Abstract
Cyclophosphamide (CP) is a chemotherapeutic agent that causes pulmonary damage by generating free radicals and pro-inflammatory cytokines. Pulmonary damage has a high mortality rate due to the severe inflammation and edema occurred in lung. PPARγ/Sirt 1 signaling has been shown to be cytoprotective effect against cellular inflammatory stress and oxidative injury. Protocatechuic acid (PCA) is a potent Sirt1 activator and exhibits antioxidant as well as anti-inflammatory properties. The current study aims to investigate the therapeutic impacts of PCA against CP-induced pulmonary damage in rats. Rats were assigned randomly into 4 experimental groups. The control group was injected with a single i.p injection of saline. CP group was injected with a single i.p injection of CP (200 mg/kg). PCA groups were administered orally with PCA (50 and 100 mg/kg; p.o.) once daily for 10 consecutive days after CP injection. PCA treatment resulted in a significant decrease in the protein levels of MDA, a marker of lipid peroxidation, NO and MPO along with a significant increase in GSH and catalase protein levels. Moreover, PCA downregulated anti-inflammatory markers as IL-17, NF-κB, IKBKB, COX-2, TNF-α, and PKC and upregulated cytoprotective defenses as PPARγ, and SIRT1. In addition, PCA administration ameliorated FoxO-1 elevation, increased Nrf2 gene expression, and reduced air alveoli emphysema, bronchiolar epithelium hyperplasia and inflammatory cell infiltration induced by CP. PCA might represent a promising adjuvant to prevent pulmonary damage in patients receiving CP due to its antioxidant and anti-inflammatory effects with cytoprotective defenses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary damage can result from sepsis, trauma, microbial infection, ischemia/reperfusion, or some drugs that induce acute respiratory failure with a mortality rate of approximately 40% (Righetti et al. 2018). The pathophysiology of pulmonary damage is characterized by infiltration of polymorphonuclear cells (PMNs) and epithelial integrity disruption which leads to interstitial edema and alveolar collapse (Castillo et al. 2015). Macrophages and neutrophils are vital mediators in stimulating the inflammatory reaction, controlling oxidative stress, and fibroblast function in pulmonary damage. In addition, macrophages and neutrophils release pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin-1beta (IL-1β), and IL-6 (IL-6), as well as transforming growth factor beta (TGF-β) (Blondonnet et al. 2016). These inflammatory cytokines suppressed by the peroxisome proliferator-activated receptors (PPARs) hormone in the lung (Kaplan et al. 2005). In addition, Sirtuin has a critical role in regulating macrophage mitochondrial bioenergetics (Kurundkar et al. 2019). Silent information regulator type-1 (SIRT1) controls gene expression for apoptosis, stress responses, and cellular senescence. SIRT1 exhibits antioxidative, antiangiogenic, antitumorigenic, and anti-inflammatory effects (Li et al. 2013). It has received much attention due to its role in resistance to oxidative stress, which is one of the mechanisms involved in the SIRT1/Forkhead box Protein (FOXOs) pathway (Mahlooji et al. 2022). FoxO1 is the main transcription factor of fundamental cellular processes, mainly regulating oxidative stress, cell proliferation, inflammation, immune homeostasis, and cell apoptosis (Arcidiacono et al. 2018).
Cyclophosphamide (CP), a synthetic alkylating agent, treats autoimmune diseases and cancer, as well as prevents organ transplantation rejection (Emadi et al. 2009). Despite its wide range of clinical applications, it produces multiorgan damage, which in turn causes severe morbidity and mortality (Haubitz 2007). Most reports focused on its hepatotoxicity (SNYDER et al. 1993), cardio- and gonadotoxicity (Swamy et al. 2013), and lung toxicity (Alsemeh and Abdullah 2022). It has been proposed that CP administration causes cellular respiration impairment through damage to mitochondria (Souid et al. 2003), which leads to liberation of reactive-oxygen species (ROS) (Suddek et al. 2013; Zarei and Shivanandappa 2013) and triggers the nuclear factor-κB (NF-κB) inflammatory pathway (Hamsa and Kuttan 2010).
Protocatechuic acid (PCA), is a polyphenolic compound, present in many vegetables and nuts (Vitaglione et al. 2007). It has anti-inflammatory, antioxidant, and hepatoprotective properties, mainly by inhibiting stress signal transduction as it suppresses Cyclooxygenase-2 (COX-2), nitric oxide synthase, and myeloperoxidase (MPO) in CCl4-induced liver injury (Hsu et al. 2009; Liu et al. 2002). In addition, it has a protective effect in lung injury (Alsharif et al. 2021).
In this regard, the current study investigates the effect of protocatechuic acid on PPARs/SIRT/FOX to mitigate CP-induced pulmonary damage in rats.
Materials and methods
Animals
Adult male Wister albino rats (150–200 g) were provided by the Animal House of the National Research Centre (Cairo, Egypt). The rats were group-housed with free access to standard laboratory rodent chow and water under temperature−light-controlled conditions (24 ± 2 °C under a 12 h light/dark cycle). The animal experiments were performed in according to recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH No. 85:23 revised 1985) in accordance with the guidelines of the Institutional Animal Ethics Committee (Medical Research Ethics Committee (MREC) of the NRC, Cairo, Egypt.
Drugs, chemicals, and kits
Cyclophosphamide and protocatechuic acid were purchased from (Santa Cruz, CA, USA). Glutathione (GSH), malondialdehyde (MDA), nitric oxide (NO), and catalase colorimetric kits were determined using Biodiagnostic kit, Cairo, Egypt. Sirtuin 1 (SIRT1) was determined using ELISA kits procured from (SunRed Biotech Co., Ltd, China). Tumor necrosis factor alpha (TNF-α), Interleukin-17 (IL-17), nuclear factor kappa B (NF-κB), inhibitor of nuclear factor kappa beta kinase subunit beta (IKBKB), proxisome proliferator-activated receptor gamma (PPARγ), cyclooxegnase-2 (COX-2), protein kinase C (PKC), and myeloperoxidase (MPO) were determined using ELISA kits procured from Sunlong Biotech Co., Ltd, China.
Experimental design
Wister albino male rats were randomly allocated into four groups (n = 8) as follows: Control group: Rats were injected with a single i.p injection of normal saline and received normal saline orally for 10 consecutive days. CP group: Rats were injected with a single i.p injection (200 mg/kg) (Saghir et al. 2020). Protocatechuic acid groups: Rats were administered protocatechuic acid (50 &100 mg/kg orally) (Krzysztoforska et al. 2019) once daily for 10 consecutive days after CP injection.
Biochemical analysis
At the end of the experimental period, the animals were sacrificed by decapitation; the lung from each rat was immediately dissected out, washed with ice‐cooled physiological saline, and homogenized in phosphate-buffered saline (PBS)(pH 7.4) as 20% (w/v) for the biochemical measurements of GSH, MDA, NO, and catalase were determined using Biodiagnostic colorimetric kits. Moreover, SIRT1, IL-17, NF-κB, IKBKB, PPARγ, COX-2, TNF-α, MPO, and PKC were determined using ELISA kits. The other lung was kept for histopathological assessment.
Estimation of GSH, MDA, NO, and catalase
GSH lung content was estimated according to the method that depends on the fact that protein and nonprotein SH-groups (mainly GSH) react with Ellman’s reagent [5,5′-dithio-bis(2 nitrobenzoic acid)] to form a stable yellow color of 5-mercapto-2-nitrobenzoic acid, which can be measured colorimetrically at 412 nm. In order to determine the GSH level in tissue, precipitation of protein SH-groups before the addition of Ellman’s reagent is necessary using precipitating solution (5% sulfosalicylic acid in bi-distilled water) (Basha et al. 2020).
Lung homogenate was used for the assessment of lipid peroxidation (LPO) as MDA and catalase activity according to the methods described by Ohkawa et al and Salama and Elgohary (2021), (Ohkawa et al. 1979; Salama and Elgohary 2021), respectively. In addition, NO was measured according to the method described by Tarpey et al. 2004. Briefly, nitrate was reduced to nitrite using the nitrate reductase enzyme. Then, the produced nitrite was assayed using Griess reagent at an optical density of 550 nm.
Estimation of SIRT1, IL-17, NF-κB, IKBKB, PPARγ, COX-2, TNF-α, and PKC
Lung contents of SIRT1, IL-17, NF-κB, IKBKB, PPARγ, COX-2, TNF-α, MPO, and PKC were determined using ELISA kits. Standards and samples were pipetted into wells with immobilized antibodies specific for rat SIRT1, IL-17, NF-κB, IKBKB, PPARγ, COX-2, TNF-α, MPO, and PKC and then were incubated for 30 min at 37 °C. After incubation and washing, horseradish peroxidase-conjugated streptavidin was pipetted into the wells, and incubated for 30 min at 37 °C, which was washed once again. Tetramethylbenzidine (TMB) substrate solution was added to the wells and incubated for 15 min at 37 °C; a color was developed proportionally to the amount of SIRT1, IL-17, NF-κB, IKBKB, PPARγ, COX-2, TNF-α, MPO, and PKC bound. Color development was discontinued (stop solution) and after 10-min color intensity was measured at 450 nm (Salama et al. 2021).
Quantitative real-time PCR gene expression of Nrf2 and FoxO1 in rats` lung tissues
Rats` lung tissue homogenates were used for total RNA extraction by RNeasy Purification Reagent (Promega, Madison, WI, USA) according to the manufacturers’ protocol. The extracted RNA was then quantified by spectrophotometry (JENWAY, USA) at 260 nm. The obtained RNA is then used to assess the expression levels of Nrf2 and FoxO1 genes with quantitative RT-PCR based on the SYBR Green (TransScript™ II Green One-Step qRT-PCR SuperMix; TransGen. Cycling conditions were adjusted as described by the manufacturer. PCR primers were designed with Gene Runner Software (Hastings Software Inc., Hastings, NY, USA) with RNA sequences obtained from GenBank, using Beta Actin as a housekeeping gene (Table1). The obtained data were analyzed using the 2−ΔΔ CT method (Livak and Schmittgen 2001).
Histological examination
Various groups' lung tissue was fixed in 10% formalin after being dissected. After being fixed for a day or two, the tissue was dehydrated progressively by stronger alcohol (70%, 90%, and three changes in absolute alcohol), cleared with xylene, impregnated in three changes of soft paraffin wax at 50 °C, and lastly embedded in paraffin wax to form solid blocks. A 7-μm thick transverse section was cut in a serial cross-section. Hematoxylin and eosin were used to stain paraffin slices mounted on glass slides with a layer of albumin glycerin. Light microscopy was used to qualitatively analyze the stained tissue slices (Carleton et al. 1980).
Histomorphometric analysis
Based on the previous studies, quantify the extent of inflammatory infiltration and tissue damage (Patel et al. 2012). Whole-lung sections were scored by observers who were blinded for groups. The degree of inflammatory cell infiltration and bronchiolar epithelium hyperplasia were assessed in alveolar septa and lumens. The scores were derived using light microscopy and scored based on the intensity of alterations: 0, absent; 1, mild; 2, moderate; 3 severe. Scores of rats from the same group were pooled and calculated as mean ± standard deviation of the mean (SD).
Statistical analysis
All the values are presented as means ± SD. The data of this study were evaluated by one-way analysis of variance followed by Tukey’s multiple comparisons test. Graph pad Prism software, version 5 (Inc., San Diego, USA) was used to carry out these statistical tests. The difference was considered significant when P < 0.05.
Results
Effect of protocatechuic acid on PPARγ, COX-2, NF-κB, and IKBKB protein levels
CP injection significantly decreased the lung protein levels of PPARγ by 77% and increased COX-2 by 305% respectively, as compared to control group (P value < 0.05). Meanwhile, in protocatechuic acid 50 and 100 mg/kg significantly increased PPARγ by 79 and 250% as well as reduced COX-2 levels by 24 and 39% respectively, as compared to the CP-treated group (P value < 0.05). Moreover, CP treatment significantly increased NF-κB and IKBKB lung protein levels by 105 and 70.3% respectively, in comparison with the control group. However, administration of protocatechuic acid 50 and 100 mg/kg significantly decreased NF-κB by 27 and 38%, along with IKBKB by 22 and 30% respectively, when compared with that of the CP group (P value < 0.05) (Fig. 1).
Effect of protocatechuic acid on PPARγ, COX-2, NF-Κβ, and IKBKB proteins levels. The effect of treatment with protocatechuic acid (50, 100 mg/kg; p.o.) once daily for 10 consecutive days after cyclophosphamide (200 mg/kg; i.p.) single dose on a PPARγ b COX-2 c NF-Κβ d IKBKB proteins levels. The data were expressed as mean ± SD, (n = 8). Statistical analysis was carried out by one-way ANOVA followed by Tukey HSD test for multiple comparisons. aSignificantly different from normal control. bSignificantly different from cyclophosphamide control. cSignificantly different from cyclophosphamide + protocatechuic acid 50 mg/kg at P < 0.05
Effect of protocatechuic acid on IL-17 protein levels
CP treatment significantly increased IL-17 lung protein levels by 167% in comparison with the control group (P value < 0.05). However, administration of protocatechuic acid 50 and 100 mg/kg significantly decreased IL-17 by 58 and 66%, respectively, when compared with that of the CP group (P value < 0.05). The treatment with 100 mg/kg of protocatechuic acid reduced the contents of IL-17 to their normal value, compared to the CP group (Fig. 2a).
Effect of protocatechuic acid on IL-17, TNF-α, and PKC proteins levels. The effect of treatment with protocatechuic acid (50, 100 mg/kg; p.o.) once daily for 10 consecutive days after cyclophosphamide (200 mg/kg; i.p.) single dose on a IL-17 b TNF-α c PKC proteins levels. The data were expressed as mean ± SD, (n = 8). Statistical analysis was carried out by one-way ANOVA followed by Tukey HSD test for multiple comparisons. aSignificantly different from normal control. bSignificantly different from cyclophosphamide control. cSignificantly different from cyclophosphamide + protocatechuic acid 50 mg/kg at P < 0.05
Effect of protocatechuic acid on TNF-α and PKC protein levels
Lung protein levels of TNF-α and PKC were significantly raised by 312 and 410% respectively, compared to the control rats (P value < 0.05). While, in protocatechuic acid 50 and 100 mg/kg caused a significant decline in the TNF-α level by 42 and 75% besides the diminution of PKC levels by 19 and 75%, respectively, as compared to CP-treated group (P value < 0.05). Treatment of protocatechuic acid 100 mg/kg reduced pulmonary levels of TNF-α to their normal value, compared to the CP group (Fig. 2b, c).
Effect of protocatechuic acid on SIRT1 protein level
CP injection significantly decreased the lung protein level of SIRT1 by 84% compared to the control group (P value < 0.05). Meanwhile, in protocatechuic acid 50 and 100 mg/kg SIRT1 protein levels were significantly increased the by 87 and 205% respectively, as compared to the CP-treated group (P value < 0.05) (Fig. 3).
Effect of protocatechuic acid on SIRT1 proteins level. The effect of treatment with protocatechuic acid (50, 100 mg/kg; p.o.) once daily for 10 consecutive days after cyclophosphamide (200 mg/kg; i.p.) single dose on SIRT1 proteins level. The data were expressed as mean ± SD, (n = 8). Statistical analysis was carried out by one-way ANOVA followed by Tukey’s HSD test for multiple comparisons. aSignificantly different from normal control. bSignificantly different from cyclophosphamide control. cSignificantly different from cyclophosphamide + protocatechuic acid 50 mg/kg at P < 0.05
Effect of protocatechuic acid on GSH, catalase, MDA, NO, and MPO pulmonary protein levels
The protein levels of GSH and catalase were significantly lower in the CP group by 24 and 88% respectively, as compared to control group (P value < 0.05). The levels of MDA, NO and MPO were significantly higher in CP group by 98, 100 and 156%, respectively, than the control group (P < 0.05). Although protein levels of GSH and catalase were significantly higher in protocatechuic acid 50 and 100 mg/kg by 8, 12.3 and 371, 457% respectively, when compared with CP-treated group (P value < 0.05). Moreover, protocatechuic acid 50 and 100 mg/ kg significantly decreased the levels of MDA, NO and MPO by 20, 54 and 30%, 38, 29, 48%, respectively, when compared with CP-treated group (P value < 0.05) (Fig. 4).
Effect of protocatechuic acid on GSH, MDA, NO, MPO, and catalase proteins levels. The effect of treatment with protocatechuic acid (50, 100 mg/kg; p.o.) once daily for 10 consecutive days after cyclophosphamide (200 mg/kg; i.p.) single dose on a GSH b MDA c NO d MPO AND e catalase proteins levels. The data were expressed as mean ± SD, (n = 8). Statistical analysis was carried out by one-way ANOVA followed by Tukey HSD test for multiple comparisons. aSignificantly different from normal control. bSignificantly different from cyclophosphamide control. cSignificantly different from cyclophosphamide + protocatechuic acid 50 mg/kg at P < 0.05
Effect of protocatechuic acid on FoxO-1 and Nrf-2 gene expression
Administration of CP showed a significant decrease in the Nrf-2 gene (P value < 0.05) with a marked increase in foxo-1, when compared with the control group (P value < 0.05). Although protocatechuic acid 50 and 100 mg/kg administration resulted in a significant increase in Nrf-2 compared with cyclophosphamide group (P value < 0.05), moreover, the Foxo-1 gene was downregulated appreciably in a dose-dependent manner as compared to the CP group (P value < 0.05) (Fig. 5).
Effect of protocatechuic acid on FoxO-1 and Nrf-2 gene expression. The effect of treatment with protocatechuic acid (50, 100 mg/kg; p.o.) once daily for 10 consecutive days after cyclophosphamide (200 mg/kg; i.p.) single dose on a FoxO-1 b Nrf-2 gene expression. The data were expressed as mean ± SD, (n = 8). Statistical analysis was carried out by one-way ANOVA followed by Tukey HSD test for multiple comparisons. aSignificantly different from normal control. bSignificantly different from cyclophosphamide control. cSignificantly different from cyclophosphamide + protocatechuic acid 50 mg/kg at P < 0.05
Histopathological study
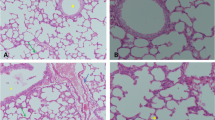
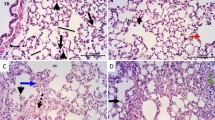
Histological pictures of lung sections from normal control rats showed no histopathological alteration and the normal histological structure of the bronchioles and the surrounding air alveoli were recorded in (Fig. 6a). The CP group showed collapse and emphysema detected in the air alveoli associated with bronchiolar epithelium hyperplasia and inflammatory cells infiltration in between all (Fig. 6b). Protocatechuic acid 50 group show congestion in the blood vessels associated with emphysema of the air alveoli. The bronchioles showed moderate epithelial lining hyperplasia with inflammatory cells infiltration in the bronchiolar lumen (Fig. 6c). In the protocatechuic acid 100 group the bronchioles showed less inflammatory cells infiltration in the bronchiolar lumen (Fig. 6d).
Effect of treatment with protocatechuic acid on lung histopathology. a Lung sections from normal control rats showed no histopathological alteration and the normal histological structure of the bronchioles and the surrounding air alveoli were recorded. b Cyclophosphamide group showed collapse and emphysema were detected in the air alveoli associated with bronchiolar epithelium hyperplasia and inflammatory cells infiltration in between all. c Protocatechuic acid 50 group showing congestion in the blood vessels associated with emphysema of the air alveoli. The bronchioles showed moderate epithelial lining hyperplasia with inflammatory cells infiltration in the bronchiolar lumen. d Protocatechuic acid 100 group showed less inflammatory cells infiltration in the bronchiolar lumen (H&E X 200)
Histomorphometric results
As shown in Fig. 7, results of negative control showed normal brain architecture. Conversely, CP group exhibited severe histopathological alterations, such as degree of inflammatory cell infiltration and bronchiolar epithelium hyperplasia. Interestingly, protocatechuic acid 50 and 100 groups significantly showed moderate and mild inflammatory cell infiltration and bronchiolar epithelium hyperplasia, respectively, as compared to the CP group.
Effect of treatment with protocatechuic acid on histomorphometric analysis. The data were presented as the mean ± SD of (n = 4) for each group. Statistical analysis was carried out by one-way analysis of variance followed by Tukey’s multiple comparisons test. aStatistically significant from the control group. bStatistically significant from the cyclophosphamide group at P < 0.05
Discussion
Peroxisome proliferator-activated receptors (PPARs) are nuclear hormone and regulate the genes expression implicated in several biological processes (Corton et al. 2000; Vamecq and Latruffe 1999). PPAR mediated transcription factors NF-κβ inhibition and enhanced IκBα expression (Gervois et al. 2000) leading to reduced inflammatory cell influx and injury to the alveolar capillary and attenuated chemokine, cytokine, adhesion molecule expression and eicosanoid production(Ishii et al. 2000). Moreover, it has suppressive effect on many proinflammatory gene expression as IL-6 and COX-2 (Delerive et al. 1999).
In the current study, CP decreased PPARγ with an elevation in NF-κB/COX-2 lung contents when compared with normal group, while PCA treatment can be attributed to stimulated PPARγ and reduced NF-κB/COX-2 lung contents as compared to CP-treated group. These present experimental findings demonstrate the promising anti-inflammatory and lung protective activity of PCA. Previously, PCA had anti-inflammatory and analgesic activity against carrageenan via inhibition of arthritis index and the synthesis or release of prostaglandins (Lende et al. 2011). Moreover, protective effects of PCA have been reported on doxorubicin-induced kidney damage as it is considered as iNOS and COX2 inhibitor (Molehin et al. 2019).
In addition, T-helper (Th)-17 cells induced the production of pro-inflammatory IL-17 (Ghoreschi et al. 2010), and are implicated in the pathogenesis of lung disease (Yanagisawa et al. 2017), and their activation in the presence of a combination of TGF-β, IL-6, and IL-1β mediated these pro-inflammatory differentiation (Ghoreschi et al. 2010). IL-6 with TGF-β induces Th17 development by activating iNOS and stimulating the expression of macrophage, IL-1 β, IL-6, TNF-α, and several chemokines, which potentiates the inflammatory response (Gonçalves-de-Albuquerque et al. 2017). The present study showed that PCA decreased IL-17 with the inhibition of NF-κB/IKBKB and TNF-α/PKC lung contents when compared with lung injury group. PCA, in another study, attenuated cerebral aneurysm progression by blocking the activation of TNF-α/NF-κB/ IL-17 signaling pathways (Molehin et al. 2019). Moreover, PCA suppressed inflammation and apoptosis in lung injury induced by LPS via modulating TNF-α, NF-κB, and IL-1β in lung tissue (Alsharif et al. 2021).
Another vital molecule in pulmonary damage is SIRT1, which has antioxidative and anti-inflammatory effects (Bai et al. 2015). SIRT enhances mitochondrial function, inhibiting the release of ROS (Yang et al. 2021). A high level of SIRT1 was associated with GSH and CAT overexpression (Ding et al. 2016). Moreover, SIRT1 protected H2O2-induced senescent endothelium (Ren et al. 2019). The present results revealed that CP suppressed SIRT1 lung content with a decrease in GSH and CAT as well as an elevation in NO, MDA (lipid peroxidation), and MPO (indicator of neutrophil accumulation), while treatment with PCA activated SIRT1 enhancing cellular responses to oxidative stress via upregulation of GSH, CAT as well as downregulation of NO, MDA, MPO, reducing acute pulmonary damage in this rat model. Previously, PCA regulated the SIRT1/NF-κB pathway in brain microglial activation induced by lipopolysaccharide (LPS) (Kaewmool et al. 2020). Moreover, FoxO1 is one of the family members of forkhead box protein (FoxO), it is a transcription factor that regulates several biological processes such as oxidative stress, cell cycle arrest, and apoptosis (Kim et al. 2017).
FoxO1 is one of the substrates of SIRT1 which deacetylates FoxO1 (Lee et al. 2018), leading to its inactivation (Sedding and Haendeler 2007) mediating its pro-survival action, while the acetylation of FoxO1 enhances the apoptotic action (Papanicolaou et al. 2008), and oxidative injury (Elrashidy and Hasan 2021). The recent studies exploring the oxidative stress due to CP administration showed it decreased the expression of SIRT1(Salama et al. 2020), which in turn decreases both phosphorylated and deacetylated FoxO1 and in turn enhanced FoxO1 gene expression (Dong et al. 2021; Elrashidy and Hasan 2021). In agreement with the previous research, our results revealed that administration of CP markedly increased FoxO-1 expression, whereas, PCA decreased it. Previously, PCA can phosphorylate several substrates, including FoxO1 (Tia et al. 2018), mitigating oxidative stress, and inhibiting apoptosis (Kaewmool et al. 2020; Salama et al. 2020). Thus, simultaneously targeting FoxO1 and SIRT1 will greatly impact the alleviation of pulmonary damage associated with organ toxicity and promotion of cell survival.
Pulmonary damage induced by CP also associated with oxidative stress and a reduction of nuclear factor E2-related factor 2 (Nrf2) transcription factor that regulate the cellular antioxidative responses by promoting the expression of series of antioxidant genes (Tonelli et al. 2018). Our results exhibited a significant decrease in Nrf2 gene expression in the CP- treated group, in accordance with previous studies showing decreased levels of Nrf2 in response to CP administration (Aladaileh et al. 2019; ALHaithloul et al. 2019). On the other hand, our results demonstrated a significant increase in Nrf2 gene expression in PCA-treated groups compared to the CP group in a dose dependent manner, this was supported by results of previous research (Ibitoye and Ajiboye 2020). Revealing that PCA enhanced Nrf2 expression, also, Je and Lee (2015) reported that a plant extract containing PCA activated Nrf2. Together, these findings imply that PCA can reduce the oxidative stress and pulmonary damage brought on by CP, as indicated by the increased expression of Nrf2.
Conclusion
The current study exhibited that PCA has a vital role in cellular inflammatory, oxidative, and immune responses through modulating PPARs/IL-17/Nrf2 to mitigate CP-induced acute lung injury in the rat. In addition, PCA regulates the SIRT1/FoxO1 pathway that helps in the treatment of lung inflammatory diseases. Further investigations are required to prove PCA's ability to prevent pulmonary damage associated with CP in the clinical setting and using it in a treatment regime will be highly worthwhile.
Data availability statement
The data presented in this study are available within the text and figures of the paper. Original data are available from the corresponding authors.
References
Aladaileh SH, Abukhalil MH, Saghir SA et al (2019) Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 9(8):346
AlHaithloul HA, Alotaibi MF, Bin-Jumah M, Elgebaly H, Mahmoud AM (2019) Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed Pharmacother 111:676–685
Alsemeh AE, Abdullah DM (2022) Protective effect of alogliptin against cyclophosphamide-induced lung toxicity in rats: Impact on PI3K/Akt/FoxO1 pathway and downstream inflammatory cascades. Cell Tissue Res 388(2):417–438
Alsharif KF, Almalki AA, Alsanie WF et al (2021) Protocatechuic acid attenuates lipopolysaccharide-induced septic lung injury in mice: The possible role through suppressing oxidative stress, inflammation and apoptosis. J Food Biochem 45(10):e13915
Arcidiacono B, Chiefari E, Messineo S et al (2018) HMGA1 is a novel transcriptional regulator of the FoxO1 gene. Endocrine 60(1):56–64
Bai X, Fan L, He T et al (2015) SIRT1 protects rat lung tissue against severe burn-induced remote ALI by attenuating the apoptosis of PMVECs via p38 MAPK signaling. Sci Rep 5(1):1–13
Basha M, Salama A, Noshi SH (2020) Soluplus® based solid dispersion as fast disintegrating tablets: a combined experimental approach for enhancing the dissolution and antiulcer efficacy of famotidine. Drug Dev Ind Pharm 46(2):253–263
Blondonnet R, Constantin J-M, Sapin V, Jabaudon M (2016) A pathophysiologic approach to biomarkers in acute respiratory distress syndrome. Dis Markers 2016:3501373
Carleton HM, Drury RAB, Wallington EA (1980) Carleton’s histological technique. Oxford University Press, USA
Castillo R, Loza RC, Romero-Dapueto C (2015) Suppl 2: M2: pathophysiological approaches of acute respiratory distress syndrome: novel bases for study of lung injury. Open Respir Med J 9:83
Corton JC, Anderson SP, Stauber A (2000) Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annu Rev Pharmacol Toxicol 40:491
Delerive P, De Bosscher K, Besnard S et al (1999) Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J Biol Chem 274(45):32048–32054
Ding Y-W, Zhao G-J, Li X-L et al (2016) SIRT1 exerts protective effects against paraquat-induced injury in mouse type II alveolar epithelial cells by deacetylating NRF2 in vitro. Int J Mol Med 37(4):1049–1058
Dong P, Xia L, Hu L, Yang K, Wang H, Ye P (2021) Runjing decoction alleviated cyclophosphamide-induced oligoasthenospermia rats by inhibiting cell apoptosis via RXFP1/AKT/FOXO1 pathway. Andrologia 53(11):e14216
Elrashidy RA, Hasan RA (2021) Cilostazol preconditioning alleviates cyclophosphamide-induced cardiotoxicity in male rats: mechanistic insights into SIRT1 signaling pathway. Life Sci 266:118822
Emadi A, Jones RJ, Brodsky RA (2009) Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol 6(11):638–647
Gervois P, Fruchart J-C, Delerive P, Staels B (2000) Induction of IκBα expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-α activators. J Biol Chem 275(47):36703–36707
Ghoreschi K, Laurence A, Yang X-P et al (2010) Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467(7318):967–971
Gonçalves-de-Albuquerque SdC, Pessoa-e-Silva R, Trajano-Silva LA et al (2017) The equivocal role of Th17 cells and neutrophils on immunopathogenesis of leishmaniasis. Front Immunol 8:1437
Hamsa T, Kuttan G (2010) Ipomoea obscura ameliorates cyclophosphamide-induced toxicity by modulating the immune system and levels of proinflammatory cytokine and GSH. Can J Physiol Pharmacol 88(11):1042–1053
Haubitz M (2007) Acute and long-ter toxicity of cyclophosphamide. Transplantationsmedizin 19(2):26
Hsu C-C, Hsu C-L, Tsai S-E, Fu TY-C, Yen G-C (2009) Protective effect of Millettia reticulata Benth against CCl4-induced hepatic damage and inflammatory action in rats. J Med Food 12(4):821–828
Ibitoye O, Ajiboye T (2020) Protocatechuic acid protects against menadione-induced liver damage by up-regulating nuclear erythroid-related factor 2. Drug Chem Toxicol 43(6):567–573
Ishii H, Ishibashi M, Takayama M, Nishida T, Yoshida M (2000) The role of cytokine-induced neutrophil chemoattractant-1 in neutrophil-mediated remote lung injury after intestinal ischaemia/reperfusion in rats. Respirology 5(4):325–331
Je J-Y, Lee D-B (2015) Nelumbo nucifera leaves protect hydrogen peroxide-induced hepatic damage via antioxidant enzymes and HO-1/Nrf2 activation. Food Funct 6(6):1911–1918
Kaewmool C, Kongtawelert P, Phitak T, Pothacharoen P, Udomruk S (2020) Protocatechuic acid inhibits inflammatory responses in LPS-activated BV2 microglia via regulating SIRT1/NF-κB pathway contributed to the suppression of microglial activation-induced PC12 cell apoptosis. J Neuroimmunol 341:577164
Kaplan JM, Cook JA, Hake PW, O’Connor M, Burroughs TJ, Zingarelli B (2005) 15-Deoxy-Δ12, 14-prostaglandin J2 (15d-PGJ2), a peroxisome proliferator activated receptor γ ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock 24(1):59–65
Kim M-k, Shin HM, Jung H et al (2017) Comparison of pancreatic beta cells and alpha cells under hyperglycemia: Inverse coupling in pAkt-FoxO1. Diabetes Res Clin Pract 131:1–11
Krzysztoforska K, Piechal A, Blecharz-Klin K et al (2019) Administration of protocatechuic acid affects memory and restores hippocampal and cortical serotonin turnover in rat model of oral D-galactose-induced memory impairment. Behav Brain Res 368:111896. https://doi.org/10.1016/j.bbr.2019.04.010
Kurundkar D, Kurundkar AR, Bone NB et al (2019) SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight. https://doi.org/10.1172/jci.insight.120722
Lee Y, Jeong GS, Kim KM, Lee W, Bae JS (2018) Cudratricusxanthone A attenuates sepsis-induced liver injury via SIRT1 signaling. J Cell Physiol 233(7):5441–5446
Lende AB, Kshirsagar AD, Deshpande AD et al (2011) Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacology 19(5):255–263
Li T, Zhang J, Feng J et al (2013) Resveratrol reduces acute lung injury in a LPS-induced sepsis mouse model via activation of Sirt1. Mol Med Rep 7(6):1889–1895
Liu C-L, Wang J-M, Chu C-Y, Cheng M-T, Tseng T-H (2002) In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem Toxicol 40(5):635–641
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Mahlooji MA, Heshmati A, Kheiripour N et al (2022) Evaluation of protective effects of curcumin and nanocurcumin on aluminium phosphide-induced subacute lung injury in rats: modulation of oxidative stress through SIRT1/FOXO3 signalling pathway. Drug Res 72(02):100–108
Molehin OR, Adeyanju AA, Adefegha SA, Oyeyemi AO, Idowu KA (2019) Protective mechanisms of protocatechuic acid against doxorubicin-induced nephrotoxicity in rat model. J Basic Clin Physiol Pharmacol. https://doi.org/10.1515/jbcpp-2018-0191
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Papanicolaou KN, Izumiya Y, Walsh K (2008) Forkhead transcription factors and cardiovascular biology. Circ Res 102(1):16–31
Patel BV, Wilson MR, Takata M (2012) Resolution of acute lung injury and inflammation: a translational mouse model. Eur Respir J 39(5):1162–1170. https://doi.org/10.1183/09031936.00093911
Ren Z, He H, Zuo Z, Xu Z, Wei Z, Deng J (2019) The role of different SIRT1-mediated signaling pathways in toxic injury. Cell Mol Biol Lett 24(1):1–10
Righetti RF, Santos TMd, Camargo LdN et al (2018) Protective effects of anti-IL17 on acute lung injury induced by LPS in mice. Front Pharmacol 9:1021
Saghir SA, Alharbi SA, Al-Garadi MA et al (2020) Curcumin prevents cyclophosphamide-induced lung injury in rats by suppressing oxidative stress and apoptosis. Processes 8(2):127
Salama A, Elgohary R (2021) L-carnitine and Co Q10 ameliorate potassium dichromate -induced acute brain injury in rats targeting AMPK/AKT/NF-κβ. Int Immunopharmacol 101:107867. https://doi.org/10.1016/j.intimp.2021.107867
Salama RM, Mohamed AM, Hamed NS, Ata RM, NourelDeen AS, Hassan MA (2020) Alogliptin: a novel approach against cyclophosphamide-induced hepatic injury via modulating SIRT1/FoxO1 pathway. Toxicol Res 9(4):561–568
Salama A, Fayed HM, Elgohary R (2021) L-carnitine alleviated acute lung injuries induced by potassium dichromate in rats: involvement of Nrf2/HO-1 signaling pathway. Heliyon 7(6):e07207
Sedding D, Haendeler J (2007) Do we age on Sirt1 expression? Am Heart Assoc 100:1396–1398
Snyder LS, Heigh RI, Anderson ML (1993) Cyclophosphamide-induced hepatotoxicity in a patient with Wegener’s granulomatosis. Mayo Clin Proc 68:1203–1204
Souid A-K, Tacka KA, Galvan KA, Penefsky HS (2003) Immediate effects of anticancer drugs on mitochondrial oxygen consumption. Biochem Pharmacol 66(6):977–987
Suddek GM, Ashry NA, Gameil NM (2013) Thymoquinone attenuates cyclophosphamide-induced pulmonary injury in rats. Inflammopharmacology 21(6):427–435
Swamy AV, Patel U, Koti B, Gadad P, Patel N, Thippeswamy A (2013) Cardioprotective effect of Saraca indica against cyclophosphamide induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Indian J Pharmacol 45(1):44
Tarpey MM, Wink DA, Grisham MB (2004) Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. A J Physiol-Regulatory Integr Compa Physiol 286(3):R431–R444
Tia N, Singh AK, Pandey P, Azad CS, Chaudhary P, Gambhir IS (2018) Role of Forkhead Box O (FOXO) transcription factor in aging and diseases. Gene 648:97–105
Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional regulation by Nrf2. Antioxid Redox Signal 29(17):1727–1745
Vamecq J, Latruffe N (1999) Medical significance of peroxisome proliferator-activated receptors. Lancet 354(9173):141–148
Vitaglione P, Donnarumma G, Napolitano A et al (2007) Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr 137(9):2043–2048
Yanagisawa H, Hashimoto M, Minagawa S et al (2017) Role of IL-17A in murine models of COPD airway disease. Am J Physiol-Lung Cell Mol Physiol 312(1):L122–L130
Yang C, Yang W, He Z et al (2021) Kaempferol alleviates oxidative stress and apoptosis through mitochondria-dependent pathway during lung ischemia-reperfusion injury. Front Pharmacol 12:624402
Zarei M, Shivanandappa T (2013) Amelioration of cyclophosphamide-induced hepatotoxicity by the root extract of Decalepis hamiltonii in mice. Food Chem Toxicol 57:179–184
Acknowledgements
We would like to thank Dr. Adel Bakeer, Pathology Department, Faculty of Veterinary Medicine, Cairo University for advice on the pathological study
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received to conducting this study.
Author information
Authors and Affiliations
Contributions
AS: conceptualization, methodology, validation, formal analysis, investigation, resources, writing, review, and editing. RE: conceptualization, methodology, resources, writing—original draft. MMA: methodology, resources. SAE: methodology, validation, and writing real-time PCR technique.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salama, A., Elgohary, R., Amin, M.M. et al. Impact of protocatechuic acid on alleviation of pulmonary damage induced by cyclophosphamide targeting peroxisome proliferator activator receptor, silent information regulator type-1, and fork head box protein in rats. Inflammopharmacol 31, 1361–1372 (2023). https://doi.org/10.1007/s10787-023-01156-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01156-6