gov/study/NCT05291039 (First Posted: March 22, 2022).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is a long-term inflammatory condition that affects the digestive system, which includes ulcerative colitis (UC) and Crohn’s disease (CD). It can affect people of all ages and genders (Aardoom et al. 2019). Symptoms of IBD include ulceration, inflammation of the tissues of the GIT, diarrhea, abdominal pain, anemia, weight loss, and rectal bleeding (Aardoom et al. 2019).
Diagnosis of IBD depends on inflammatory markers, clinical findings, and endoscopic evaluation. An inflammatory marker is C-reactive protein (CRP), which rises when there is inflammation in the intestine (Afif et al. 2009).
IBD complications are divided into two categories, extra-intestinal and intestinal complications. Extra-intestinal complications are osteoporosis, arthritis, anemia, aphthous ulcers, uveitis, anal fistulas, and erythema nodosum. Intestinal complications include colon cancer and colon perforation (Afif et al. 2009).
The severity of CD is determined using the Crohn’s Disease Activity Index (CDAI), which helps to evaluate the stage of CD. In contrast, UC is evaluated based on the Mayo score/disease activity index (DAI). CDAI ranges from 0 to 600. CDAI scores are based on symptoms, signs, blood test results, patient demographic characteristics, and extra-intestinal findings (Afify et al. 2021; Albader et al. 2021).
The DAI is a scale that goes from 0 to 12, and each part is ranked from 0 to 3. The DAI score is calculated by looking at symptoms like rectal bleeding, stool frequency, doctor’s evaluation, and endoscopy results (Albader et al. 2021; Cholapranee et al. 2017).
The treatment approach for IBD aims to relieve symptoms and achieve mucosal healing through a step-up approach of medication. Amino-salicylates are the first-line treatment, followed by corticosteroids if there is no response. If symptoms persist or recur during corticosteroid tapering, a purified protein derivative test is conducted to rule out latent tuberculosis before starting immune-modifying agents (Anti-TNF agents). If no response is achieved, surgery may be necessary (Colombel et al. 2009; Da et al. 2013).
Infliximab (IFX) and adalimumab (ADA) are examples of approved anti-TNF agents (biologic drugs) that are used in the management of IBD. Both IFX and ADA are effective and safe for managing IBD in the short and long term (Domenicantonio et al. 2018).
Soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), a protein found on specific immune cells, is known to increase in inflammatory situations. Recent research has shown higher levels of sTREM-1 in individuals with IBD which is potentially tied to the severity of the disease (Doecke et al. 2017).
The Egyptian Ministry of Health introduced recently IFX and ADA in the treatment protocol for IBD. Although the prevalence of IBD is considered at a low rate in Egypt, the curve of newly diagnosed cases is increasing and there are a few data regarding the management of biologic treatments in Egyptian IBD patients (Gisbert and Chaparro 2021). Therefore, this research aims to assess the efficacy and safety of IFX and ADA in treating IBD Egyptian patients.
Design and methods
Our study was conducted on patients with IBD who were split into two treatment groups, IFX and ADA, in a prospective, parallel, randomized manner. The study assessed trough concentrations of IFX and ADA, sTREM-1 level, CRP levels, CDAI, and DAI score. Trough concentrations of IFX and ADA were assessed after 14 weeks of starting treatments. Then Patients were further followed up for 2 months. Follow-ups were performed via scheduled hospital visits and phone calls for all enrolled patients.
Study population and eligibility
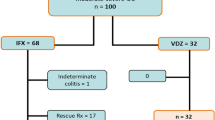
All patients with IBD were selected from the Outpatient Clinic at Ain Shams University Hospitals in Cairo, Egypt, and were evaluated for eligibility. The criteria for inclusion and exclusion can be found in Table 1. Six hundred and seven patients presented to the clinic. Eligible patients were randomly assigned based on the appointment number.
All eligible patients were asked to provide a written informed consent for participation in the study. Figure 1 is a flow diagram for the patients included in the study.
Intervention
The loading dose of ADA (Humira®, AbbVie pharmaceuticals, North Chicago, USA) is 160 mg subcutaneously, given either as 4 injections of 40 mg on the first day or as 2 injections of 40 mg daily for 2 consecutive days, followed by a dose of 80 mg subcutaneously 2 weeks later. The maintenance dose is 40 mg subcutaneously every 2 weeks.
For IFX (Remicade®, Janssen Biotech pharmaceuticals, Horsham Township, Pennsylvania, USA), the loading dose is 5 mg/kg intravenously at 0, 2, and 6 weeks, with a maintenance dose of 5 mg/kg intravenously every 2 months.
In patients previously treated with biologic drugs there were washout periods of 4 weeks in the case of IFX and 5 weeks in the case of ADA or golimumab (Han et al. 2020).
Compliance
During the trial, a clinical pharmacist monitored compliance by ensuring patients received their scheduled doses at each visit. In addition, two phone calls were made to follow up with the patients. A colonoscopy was performed at baseline and week 14, using Olympus® CF-H180, Japan, to assess the patient’s condition in both treatment groups.
Assessment plan
Baseline assessments included patient history and current symptoms, CRP levels, CDAI, DAI, and colonoscopy. At week 14, CRP, CDAI, DAI, and colonoscopy were reassessed for all patients. sTREM-1 and trough concentration levels of IFX and ADA were also assessed at week 14. Patients were further followed up for 2 months for any side effects.
Primary endpoint
Assessment of efficacy was performed according to; trough concentrations of IFX and ADA, CRP, CDAI score, DAI score, and serum TREM-1 for all included patients.
Determination of trough concentrations of the biologic drugs (infliximab and adalimumab) and level of soluble triggering receptor expressed on myeloid cells-1 in the serum
After 14 weeks of treatment with either IFX or ADA regimens, blood samples (5 ml) were collected before the next dose (trough concentration). Serum trough concentration of IFX and ADA were measured using ELISA kits (RIDASCREEN® IFX Monitoring, R-Biopharm AG®, Darmstadt, Germany, and RIDASCREEN® ADA Monitoring, R-Biopharm AG®, Darmstadt, Germany, respectively). The serum TREM-1 was measured using the Human Triggering Receptor Expressed on Myeloid Cells-1 ELISA Kit (Bioassay Technology Laboratory, Shanghai, China). ELISA kits were used according to the manufacturer’s rules.
Determination of efficacy by crohn’s disease activity index
Patients diagnosed with moderate to severe active CD underwent treatment with either IFX or ADA and were assessed for effectiveness using the CDAI. Clinical response (CR) was evaluated based on CR-70 and CR-100 criteria, signifying a decrease of at least 70 points and 100 points, respectively in CDAI scores from the initial assessment (Han et al. 2020).
Determination of efficacy by mayo score/disease activity index
The CR was determined by a decrease of at least 3 points and 30% from the initial Mayo score, as well as a decrease of at least 1 point in the rectal bleeding sub-score or reaching an absolute rectal bleeding sub-score of 0 or 1 (Kamat et al. 2019).
Secondary endpoint
Any adverse effect was recorded during the follow-up visits or calls for the included patients in both regimens as summarized in Table 2.
Statistical analysis and sample size
Statistical analysis was conducted using the Statistical Package of Social Sciences (SPSS) software version 20. A normality test was carried out for continuous variables. If the data was normally distributed, paired samples t tests were used to compare the mean and standard deviation before and after treatments. Unpaired t tests were used for comparisons between groups. Otherwise, non-parametric data was compared using the Mann–Whitney U test and the Wilcoxon signed-rank test. For non-parametric data, patient demographics and baseline characteristics were described using descriptive statistics. Continuous variables were presented as means ± SD, while categorical variables were presented as frequencies and percentages. The Chi-square test was used to compare responses between the two treatment groups for categorical data. The level of significance was considered significant when P values < 0.05.
Ethical considerations
The trial had no impact on the patient’s treatment or follow-up.
Trial status
This study followed the ethical guidelines outlined in the Declaration of Helsinki and the obtained approval from the Faculty of Medicine at Ain Shams University Hospitals, and Damanhur University—Faculty of Pharmacy—Research Ethics Committee with an ethical approval number FMASU R175/2023 and 923PP68M, respectively. The study is registered on ClinicalTrials.gov with the identifier NCT05291039. Participants in the study provided informed consent to participate. (First Posted: March 22, 2022). Visit the study page at: https://clinicaltrials.gov/study/NCT05291039. Patients’ inclusion began in December 2021 and ended in March 2023.
Results
Tables 3 and 4 provide an overview of the baseline clinical features and demographics of the studied patients. Meanwhile, Table 5 outlines the clinical characteristics of the treatment groups receiving IFX and ADA before and after therapy. In our study, four patients out of the 56 included in both groups IBD patients who needed hemicolectomy, two CD patients, and two UC patients. Similarly, two CD patients needed bowel resection. In addition, one patient out of the 12 CD patients underwent ileocecal mass removal surgery.
In our study, two IBD patients were diagnosed with rheumatoid arthritis, one patient had myelophthisic anemia, one patient had Mediterranean fever with thalassemia, and one patient had hypothyroidism.
Efficacy
Crohn’s disease activity index
Figure 2 displays the number of patients who achieved CDAI response with IFX and ADA after 14 weeks in patients with CD. There was a significant decrease in scores for IFX (P = 0.045) and ADA (P = 0.001). ADA displayed effectiveness rates of 100 and 71.4% based on CR-70 and CR-100, respectively. In comparison, IFX exhibited effectiveness rates of 50 and 25% according to CR-70 and CR-100, respectively.
Mayo score/disease activity index
According to the Mayo score, ADA and IFX showed efficacy with 46.66 and 36.36%, respectively. Figure 2 illustrates the number of patients who achieved a clinical response to IFX and ADA after 14 weeks of treatment for UC. Both groups showed a significant decrease in their scores, with IFX having a P value of 0.026 and ADA having a P value of 0.032.
Medication Concentration
Figure 3 shows the levels of IFX in CD patients who responded and didn’t respond to treatment. Patients who achieved a clinical response based on CDAI-70 had significantly higher levels of IFX (5.8 ± 2.6 μg/mL) compared to non-responding patients (1.1 ± 0.7 μg/mL) group (P = 0.001). The same trend was observed with UC patients treated with IFX as seen in Fig. 4.
In Fig. 5, it is shown that patients with ulcerative colitis who experienced an improvement in their symptoms had significantly elevated serum levels of ADA (7.3 ± 5 μg/mL) in comparison to those who did not show a similar response. (0.9 ± 0.9 μg/mL) according to DAI (P < 0.001). One outlier was noticed in the responded group with a serum level of 18.7 μg/mL of ADA.
It was noticed that ADA’s mean level in CD patients was 6.5 ± 3.7 μg/mL with no “non-response” patients.
Levels of C-reactive protein
In Table 5, the levels of CRP before and after treatments for patients with IBD were compared.
After treatment, the CRP levels significantly decreased in both the IFX and ADA groups, with a P value less than 0.05.
Soluble triggering receptor expressed on myeloid cells-1 (sTREM-1)
Table 5 also shows the levels of sTREM-1 for patients with UC and CD. Patients who received IFX and showed clinical response had significantly lower levels of sTREM-1 compared to those who did not respond. ADA also had a similar impact on patients with CD and UC.
Adalimumab and infliximab
The clinical response to ADA and IFX was measured using CDAI and DAI for CD and UC patients, respectively. In CD patients, ADA showed a higher clinical response compared to IFX (P = 0.034). In UC patients, both ADA and IFX showed similar effectiveness (P = 0.386).
Safety
The main side effects that were reported included headache, tiredness, muscle pain, and reactions from the infusion. Out of the 34 people who received IFX, 6 (17.6%) felt fatigued after the treatment, while only 1 out of 22 (4.5%) ADA recipients experienced fatigue. Infusion reactions, which showed symptoms like headache, dizziness, nausea, irritation at the injection site, flushing, chest pain, and difficulty in breathing, happened in 4 out of 34 people who received IFX (11.8%). Most of these reactions were not severe. Pre-treatment with steroids effectively managed this side effect. Since ADA was injected under the skin, reactions at the injection site included pain, sensitivity, redness, hardening, and swelling. This happened in 2 out of 22 (9.1%) patients. Muscle pain was observed in 17.6% of patients in the IFX group and in 9.1% of patients in the ADA group. The pain was felt in different parts of the body like the back, neck, and limbs. Headache was also noted in 11.8% of patients in the IFX group and in 9.1% of patients in the ADA group, occurring several hours after treatment.
The pregnant patient who received IFX did not experience any negative effects during her pregnancy. She responded well to IFX and stopped taking the medication 2 months before giving birth without any issues.
Discussion
The Egyptian Ministry of Health recently approved the use of IFX and ADA for treating moderate to severe IBD. This research project aims to evaluate the effectiveness and safety of both treatments in Egyptian IBD patients.
Our findings show that IBD patients treated with IFX or ADA experienced a significant reduction in sTREM-1, CRP levels, CDAI, and DAI. Patients who responded well to the treatment had serum therapeutic levels of IFX and ADA, while those who did not respond adequately had sub-therapeutic levels. Furthermore, our research indicated that the effectiveness of IFX and ADA in patients with CD and UC is dependent on the concentration of the drugs in their body. This discovery aligns with previous studies conducted on a larger scale (Kamat et al. 2019; Kawalec et al. 2013; Kim et al. 2020).
It was noted that only one of the patients treated with ADA (4.5%) showed a highly elevated level of serum ADA reaching 18.7 µg/mL. This finding aligns with existing literature supporting the association between higher ADA levels and the attainment of biologic remission (Kutlu et al. 2021). This patient had experienced side effects such as hair loss, and skin lesions, which led to the physician’s decision of drug discontinuation.
In recent systematic reviews and meta-analyses, the effectiveness of IFX and ADA for patients with CD and UC was examined. The findings indicated that both medications proved to be successful in treating patients with CD and UC (Lee et al. 2021; Liefferinckx et al. 2020) which is consistent with our findings in Egyptian IBD patients.
The finding of this study reveals a significant reduction in disease activity, as evidenced by lowered levels of CRP in both IFX and ADA-treated patients and this was in agreement with three recent studies (Kutlu et al. 2021; Maaser et al. 2019; Mizoshita et al. 2017).
The effectiveness of both IFX and ADA in CD patients was assessed by the significant decrease in the CDAI’s scores after treatment when compared with baseline values. This reduction aligns with the existing literature, as documented by Paweł Kawalec et al. (Mogilevski and Sparrow 2018).
Yong Il Lee et al. 2021 reported a significantly lower DAI score after treatment when compared with baseline values in patients receiving either IFX or ADA (Nielsen et al. 2022) and this coincides with our data.
In our study, there was a significant decrease in sTREM-1 levels following treatment with both IFX and ADA. This result was reported by Verstockt et al., 2019, confirming a shared consensus regarding the anti-inflammatory effects of these biologics (Okobi et al. 2021). On the contrary, Kutlu et al., 2021 reported a conflicting result with the present study on sTREM-1 levels (Pabla and Schwartz 2020) This discrepancy underscores the complexity of the inflammatory response and highlights the need for further investigation to unravel the nuanced effects of IFX and ADA on sTREM-1 in the context of IBD.
Our study also compared the efficacy of ADA and IFX in treating CD and UC. In UC patients,
both ADA and IFX demonstrated comparable efficacy. This result is consistent with the results of recent clinical trials (Papamichael et al. 2019; Patel and Yarur 2023; Plevris et al. 2019; Prins et al. 2021). On the contrary, a clinical trial and a meta-analysis had reported conflict results with our data (Lee et al. 2021; Razzaq 2017).
Our study found that ADA was more effective than IFX in treating CD patients. However, other studies have shown similar effectiveness between IFX and ADA in treating CD patients (Plevris et al. 2019; Seyedian et al. 2019; Shamkh et al. 2022). This difference in results may be due to the smaller size of our study group and the diverse ethnic backgrounds of the patients included in the previous studies.
In addition, our study involved 7 CD patients treated successfully with ADA, 4 of them had previously experienced treatment failure with IFX. This finding provides additional support for the superior efficacy of ADA over IFX. This result is consistent with previous research (Singh et al. 2021; Sostegni et al. 2003; Sturm et al. 2019; Su et al. 2019).
Our study has identified mild adverse events in patients receiving either IFX or ADA. In accordance with our data, other studies have shown a good safety profile for IFX and ADA in the management of moderate to severe IBD (Thorlund et al. 2015; Tursi et al. 2021; Verstockt et al. 2019).
The safety of biologic treatment during pregnancy is of crucial concern. Our unique observation of a successful pregnancy in an IFX-treated patient aligns with reassuring data from (Wang et al. 2022).
According to a recent study by Ole Haagen Nielsen et al. in 2022, it was discovered that biologics had more advantages than risks for pregnant women with IBD. The research also determined that the occurrence of negative pregnancy outcomes in IBD pregnant women using TNF inhibitors is not greater than that of the general population. (Yang et al. 2022).
Limitations
One major limitation of our study is that we did not assess immunogenicity, specifically the presence of antibodies. In clinical practice, routine monitoring of serum trough levels and antibody formation at every visit is uncommon, primarily due to financial constraints. In additiony, the small sample size of our study is attributed to the low prevalence of IBD in Egypt.
Conclusion
In conclusion, both IFX and ADA are safe and effective for treating moderate to severe IBD patients. It is important to monitor drug levels and manage potential side effects for best results. ADA is more effective than IFX in CD patients, while both treatments are equally effective in UC patients. These findings highlight the importance of individualization of IBD treatment to balance effectiveness and safety.
Recommendation
To improve treatment strategies and overall quality of life for Egyptian patients with IBD, it is essential to conduct additional large-scale multicenter studies with longer follow-up periods. Baseline measurements of sTREM-1 are needed to accurately assess if treatment has an effect on this parameter.
Data availability
If you need data, just ask the corresponding author.
References
Aardoom MA, Veereman G, de Ridder L (2019) A Review on the use of anti-TNF in children and adolescents with inflammatory bowel disease. Int J Mol Sci. https://doi.org/10.3390/ijms20102529
Afif W, Leighton JA, Hanauer SB, Loftus EV Jr, Faubion WA, Pardi DS et al (2009) Open-label study of adalimumab in patients with ulcerative colitis including those with prior loss of response or intolerance to infliximab. Inflamm Bowel Dis 15(9):1302–1307
Afify A, Gado M, Gohar M, Wadea F (2021) Clinical and biochemical response of treatment with anti-TNFα in egyptian IBD patients: zagazig university IBD clinic experience. Zagazig Univ Med J 10(4):56–78
Albader F, Golovics PA, Gonczi L, Bessissow T, Afif W, Lakatos PL (2021) Therapeutic drug monitoring in inflammatory bowel disease: the dawn of reactive monitoring. World J Gastroenterol 27(37):6231–6247
Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN (2017) Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther 45(10):1291–1302
Colombel JF, Sandborn WJ, Panaccione R, Robinson AM, Lau W, Li J et al (2009) Adalimumab safety in global clinical trials of patients with crohn’s disease. Inflamm Bowel Dis 15(9):1308–1319
Da W, Zhu J, Wang L, Lu Y (2013) Adalimumab for crohn’s disease after infliximab treatment failure: a systematic review. Eur J Gastroenterol Hepatol 25(8):885–891
Di Domenicantonio R, Trotta F, Cascini S, Agabiti N, Kohn A, Gasbarrini A et al (2018) Population-based cohort study on comparative effectiveness and safety of biologics in inflammatory bowel disease. Clin Epidemiol 10:203–213
Doecke JD, Hartnell F, Bampton P, Bell S, Mahy G, Grover Z et al (2017) Infliximab vs. adalimumab in crohn’s disease: results from patients in an australian and new zealand observational cohort study. Aliment Pharmacol Ther. https://doi.org/10.1111/apt.13880
Gisbert JP, Chaparro M (2021) Primary failure to an anti-TNF Agent in inflammatory bowel disease: switch (to a second anti-TNF agent) or swap (for another mechanism of action)? J Clin Med 10(22):5318
Han M, Jung YS, Cheon JH, Park S (2020) Comparison of real-world outcomes of infliximab versus adalimumab in biologic-naive korean patients with ulcerative colitis: a population-based study. Yonsei Med J 61(1):48–55
Kamat N, Kedia S, Ghoshal UC, Nehra A, Makharia G, Sood A et al (2019) Effectiveness and safety of adalimumab biosimilar in inflammatory bowel disease: a multicenter study. Indian J Gastroenterol 38(1):44–54
Kawalec P, Mikrut A, Wisniewska N, Pilc A (2013) Tumor necrosis factor-alpha antibodies (infliximab, adalimumab and certolizumab) in crohn’s disease: systematic review and meta-analysis. Arch Med Sci 9(5):765–779
Kim SY, An S, Park DK, Kwon KA, Kim KO, Chung JW et al (2020) Efficacy of iron supplementation in patients with inflammatory bowel disease treated with anti-tumor necrosis factor-alpha agents. Therap Adv Gastroenterol 13:1756284820961302
Kutlu Y, Toprak İ, Gökden Y, Eruzun H, Arman Y, Aydın Yoldemir Ş et al (2021) THE relationship between strem-1 and activation of inflammatory bowel diseases. İstanbul Tıp Fakültesi Dergisi. 10(4):56–78
Lee YI, Park Y, Park SJ, Kim TI, Kim WH, Cheon JH (2021) Comparison of long-term outcomes of infliximab versus adalimumab treatment in biologic-naive patients with ulcerative colitis. Gut Liver 15(2):232–242
Liefferinckx C, Cremer A, Franchimont D (2020) Switching biologics used in inflammatory bowel diseases: how to deal with in practice? Curr Opin Pharmacol 55:82–89
Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V et al (2019) ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 13(2):144–164
Mizoshita T, Katano T, Tanida S, Hirano A, Miyaki T, Ozeki K et al (2017) Prospective comparison of preference and efficacy of adalimumab and infliximab for treating ulcerative colitis naive to antitumor necrosis factor therapy. Medicine (baltimore) 96(32):e7800
Mogilevski T, Sparrow MP (2018) Infliximab versus adalimumab in patients with biologic-naïve crohn’s disease: is the difference real? Dig Dis Sci 63(5):1094–1096
Nielsen OH, Gubatan JM, Juhl CB, Streett SE, Maxwell C (2022) Biologics for inflammatory bowel disease and their safety in pregnancy: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. https://doi.org/10.1016/j.cgh.2020.09.021
Okobi OE, Udoete IO, Fasehun OO, Okobi T, Evbayekha EO, Ekabua JJ et al (2021) A review of four practice guidelines of inflammatory bowel disease. Cureus 13(8):e16859
Pabla BS, Schwartz DA (2020) Assessing severity of disease in patients with ulcerative colitis. Gastroenterol Clin North Am 49(4):671–688
Papamichael K, Lin S, Moore M, Papaioannou G, Sattler L, Cheifetz AS (2019) Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis 10:2040622319838443
Patel S, Yarur AJ (2023) A Review of therapeutic drug monitoring in patients with inflammatory bowel disease receiving combination therapy. J Clin Med. https://doi.org/10.3390/jcm12206577
Plevris N, Lyons M, Jenkinson PW, Chuah CS, Merchant LM, Pattenden RJ et al (2019) Higher adalimumab drug levels during maintenance therapy for crohn’s disease are associated with biologic remission. Inflamm Bowel Dis 25(6):1036–1043
Prins MM, Verstockt B, Ferrante M, Vermeire S, Wildenberg ME, Koelink PJ (2021) Monocyte TREM-1 levels associate with anti-TNF responsiveness in IBD through autophagy and fcgamma-receptor signaling pathways. Front Immunol 12:627535
Razzaq B (2017) Efficacy and safety of adalimumab versus infliximab in patients suffered from moderate to severe active ulcerative colitis. Asian J Pharm Clin Res 10:300–307
Seyedian SS, Nokhostin F, Malamir MD (2019) A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life 12(2):113–122
Shamkh MAA, Sakr MA, Abd Alaty WH, Kamel SY, Eltabbakh MM, Sherief AF et al (2022) A decade of inflammatory bowel disease: a single center experience in Egypt. The Egyptian Journal of Internal Medicine. https://doi.org/10.1186/s43162-022-00115-x
Singh S, Murad MH, Fumery M, Sedano R, Jairath V, Panaccione R et al (2021) Comparative efficacy and safety of biologic therapies for moderate-to-severe crohn’s disease: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 6(12):1002–1014
Sostegni R, Daperno M, Scaglione N, Lavagna A, Rocca R, Pera A (2003) Review article: crohn’s disease: monitoring disease activity. Aliment Pharmacol Ther 17(Suppl 2):11–17
Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T et al (2019) ECCO-ESGAR guideline for diagnostic assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis 13(3):273–284
Su HJ, Chiu YT, Chiu CT, Lin YC, Wang CY, Hsieh JY et al (2019) Inflammatory bowel disease and its treatment in 2018: global and taiwanese status updates. J Formos Med Assoc 118(7):1083–1092
Thorlund K, Druyts E, Toor K, Mills EJ (2015) Comparative efficacy of golimumab, infliximab, and adalimumab for moderately to severely active ulcerative colitis: a network meta-analysis accounting for differences in trial designs. Expert Rev Gastroenterol Hepatol 9(5):693–700
Tursi A, Mocci G, Lorenzetti R, Allegretta L, Brandimarte G, Cassieri C et al (2021) Long-term real-life efficacy and safety of infliximab and adalimumab in the treatment of inflammatory bowel diseases outpatients. Eur J Gastroenterol Hepatol 33(5):670–679
Verstockt B, Verstockt S, Blevi H, Cleynen I, de Bruyn M, Van Assche G et al (2019) TREM-1, the ideal predictive biomarker for endoscopic healing in anti-TNF-treated crohn’s disease patients? Gut 68(8):1531–1533
Wang H, Hu Y, Chen F, Shen M (2022) Comparative safety of infliximab and adalimumab on pregnancy outcomes of women with inflammatory bowel diseases: a systematic review & meta-analysis. BMC Pregnancy Childbirth 22(1):854
Yang HH, Huang Y, Zhou XC, Wang RN (2022) Efficacy and safety of adalimumab in comparison to infliximab for crohn’s disease: a systematic review and meta-analysis. World J Clin Cases 10(18):6091–6104
Acknowledgements
The authors wish to thank the participants and medical staff of the Inflammatory Bowel Disease Outpatient Clinic at Ain Shams University Hospitals, Cairo, Egypt, for their help and support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). There were no specific grants received for this research.
Author information
Authors and Affiliations
Contributions
All authors worked on the literature review, study design, writing of the manuscript, and revision before submission.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest were declared by the authors.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamal, M.E., Werida, R.H., Radwan, M.A. et al. Efficacy and safety of infliximab and adalimumab in inflammatory bowel disease patients. Inflammopharmacol (2024). https://doi.org/10.1007/s10787-024-01508-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10787-024-01508-w