Abstract
Background
Melatonin is a neurohormone secreted predominantly by the pineal gland that is demonstrated to be associated with the pathogenesis of multiple sclerosis (MS). This research desires to evaluate the tolerability and beneficial effects of exogenous melatonin supplementations in patients with MS.
Methods
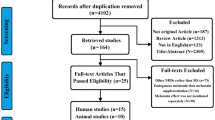
This study was executed following the PRISMA 2020 statement. Both observational and interventional studies which reported the clinical effectiveness and/or safety of melatonin supplementation in patients with MS were included in this systematic review. Ovid, PubMed, Scopus, Embase, and Web of Science databases were searched and the risk of bias in included studies was assessed using the Joanna Briggs Institute (JBI) critical appraisal tools based on study design.
Results
Out of 1304 results of database searches, finally, 14 articles, including 7 randomized controlled trials (RCTs), 6 case–control studies, and one quasi-experimental study, were included based on the full-text review. Included phenotypes of MS were mostly relapsing-remitting MS (RRMS) (in 11 studies); it was secondary progressive MS (SPMS) in only one study, and two other studies had a mixture of the different phenotypes. The course of treatment with melatonin supplementation was between 2 weeks and 12 months. There were no substantial safety issues. Although melatonin was associated with enhanced oxidative stress and inflammation status, concerning the clinical benefits, limited studies suggested improvements in sleep conditions, cognitive outcomes, and fatigue in MS.
Discussion
There are insufficient data to support the regular melatonin prescription in MS. Limitations such as the small number of included studies, the diversity of the dosage, route, and duration of melatonin administration, and the diversity of assessment tests lead to unconvincing findings in this study. There is a need for future studies to achieve a comprehensive judgment on this subject.

Similar content being viewed by others
Availability of data and materials
This published article and its supplementary files include all data generated or analyzed during this study.
References
Adamczyk-Sowa M, Pierzchala K, Sowa P, Mucha S, Sadowska-Bartosz I, Adamczyk J, Hartel M (2014a) Melatonin acts as antioxidant and improves sleep in MS patients. Neurochem Res 39(8):1585–1593. https://doi.org/10.1007/s11064-014-1347-6
Adamczyk-Sowa M, Galiniak S, Zyracka E, Grzesik M, Naparło K, Sowa P, Bartosz G, Sadowska-Bartosz I (2017) Oxidative modification of blood serum proteins in multiple sclerosis after interferon beta and melatonin treatment. Oxidative Med Cell Longev. https://doi.org/10.1155/2017/7905148
Adamczyk-Sowa M, Pierzchala K, Sowa P, Polaniak R, Kukla M, Hartel M (2014b) Influence of melatonin supplementation on serum antioxidative properties and impact of the quality of life in multiple sclerosis patients. J Physiol Pharmacol 65(4), 543–550. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med11&NEWS=N&AN=25179086
Adamczyk-Sowa M, Sowa P, Adamczyk J, Niedziela N, Misiolek H, Owczarek M, Zwirska-Korczala K (2016a) Effect of melatonin supplementation on plasma lipid hydroperoxides, homocysteine concentration and chronic fatigue syndrome in multiple sclerosis patients treated with interferons-beta and mitoxantrone. J Physiol Pharmacol 67(2), 235–242. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med13&NEWS=N&AN=27226183
Adamczyk-Sowa M, Sowa P, Mucha S, Zostawa J, Mazur B, Owczarek M, Pierzchala K (2016b) Changes in serum ceruloplasmin levels based on immunomodulatory treatments and melatonin supplementation in multiple sclerosis patients. Med Sci Monitor 22:2484–2491. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med13&NEWS=N&AN=27420299https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4958372/pdf/medscimonit-22-2484.pdf
Alvarez-Sanchez N, Cruz-Chamorro I, Diaz-Sanchez M, Sarmiento-Soto H, Medrano-Campillo P, Martinez-Lopez A, Lardone PJ, Guerrero JM, Carrillo-Vico A (2017) Melatonin reduces inflammatory response in peripheral T helper lymphocytes from relapsing-remitting multiple sclerosis patients. J Pineal Res. https://doi.org/10.1111/jpi.12442
Balcer LJ, Frohman EM (2010) Evaluating loss of visual function in multiple sclerosis as measured by low-contrast letter acuity. Neurology 74(Suppl 3):S16-23. https://doi.org/10.1212/WNL.0b013e3181dbb664
Besag FMC, Vasey MJ, Lao KSJ, Wong ICK (2019) Adverse events associated with melatonin for the treatment of primary or secondary sleep disorders: a systematic review. CNS Drugs 33(12):1167–1186. https://doi.org/10.1007/s40263-019-00680-w
Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Baker G, Klassen TP, Vohra S (2005) The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J Gen Intern Med 20(12):1151–1158. https://doi.org/10.1111/j.1525-1497.2005.0243.x
Chan V, Lo K (2022) Efficacy of dietary supplements on improving sleep quality: a systematic review and meta-analysis. Postgrad Med J 98(1158):285–293. https://doi.org/10.1136/postgradmedj-2020-139319
Cho JH, Bhutani S, Kim CH, Irwin MR (2021) Anti-inflammatory effects of melatonin: a systematic review and meta-analysis of clinical trials. Brain Behav Immun 93:245–253. https://doi.org/10.1016/j.bbi.2021.01.034
Cipolla-Neto J, Amaral FGD (2018) Melatonin as a hormone: new physiological and clinical insights. Endocr Rev 39(6):990–1028. https://doi.org/10.1210/er.2018-00084
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372(9648):1502–1517. https://doi.org/10.1016/s0140-6736(08)61620-7
Delgado D, Canham L, Cotterill N, Cottrell D, Drake MJ, Inglis K, Owen D, White P (2017) Protocol for a randomized, double blind, placebo controlled, crossover trial of Melatonin for treatment of Nocturia in adults with Multiple Sclerosis (MeNiMS). BMC Neurol. https://doi.org/10.1186/s12883-017-0845-y
Drake MJ, Canham L, Cotterill N, Delgado D, Homewood J, Inglis K, Johnson L, Kisanga MC, Owen D, White P, Cottrell D (2018) Results of a randomized, double blind, placebo controlled, crossover trial of melatonin for treatment of Nocturia in adults with multiple sclerosis (MeNiMS). BMC Neurol 18(1):107. https://doi.org/10.1186/s12883-018-1114-4
Emamgholipour S, Hossein-Nezhad A, Ansari M (2016a) Can melatonin act as an antioxidant in hydrogen peroxide-induced oxidative stress model in human peripheral blood mononuclear cells? Biochem Res Int. https://doi.org/10.1155/2016/5857940
Emamgholipour S, Hossein-Nezhad A, Sahraian MA, Askarisadr F, Ansari M (2016b) Evidence for possible role of melatonin in reducing oxidative stress in multiple sclerosis through its effect on SIRT1 and antioxidant enzymes. Life Sci 145:34–41. https://doi.org/10.1016/j.lfs.2015.12.014
Fatemeh G, Sajjad M, Niloufar R, Neda S, Leila S, Khadijeh M (2022) Effect of melatonin supplementation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. J Neurol 269(1):205–216. https://doi.org/10.1007/s00415-020-10381-w
Fuladi Targhi F, Faraji F, Maleki Rad AA, Ghassami K, Talaei A (2018) Study the effect of melatonin on fatigue in patients with multiple sclerosis [Original Atricle]. J Arak Univ Med Sci 21(6):67–75. http://jams.arakmu.ac.ir/article-1-5562-en.html
Galley HF, Lowes DA, Allen L, Cameron G, Aucott LS, Webster NR (2014) Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res 56(4):427–438. https://doi.org/10.1111/jpi.12134
Ghareghani M, Scavo L, Arnoult D, Zibara K, Farhadi N (2018) Melatonin therapy reduces the risk of osteoporosis and normalizes bone formation in multiple sclerosis. Fund Clin Pharmacol 32(2):181–187. https://doi.org/10.1111/fcp.12337
Golan D, Staun-Ram E, Glass-Marmor L, Lavi I, Rozenberg O, Dishon S, Barak M, Ish-Shalom S, Miller A (2013) The influence of vitamin D supplementation on melatonin status in patients with multiple sclerosis. Brain Behav Immun 32:180–185. https://doi.org/10.1016/j.bbi.2013.04.010
Hawkes CH, Baker MD, Pohl D, Lechner-Scott J, Levy M, Giovannoni G (2021) Melatonin and multiple sclerosis. Mult Scler Relat Disord 51:103032. https://doi.org/10.1016/j.msard.2021.103032
Hosseini S, Yazdchi M, Sahraian MA, Majidi S, Taher S, Hassannezhad S, Mosaddeghi Heris R (2022) Fatigue in multiple sclerosis is a diagnostic challenge: a cross-sectional study. J Res Clin Med 10(1):23–23. https://doi.org/10.34172/jrcm.2022.023
Hsu W-Y, Anderson A, Rowles W, Peters KE, Li V, Stone KL, Ashbrook LH, Gelfand AA, Bove RM (2021) Effects of melatonin on sleep disturbances in multiple sclerosis: a randomized, controlled pilot study. Mult Scler J Exp Transl Clin 7(4):20552173211048756. https://doi.org/10.1177/20552173211048756
Jallouli S, Ghroubi S, Dhia IB, Yahia A, Elleuch MH, Sakka S, Mhiri C, Hammouda O (2022) Effect of melatonin intake on postural balance, functional mobility and fall risk in persons with multiple sclerosis: a pilot study. Int J Neurosci. https://doi.org/10.1080/00207454.2022.2090353
Jamshidi Fard A, Rafipour H, Faraji F (2013) Effects of melatonin on visual functioning of patients with multiple sclerosis. J Arak Univ Med Sci 16(5):8–18. http://jams.arakmu.ac.ir/article-1-2282-en.html
Janalipour K (2019) Multiple sclerosis symptoms. In: Neurological disorders and imaging physics, Volume 2: engineering and clinical perspectives of multiple sclerosis. IOP Publishing
Kawakami N, Nägerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flügel A (2005) Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med 201(11):1805–1814. https://doi.org/10.1084/jem.20050011
Koike H, Katsuno M (2021) Macrophages and autoantibodies in demyelinating diseases. Cells. https://doi.org/10.3390/cells10040844
Lõpez-González A, Álvarez-Sánchez N, Lardone PJ, Cruz-Chamorro I, Martínez-Lõpez A, Guerrero JM, Reiter RJ, Carrillo-Vico A (2015) Melatonin treatment improves primary progressive multiple sclerosis: a case report. J Pineal Res 58(2):173–177. https://doi.org/10.1111/jpi.12203
Lundmark PO, Pandi-Perumal SR, Srinivasan V, Cardinali DP, Rosenstein RE (2007) Melatonin in the eye: implications for glaucoma. Exp Eye Res 84(6):1021–1030. https://doi.org/10.1016/j.exer.2006.10.018
Lv T, Yan J, Lou Y, Zhang Z, Ye M, Zhou J, Luo F, Bi C, Lin H, Zhang J, Guo H, Liu Z (2022) Evaluation of melatonin therapy in patients with myocardial ischemia-reperfusion injury: a systematic review and meta-analysis. Oxid Med Cell Longev 2022:4610522. https://doi.org/10.1155/2022/4610522
Menczel Schrire Z, Phillips CL, Chapman JL, Duffy SL, Wong G, D’Rozario AL, Comas M, Raisin I, Saini B, Gordon CJ, McKinnon AC, Naismith SL, Marshall NS, Grunstein RR, Hoyos CM (2022) Safety of higher doses of melatonin in adults: a systematic review and meta-analysis. J Pineal Res 72(2):e12782. https://doi.org/10.1111/jpi.12782
Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T (2014) Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol 14:58. https://doi.org/10.1186/1471-2377-14-58
Miller E, Mrowicka M, Malinowska K, Mrowicki J, Saluk-Juszczak J, Kedziora J (2010) The effects of whole-body cryotherapy on oxidative stress in multiple sclerosis patients. J Therm Biol 35(8):406–410. https://doi.org/10.1016/j.jtherbio.2010.08.006
Miller E, Walczak A, Majsterek I, Kedziora J (2013) Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J Neuroimmunol 257(1–2):97–101. https://doi.org/10.1016/j.jneuroim.2013.02.012
Miller E, Mrowicka M, Malinowska K, Kedziora J, Majsterek I (2011) The effects of whole-body cryotherapy and melatonin supplementation on total antioxidative status and some antioxidative enzymes in multiple sclerosis patients. Wplyw krioterapii ogolnoustrojowej i suplementacji melatonina na calkowity potencjal antyoksydacyjny w osoczu oraz aktywnosc wybranych enzymow antyoksydacyjnych w erytrocytach chorych na stwardnlenie rozsiane 31(183):150–153. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med8&NEWS=N&AN=21991843
Morvaridzadeh M, Sadeghi E, Agah S, Nachvak SM, Fazelian S, Moradi F, Persad E, Heshmati J (2020) Effect of melatonin supplementation on oxidative stress parameters: a systematic review and meta-analysis. Pharmacol Res 161:105210. https://doi.org/10.1016/j.phrs.2020.105210
Mrowicka M, Garncarek P, Miller E, Kedziora J, Smigielski J, Malinowska K, Mrowicki J (2010) Effect of melatonin on activity of superoxide dismutase (CuZn-SOD) in erythrocytes of patients during short- and long-term hypokinesis. Wplyw melatoniny na aktywnosc dysmutazy ponadtlenkowej (CuZn-SOD) w krwinkach czerwonych chorych w okresie hipokinezji krotko- i dlugoterminowej 63(1), 3–9. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med8&NEWS=N&AN=20701024
Munoz-Jurado A, Escribano BM, Caballero-Villarraso J, Galvan A, Aguera E, Santamaria A, Tunez I (2022) Melatonin and multiple sclerosis: antioxidant, anti-inflammatory and immunomodulator mechanism of action. Inflammopharmacology. https://doi.org/10.1007/s10787-022-01011-0
Naseri A, Forghani N, Sadigh-Eteghad S, Shanehbandi D, Asadi M, Nasiri E, Talebi M (2022) Circulatory antioxidant and oxidative stress markers are in correlation with demographics but not cognitive functions in multiple sclerosis patients. Mult Scler Relat Disord 57:103432. https://doi.org/10.1016/j.msard.2021.103432
Oliva Ramirez A, Keenan A, Kalau O, Worthington E, Cohen L, Singh S (2021) Prevalence and burden of multiple sclerosis-related fatigue: a systematic literature review. BMC Neurol 21(1):468. https://doi.org/10.1186/s12883-021-02396-1
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Roostaei T, Sahraian MA, Hajeaghaee S, Gholipour T, Togha M, Siroos B, Mansouri S, Mohammadshirazi Z, Aghazadeh Alasti M, Harirchian MH (2015) Impact of melatonin on motor, cognitive and neuroimaging indices in patients with multiple sclerosis. Iran J Allergy Asthma Immunol 14(6):589–595. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med12&NEWS=N&AN=26725556https://ijaai.tums.ac.ir/index.php/ijaai/article/download/611/576
Samanta S (2022) Physiological and pharmacological perspectives of melatonin. Arch Physiol Biochem 128(5):1346–1367. https://doi.org/10.1080/13813455.2020.1770799
Sánchez-López AL, Ortiz GG, Pacheco-Moises FP, Mireles-Ramírez MA, Bitzer-Quintero OK, Delgado-Lara DLC, Ramírez-Jirano LJ, Velázquez-Brizuela IE (2018) Efficacy of melatonin on serum pro-inflammatory cytokines and oxidative stress markers in relapsing remitting multiple sclerosis. Arch Med Res 49(6):391–398. https://doi.org/10.1016/j.arcmed.2018.12.004
Savage RA, Zafar N, Yohannan S, Miller JMM (2023) Melatonin. In: StatPearls. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC
Talebi M, Majdi A, Nasiri E, Naseri A, Sadigh-Eteghad S (2021) The correlation between circulating inflammatory, oxidative stress, and neurotrophic factors level with the cognitive outcomes in multiple sclerosis patients. Neurol Sci 42(6):2291–2300. https://doi.org/10.1007/s10072-020-04807-6
Tryfonos C, Mantzorou M, Fotiou D, Vrizas M, Vadikolias K, Pavlidou E, Giaginis C (2019) Dietary supplements on controlling multiple sclerosis symptoms and relapses: current clinical evidence and future perspectives. Medicines 6(3):95. https://www.mdpi.com/2305-6320/6/3/95
Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp LAE, Munn Z (2020) Chapter 3: systematic reviews of effectiveness. JBI Manual for Evidence Synthesis. Adelaide: JBI [cited 2020 Feb 10]. In.
Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, Wallin M, Helme A, Angood NC, Rijke N, Baneke P (2020) Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler 26(14):1816–1821. https://doi.org/10.1177/1352458520970841
Wekerle H, Kojima K, Lannes-Vieira J, Lassmann H, Linington C (1994) Animal models. Ann Neurol 36(Suppl):S47-53. https://doi.org/10.1002/ana.410360714
Willenborg DO, Staykova M, Fordham S, O’Brien N, Linares D (2007) The contribution of nitric oxide and interferon gamma to the regulation of the neuro-inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol 191(1–2):16–25. https://doi.org/10.1016/j.jneuroim.2007.09.007
Yazdchi M, Khanalizadeh R, Nasiri E, Naseri A, Talebi M, Talebi M (2022) Sleep status in multiple sclerosis: Role of vitamin D and body mass index. Curr J Neurol 21(2):66–73. https://doi.org/10.18502/cjn.v21i2.10489
Yeganeh Salehpour M, Mollica A, Momtaz S, Sanadgol N, Farzaei MH (2019) Melatonin and multiple sclerosis: from plausible neuropharmacological mechanisms of action to experimental and clinical evidence. Clin Drug Investig 39(7):607–624. https://doi.org/10.1007/s40261-019-00793-6
Yosefifard M, Vaezi G, Malekirad AA, Faraji F, Hojati V (2019a) A randomized control trial study to determine the effect of melatonin on serum levels of IL-1beta and TNF-alpha in patients with multiple sclerosis. Iran J Allergy Asthma Immunol 18(6):649–654. https://doi.org/10.18502/ijaai.v18i6.2177
Yosefifard M, Vaezi G, Malekirad AA, Faraji F, Hojati V (2019b) A randomized control trial study to determine the effect of melatonin on serum levels of IL-1β and TNF-α in patients with multiple sclerosis. Iran J Allergy Asthma Immunol 18(6):649–654. https://doi.org/10.18502/ijaai.v18i6.2177
Yosefi-Fard M, Vaezi G, Maleki-Rad AA, Faraji F, Hojati V (2020) Effect of melatonin on serum levels of inf-1ß and vitamin b12 in patients with multiple sclerosis: a randomized controlled trial. Iran J Toxicol 14(1):19–24. https://doi.org/10.32598/ijt.14.1.19
Zarezadeh M, Barzegari M, Aghapour B, Adeli S, Khademi F, Musazadeh V, Jamilian P, Jamilian P, Fakhr L, Chehregosha F, Ghoreishi Z, Ostadrahimi A (2022) Melatonin effectiveness in amelioration of oxidative stress and strengthening of antioxidant defense system: Findings from a systematic review and dose-response meta-analysis of controlled clinical trials. Clin Nutr ESPEN 48:109–120. https://doi.org/10.1016/j.clnesp.2022.01.038
Zhang W, Chen XY, Su SW, Jia QZ, Ding T, Zhu ZN, Zhang T (2016) Exogenous melatonin for sleep disorders in neurodegenerative diseases: a meta-analysis of randomized clinical trials. Neurol Sci 37(1):57–65. https://doi.org/10.1007/s10072-015-2357-0
Zhao CN, Wang P, Mao YM, Dan YL, Wu Q, Li XM, Wang DG, Davis C, Hu W, Pan HF (2019) Potential role of melatonin in autoimmune diseases. Cytokine Growth Factor Rev 48:1–10. https://doi.org/10.1016/j.cytogfr.2019.07.002
Acknowledgements
The research protocol was approved and supported by the Student Research Committee, Tabriz University of Medical Sciences (Grant Number: 70831).
Funding
The research protocol was approved and supported by the Student Research Committee, Tabriz University of Medical Sciences (Grant Number: 70831).
Author information
Authors and Affiliations
Contributions
ZS, SM, JK, SH, ZH, EG-K, and AN: systematic search; study selection, data extraction, risk of bias assessment, preparing the figures and writing the first draft of the manuscript; SS and MT: supervision and critically editing the manuscript. All authors approved the final version for submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethics approval
The ethics committee of Tabriz University of Medical Science reviewed and approved the study protocol (Ethics code: IR.TBZMED.VCR.REC.1401.293).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morsali, S., Sabahi, Z., Kakaei, J. et al. Clinical efficacy and safety of melatonin supplementation in multiple sclerosis: a systematic review. Inflammopharmacol 31, 2213–2220 (2023). https://doi.org/10.1007/s10787-023-01271-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01271-4