Abstract
In coronavirus disease 2019 (Covid-19) era, neuroinflammation may develop due to neuronal tropism of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) and/or associated immune activation, cytokine storm, and psychological stress. SARS-CoV-2 infection and linked cytokine storm may cause blood–brain barrier (BBB) injury through which activated immune cells and SARS-CoV-2 can pass into the brain causing activation of glial cells with subsequent neuroinflammation. Different therapeutic regimens were suggested to alleviate Covid-19-induced neuroinflammation. Since glibenclamide has anti-inflammatory and neuroprotective effects, it could be effective in mitigation of SARS-CoV-2 infection-induced neuroinflammation. Glibenclamide is a second-generation drug from the sulfonylurea family, which acts by inhibiting the adenosine triphosphate (ATP)-sensitive K channel in the regulatory subunit of type 1 sulfonylurea receptor (SUR-1) in pancreatic β cells. Glibenclamide reduces neuroinflammation and associated BBB injury by inhibiting the nod-like receptor pyrin 3 (NLRP3) inflammasome, oxidative stress, and microglial activation. Therefore, glibenclamide through inhibition of NLRP3 inflammasome, microglial activation, and oxidative stress may attenuate SARS-CoV-2-mediated neuroinflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease 2019 (Covid-19) represents a current pandemic disease caused by a novel severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) leading to formidable global effects (Al-kuraishy et al. 2022c; Al-Thomali et al. 2022). Covid-19 is regarded as a primary respiratory disease leading to a mild respiratory infection. However, in severe cases, it causes acute lung injury (ALI) and the development of acute respiratory distress syndrome (ARDS) (Al-kuraishy et al. 2022a; Al-kuraishy et al. 2022e). Besides, Covid-19 may cause extra-pulmonary complications including acute kidney injury, stroke, hepatic injury, testicular injury and neuroinflammation due to the propagation of hyper-inflammation and cytokine storm (Al-kuraishy et al. 2022b).
Furthermore, in Covid-19, neuroinflammation may develop due to neuronal tropism of SARS-CoV-2 and/or associated immune activation, cytokine storm, and psychological stress (Kempuraj et al. 2020; Ojo et al. 2021; Alorabi et al. 2022). Exaggeration of peripheral immune response and hyper-inflammation in SARS-CoV-2 infection can exacerbate and causes neuroinflammation by activating mast cells (Kempuraj et al. 2020; Koneru et al. 2021). SARS-CoV-2 infection and linked cytokine storm may cause blood–brain barrier (BBB) injury through which activated immune cells and SARS-CoV-2 can pass into the brain causing activation of glial cells with subsequent neuroinflammation (Pacheco-Herrero et al. 2021). In the clinical setting, it has been reported that the latency of about one week gap between the onset of severe Covid-19 and onset of neuroinflammation due to SARS-CoV-2 and alteration in the function of BBB (Pacheco-Herrero et al. 2021). SARS-CoV-2-induced neuroinflammation is associated with increased biomarkers of neuronal and BBB injuries such as neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) in Covid-19 patients (Kanberg et al. 2020). A cross-sectional study included 47 Covid-19 patients with mild (n = 20), moderate (n = 9) and severe (n = 18) showed that patients with severe Covid-19 had higher levels of GFAP and NfL compared to the mild and moderate ones. The early peak of GFAP was reduced on follow-up while NfL remained high during the follow-up (Kanberg et al. 2020). This finding suggests that SARS-CoV-2-induced neuroinflammation is linked with early astrocyte activation and delayed axonal injury in Covid-19. SARS-CoV-2-induced neuroinflammation is more common in the elderly due to low-grade inflammatory changes which might explain the greater risk of Covid-19 in the elderly age group (Bossù et al. 2020).
Baig and other studies revealed that SARS-CoV-2 can be transported through general circulation and enters the brain via cerebral microcirculation where it binds angiotensin-converting enzyme 2 (ACE2) in the neurovascular unit leading to the induction of neuroinflammation (Baig et al. 2020; Abubakar et al. 2021; Babalghith et al. 2022). It has been reported that 36.4% of Covid-19 patients presented with neurological manifestations including dizziness, headache, impaired consciousness, and cerebrovascular events (Mao et al. 2020). Similarly, a prospective study showed that 13.5% of Covid-19 patients had neurological symptoms associated with poor clinical outcomes and high mortality (Frontera et al. 2021; Mathew et al. 2021).
Different therapeutic regimens were suggested to alleviate Covid-19-induced neuroinflammation. Ong and colleagues suggested that antimalarial drugs could be effective in the management of SARS-CoV-2 infection-induced neuroinflammation by inhibiting phospholipase A2 (PLA2) (Ong et al. 2021). Besides, selective serotonin reuptake inhibitor fluvoxamine which has an agonist effect on the sigma-1 receptor was confirmed in a randomized clinical trial to be effective in reducing Covid-19-induced neuroinflammation and clinical deterioration (Lenze et al. 2020; Al-kuraishy et al. 2021a). In addition, statins were proposed recently to be effective against Covid-19-induced neuroinflammation and acute brain injury by their anti-inflammatory effects (Hussien et al. 2021; Alsubaie et al. 2022). Of note, glibenclamide has anti-inflammatory and neuroprotective effects (Hussien et al. 2018) therefore we hypothesized that glibenclamide could be an effective agent in the mitigation of SARS-CoV-2 infection-induced neuroinflammation.
Glibenclamide and neuroinflammation
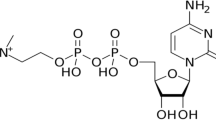
Glibenclamide is a second-generation drug from the sulfonylurea family (Fig. 1), which acts by inhibiting the adenosine triphosphate (ATP)-sensitive K channel in the regulatory subunit of type 1 sulfonylurea receptor (SUR-1) in pancreatic β cells. This effect induces membrane depolarization with increasing intracellular Ca+2 within pancreatic β cells and subsequent insulin release (Najdi et al. 2019). Glibenclamide is mainly used in the management of type 2 diabetes mellitus (T2DM); however, it is not the first-line therapy in T2DM (Najdi et al. 2019; Batiha et al. 2022).
Glibenclamide is regarded as an old drug; it was discovered in 1969 and permitted in medical use in 1984 (Katsilambros 2006). There are three isoforms of SUR, SUR-1 in the pancreatic β cells, SUR-2A, and SUR-1B in the heart and adipose tissue, respectively, though the brain expresses all types of SUR isoforms (Katsilambros 2006). It has been reported that neuroinflammation was associated with over-expression of SUR-1 and tumor necrosis factor-alpha (TNF-α) (Simard et al. 2012; Batiha et al. 2021). The use of glibenclamide can reduce neuroinflammation in experimental rats through the inhibition of SUR-1 (Tosun et al. 2013). Hussien et al (2018) proposed the neuroprotective effects of glibenclamide in reducing ischemic stroke and brain edema through induction of neurogenesis and possible anti-inflammatory effects. Similarly, glibenclamide was suggested to be a potent systemic anti-inflammatory agent against respiratory, cardiac, digestive, and neurological inflammation by its anti-inflammatory effects with reduced release of pro-inflammatory cytokines (Zhang et al. 2017). A recent experimental study observed that glibenclamide mitigated hippocampal inflammation and cognitive impairment in T2DM rats (Esmaeili et al. 2020). Zhang and colleagues revealed that glibenclamide had a protective effect against inflammation-mediated neuronal injury (Zhang et al. 2017). Similarly, glibenclamide promotes neurological recovery and neuroinflammation following intracerebral hemorrhage in rats (Jiang et al. 2021). Notably, oral treatment of glibenclamide can mitigate functional outcomes in patients with moderate to severe traumatic brain injuries (Khalili et al. 2017).
These verdicts suggest that glibenclamide is effective against neuroinflammation by its anti-inflammatory effects. Depending on these observations and suggestions, glibenclamide may be effective against viral infections including SARS-CoV-2.
Glibenclamide and Covid-19-induced neuroinflammation
In severe SARS-CoV-2 infection, different inflammatory signaling pathways are activated with the subsequent release of pro-inflammatory cytokines including TNF-α, interleukins (IL-1β, IL-6) and chemokines (Mostafa-Hedeab et al. 2022; Al-Kuraishy et al. 2022d).
Of note, in Covid-19-induced neuroinflammation, nod-like receptor pyrin 3 (NLRP3) inflammasome is activated by SARS-CoV-2 and/or activated microglial cells causing progressive neuroinflammation and brain injury (Cama et al. 2021; Ezeonuegbu et al. 2021). Thus, suppression of NLRP3 inflammasome could be an effective strategy against the development of SARS-CoV-2-induced neuroinflammation (Cama et al. 2021). Likewise, activation of the NLRP3 inflammasome by SARS-CoV-2 is linked with the development of BBB injury (Zhao et al. 2021). Severe disruption of BBB in Covid-19 leads to critical neuroinflammation and central nervous system (CNS) complications (Welcome and Mastorakis 2021). Targeting of NLRP3 inflammasome in SARS-CoV-2 by specific inhibitors can mitigate the neuroinflammation and associated BBB injury in Covid-19 patients (Freeman and Swartz 2020). As well, natural products such as Oridonin, Parthenolide and vinyl sulfone-related compounds have potential inhibitory effects on the activation of NLRP3 inflammasome (Shah 2020).
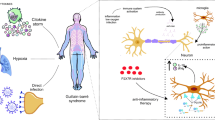
Of interest, glibenclamide reduces neuroinflammation and associated BBB injury by inhibiting NLRP3 inflammasome in mice with experimental intracerebral hemorrhage (Xu et al. 2019). Yang et al (2019) found that glibenclamide had a neuroprotective effect through inhibition of the NLRP3 inflammasome signaling pathway. In addition, glibenclamide can decrease microglial activation-induced neuroinflammation through the suppression release of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α (Esmaeili et al. 2020; Mahran et al. 2021).
Glial cells including microglia and astrocytes respond to the brain insults-induced neuroinflammation and could be a potential target of SARS-CoV-2 due to higher expression of ACE2 (McMahon et al. 2021). As well, glial cells are the major source of inflammatory cytokines in the CNS (Vargas et al. 2020). In general, glial cells are involved in viral clearance through the recruitment of immune cells and the activation release of antiviral cytokines (Amaral et al. 2021). However, over-activation of microglial cells by the persistence of viral infection or through activation of astrocytes induces the release of pro-inflammatory cytokines with the development of cytokine storm which causes synaptic loss and BBB injury (Mangale et al. 2020; Opara et al. 2021). Therefore, microglial cells might be responsible for direct neuronal injury or T cell/astrocytes-induced neurotoxicity and cytokine storm. Glibenclamide had a neuroprotective role with the reduction of cerebral edema and the release of inflammatory cytokines by inhibiting glial and microglial cells in rats (Kajimoto et al. 2022).
These findings suggest that glibenclamide may reduce Covid-19-induced neuroinflammation through its anti-inflammatory effects which are mediated by suppressing the activation of NLRP3 inflammasome and the release of pro-inflammatory cytokines.
Furthermore, the induction of oxidative stress during SARS-CoV-2 infection may cause neuroinflammation and other CNS complications through the induction of nuclear factor kappa B (NF-κB) (Karnik et al. 2021). Indeed, glibenclamide inhibits neuronal ischemic-reperfusion injury in rat hippocampus through suppression the development of oxidative stress (Abdallah et al. 2011; Yaqoob et al. 2021). Different experimental studies demonstrated that glibenclamide can attenuate acute brain injury by reducing the generation of reactive oxygen species (ROS) and inducing the expression of antioxidant enzymes (Abdallah et al. 2011; Erejuwa et al. 2010). However, the effect of glibenclamide on the redox potential cellular changes in relation to neuroinflammation is not well-defined. Thus, the glibenclamide effect on SARS-CoV-2 infection needs further studies.
Of interest, glibenclamide was not evaluated in Covid-19 patients as most critical diabetic patients switched to insulin therapy for strict glucose control (Rodrigues Ferreira et al. 2021; Al-kuraishy et al. 2021b). In addition, the use of glibenclamide in diabetic Covid-19 patients may increase the risk of hypoglycemia (Nakhleh and Shehadeh 2020). Of note, the medical use of glibenclamide is associated with the risk of hypoglycemia in diabetic and non-diabetic patients (Soydan et al. 2013). However, the use of a non-hypoglycemic dose of glibenclamide 0.4 mg/kg still has an anti-inflammatory effect (Berdugo et al. 2021). Therefore, the use of a lower effective dose of glibenclamide could be a promising therapeutic strategy in the management of SARS-CoV-2-induced neuroinflammation even in non-diabetic patients. Remarkably, glibenclamide is effective against ALI by inhibiting the release of pro-inflammatory cytokines and the generation of ROS (Nakhleh and Shehadeh 2020; Moubarak et al. 2021). In this state, glibenclamide can decrease the risk for the development of hypoxemia which is associated with the propagation of neuroinflammation and acute brain injury (Amruta et al. 2021).
Moreover, downregulation of ACE2 by SARS-CoV-2 may induce dysregulation of the renin-angiotensin system (RAS) with increasing vasoconstrictor angiotensin II (AngII) and reduction of vasodilator Ang1-7 (Al-Kuraishy et al. 2021c; Alkazmi et al. 2022). Dysregulated RAS in Covid-19 increases the risk for the development of cytokine storm and other critical complications including ALI (Al-Kuraishy et al. 2021d). As well, hyperglycemia in T2DM may trigger dysregulation of RAS causing propagation of diabetic complications (Nakhleh and Shehadeh 2020). Interestingly, insulin therapy induces the expression of a protective anti-inflammatory ACE2, which may reduce Covid-19 complications in T2DM patients (Nakhleh and Shehadeh 2020). Since glibenclamide stimulates insulin release from pancreatic β-cells (Hussien et al. 2018), a lower dose of glibenclamide may decrease Covid-19 complications through the insulin-mediated pathway.
Therefore, glibenclamide through inhibition of NLRP3 inflammasome, microglial activation, and oxidative stress may attenuate SARS-CoV-2-mediated neuroinflammation (Fig. 2).
These findings and our hypotheses provoke future preclinical and clinical studies to confirm the potential role of glibenclamide in Covid-19 and associated neuroinflammation. However, the present hypotheses had many limitations including a paucity of clinical studies regarding the use of glibenclamide in patients with neuroinflammation. Besides, glibenclamide is little to be used in T2DM patients with Covid-19 as most of them switched to insulin therapy mainly in the severe state. Therefore, experimental, preclinical and clinical studies are warranted in this regard to confirm the lower non-hypoglycemic dose of glibenclamide in Covid-19 even in non-diabetic patients.
Conclusions
Glibenclamide is an antidiabetic drug used in the management of T2DM by inhibiting ATP-sensitive K channel SUR-1 in pancreatic β cells. Glibenclamide reduces neuroinflammation and associated BBB injury by inhibiting NLRP3 inflammasome, oxidative stress, and microglial activation. Therefore, glibenclamide may attenuate SARS-CoV-2-mediated neuroinflammation.
Data availability statement
All data are included in this manuscript.
References
Abdallah DM, Nassar NN, Abd-El-Salam RM (2011) Glibenclamide ameliorates ischemia–reperfusion injury via modulating oxidative stress and inflammatory mediators in the rat hippocampus. Brain Res 1385:257–262
Abubakar MB, Usman D, El-Saber Batiha G, Cruz-Martins N, Malami I, Ibrahim KG, Abubakar B, Bello MB, Muhammad A, Gan SH, Dabai AI, Alblihed M, Ghosh A, Badr RH, Thangadurai D, Imam MU (2021) Natural products modulating angiotensin converting enzyme 2 (ACE2) as potential COVID-19 therapies. Front Pharmacol 12:629935. https://doi.org/10.3389/fphar.2021.629935 (PMID:34012391;PMCID:PMC8126690)
Al-kuraishy H, Al-Gareeb AI, Abdullah SM, Cruz-Martins N, Batiha GES (2021a) Response: commentary: case report: hyperbilirubinemia in gilbert syndrome attenuates covid-19-induced metabolic disturbances. Front Cardiovasc Med. 8:1264
Al-kuraishy H, Al-Gareeb AI, Guerreiro SG, Cruz-Martins N, Batiha GE (2021b) COVID-19 in relation to hyperglycemia and diabetes mellitus. Front Cardiovasc Med 8:335
Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Habotta OA, Batiha GE (2022a) High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons. Inflammopharmacology 30:811–820. https://doi.org/10.1007/s10787-022-00988-y
Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Aljowaie RM, Almutairi SM, Alexiou A, Batiha GE (2022b) The Prospective Effect of Allopurinol on the Oxidative Stress Index and Endothelial Dysfunction in Covid-19. Inflammation 1–7
Al-Kuraishy HM, Al-Gareeb AI, Alqarni M, Cruz-Martins N, Batiha GE (2021c) Pleiotropic effects of tetracyclines in the management of COVID-19: emerging perspectives. Front Pharmacol 12:136
Al-Kuraishy HM, Al-Gareeb AI, El-Bouseary MM, Sonbol FI, Batiha GE (2022c) Hyperviscosity syndrome in COVID-19 and related vaccines: exploring of uncertainties. Clin Exp Med 24:1
Al-Kuraishy HM, Al-Gareeb AI, Faidah H, Al-Maiahy TJ, Cruz-Martins N, Batiha GE (2021d) The looming effects of estrogen in Covid-19: a rocky rollout. Front Nutr 8:649128
Al-Kuraishy HM, Al-Gareeb AI, Welson NN, Batiha GE (2022d) Trimetazidine and COVID-19-induced acute cardiac injury: a missed key. Int J Clin Pharm 44:832–833. https://doi.org/10.1007/s11096-022-01408-5
Al-kuraishy HM, Batiha GE-S, Faidah H, Al-Gareeb AI, Saad HM, Simal-Gandara J (2022e) Pirfenidone and post-Covid-19 pulmonary fibrosis: invoked again for realistic goals. Inflammopharmacology. https://doi.org/10.1007/s10787-022-01027-6
Alkazmi L, Al-kuraishy HM, Batiha GE-S, Mostafa-Hedeab G, De Waard M, Sabatier J-M, Kabrah SM, Saad HM, Al-Gareeb AI, Simal-Gandara J (2022) Roxadustat for SARS-CoV-2 infection: old signaling raised new hopes. Drugs R&d 22:183–186
Alorabi M, Cavalu S, Al-Kuraishy HM, Al-Gareeb AI, Mostafa-Hedeab G, Negm WA, Youssef A, El-Kadem AH, Saad HM, Batiha GE-S (2022) Pentoxifylline and berberine mitigate diclofenac-induced acute nephrotoxicity in male rats via modulation of inflammation and oxidative stress. Biomed Pharmacother 152:113225
Alsubaie N, Al-kuraishy HM, Al-Gareeb AI, Alharbi B, De Waard M, Sabatier J-M, Saad HM, Batiha GE-S (2022) Statins use in alzheimer disease: bane or boon from frantic search and narrative review. Brain Sci 12:1290
Al-Thomali AW, Al-kuraishy HM, Al-Gareeb AI, Al-buhadiliy KA, De Waard M, Sabatier J-M, Khan Khalil AA, Saad HM, Batiha GE-S (2022) Role of neuropilin 1 in COVID-19 patients with acute ischemic stroke. Biomedicines 10:2032
Amaral RF, Geraldo LH, Einicker-Lamas M, e Spohr TC, Mendes F, Lima FR (2021) Microglial lysophosphatidic acid promotes glioblastoma proliferation and migration via LPA1 receptor. J Neurochem 156(4):499–512
Amruta N, Chastain WH, Paz M, Solch RJ, Murray-Brown IC, Befeler JB, Gressett TE, Longo MT, Engler-Chiurazzi EB, Bix G (2021) SARS-CoV-2 mediated neuroinflammation and the impact of COVID-19 in neurological disorders. Cytokine Growth Factor Rev 58:1–5
Babalghith AO, Al-kuraishy HM, Al-Gareeb AI, De Waard M, Sabatier J-M, Saad HM, Batiha GE-S (2022) The potential role of growth differentiation factor 15 in covid-19: a corollary subjective effect or not? Diagnostics 12:2051
Baig AM, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11(7):995–998
Batiha GES, Hussein DE, Algammal AM, George TT, Jeandet P, Al-Snafi AE, Tiwari A, Pagnossa JP, Lima CM, Thorat ND, Zahoor M, El-Esawi M, Dey A, Alghamdi S, Hetta HF, Cruz-Martins N (2021) Application of natural antimicrobials in food preservation: recent views. Food Control 126:108066
Batiha GE-S, Al-kuraishy HM, Al-Maiahy TJ, Al-Buhadily AK, Saad HM, Al-Gareeb AI, Simal-Gandara J (2022) Plasminogen activator inhibitor 1 and gestational diabetes: the causal relationship. Diabetol Metab Syndr 14:127
Berdugo M, Delaunay K, Lebon C, Naud MC, Radet L, Zennaro L, Picard E, Daruich A, Beltrand J, Kermorvant-Duchemin E, Polak M, Crisanti P, Behar-Cohen FF (2021) Long-term oral treatment with non-hypoglycemic dose of glibenclamide reduces diabetic retinopathy damage in the goto-kakizakirat model. Pharmaceutics 13(7):1095
Bossù P, Toppi E, Sterbini V, Spalletta G (2020) Implication of aging related chronic neuroinflammation on COVID-19 pandemic. J Pers Med 10(3):102
Cama VF, Marín-Prida J, Acosta-Rivero N, Acosta EF, Díaz LO, Casadesús AV et al (2021) The microglial NLRP3 inflammasome is involved in human SARS-CoV-2 cerebral pathogenicity: a report of three post-mortem cases. J Neuroimmunol 361:577728
Erejuwa OO, Sulaiman SA, Wahab MS, Sirajudeen KN, Salleh MS, Gurtu S (2010) Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin-induced diabetic rats. Int J Mol Sci 11(5):2056–2066
Esmaeili MH, Enayati M, Abkenar FK, Ebrahimian F, Salari AA (2020) Glibenclamide mitigates cognitive impairment and hippocampal neuroinflammation in rats with type 2 diabetes and sporadic Alzheimer-like disease. Behav Brain Res 379:112359
Ezeonuegbu BA, Machido DA, Whong CM, Japhet WS, Alexiou A, Elazab ST, Qusty N, Yaro CA, Batiha GE (2021) Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: Biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol Rep 30:e00614
Freeman TL, Swartz TH (2020) Targeting the NLRP3 inflammasome in severe COVID-19. Front Immunol 11:1518
Frontera JA, Sabadia S, Lalchan R, Fang T, Flusty B, Millar-Vernetti P et al (2021) A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology 96(4):e575–e586
Hussien NR, Al-Naimi MS, Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI (2018) Sulfonylurea and neuroprotection: the bright side of the moon. J Adv Pharm Technol Res 9(4):120
Hussien NR, Al-Niemi MS, Al-Kuraishy HM, Al-Gareeb AI (2021) Statins and Covid-19: The Neglected Front of bidirectional effects. J Pak Med Assoc 71(8):133
Jiang B, Zhang Y, Wang Y, Li Z, Chen Q, Tang J, Zhu G (2021) Glibenclamide attenuates neuroinflammation and promotes neurological recovery after intracerebral hemorrhage in aged rats. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2021.729652
Kajimoto R, Igarashi T, Moro N, Oshima H, Suma T, Otani N, Yoshino A (2022) Effects of glibenclamide against early brain injury in rat after subarachnoid hemorrhage. J Neurosurg Sci. https://doi.org/10.23736/S0390-5616.22.05271-7
Kanberg N, Ashton NJ, Andersson L-M, Yilmaz A, Lindh M, Nilsson S, Price RW, Blennow K, Zetterberg H, Gisslén M (2020) Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology 95(12):e1754–e1759
Karnik M, Beeraka NM, Uthaiah CA, Nataraj SM, Bettadapura AD, Aliev G et al (2021) A review on SARS-CoV-2-induced neuroinflammation, neurodevelopmental complications, and recent updates on the vaccine development. Mol Neurobiol 58(9):4535–4563
Katsilambros N (2006) Diabetes in clinical practice: questions and answers from case studies. Chichester, England, Wiley. http://www.123library.org/book_details/?id=3731.
Kempuraj D, Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Khan A et al (2020) COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist 26(5–6):402–414
Khalili H, Derakhshan N, Niakan A, Ghaffarpasand F, Salehi M, Eshraghian H, Shakibafard A, Zahabi B (2017) Effects of oral glibenclamide on brain contusion volume and functional outcome of patients with moderate and severe traumatic brain injuries: a randomized double-blind placebo-controlled clinical trial. World Neurosurg 101:130–136
Koneru G, Batiha GES, Algammal AM, Mabrok M, Magdy S, Sayed S et al (2021) BCG vaccine-induced trained immunity and COVID-19: protective or bystander? Infect Drug Resist 14:1169–1184
Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE et al (2020) Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA 324(22):2292–2300
Mahran A, Khairy M, Elkhateeb R, Hegazy AR, Abdelmeged A, Batiha GES, Hetta HF, Bahaa HA (2021) The value of serum progesterone level on day of human chorionic gonadotrophin administration/metaphase II oocyte ratio in predicting IVF/ICSI outcome in patients with normal ovarian reserve. J Ovarian Res 14:52. https://doi.org/10.1186/s13048-021-00800-5
Mangale V, Syage AR, Ekiz HA, Skinner DD, Cheng Y, Stone CL, Brown RM, O’Connell RM, Green KN, Lane TE (2020) Microglia influence host defense, disease, and repair following murine coronavirus infection of the central nervous system. Glia 68(11):2345–2360
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X (2020) Neurologicmanifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol 77(6):683–690
Mathew B, Oh JM, Baty RS, Batiha GE, Parambi DGT, Gambacorta N, Nicolotti O, Kim H (2021) Piperazine-substituted chalcones: a new class of MAO-B, AChE, and BACE-1 inhibitors for the treatment of neurological disorders. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-021-13320-y (Epub ahead of print. PMID: 33743158)
McMahon CL, Staples H, Gazi M, Carrion R, Hsieh J (2021) SARS-CoV-2 targets glial cells in human cortical organoids. Stem Cell Rep 16(5):1156–1164
Mostafa-Hedeab G, Al-Kuraishy HM, Al-Gareeb AI, Welson NN, Batiha GE, Conte-Junior CA (2022) Selinexor and COVID-19: the neglected warden. Front Pharmacol. https://doi.org/10.3389/fphar.2022.884228
Moubarak M, Kasozi KI, Hetta HF, Shaheen HM, Rauf A, Al-Kuraishy HM, Qusti S, Alshammari EM, Ayikobua ET, Ssempijja F (2021) The rise of SARS-CoV-2 variants and the role of convalescent plasma therapy for management of infections. Life 11:734
Najdi RA, Hagras MM, Kamel FO, Magadmi RM (2019) A randomized controlled clinical trial evaluating the effect of Trigonella foenum-graecum (fenugreek) versus glibenclamide in patients with diabetes. Afr Health Sci 19(1):1594–1601
Nakhleh A, Shehadeh N (2020) Interactions between antihyperglycemic drugs and the renin-angiotensin system: putative roles in COVID-19. A mini-review. Diabetes Metab Syndr Clin Res Rev 14(4):509–512
Ojo OA, Ojo AB, Okolie C, Nwakama MAC, Iyobhebhe M, Evbuomwan IO et al (2021) Deciphering the interactions of bioactive compounds in selected traditional medicinal plants against Alzheimer’s diseases via pharmacophore modeling, auto-QSAR, and molecular docking approaches. Molecules 26(7):1996
Ong WY, Go ML, Wang DY, Cheah IK, Halliwell B (2021) Effects of antimalarial drugs on neuroinflammation-potential use for treatment of COVID-19-related neurologic complications. Mol Neurobiol 58(1):106–117
Opara KN, Wilson EU, Yaro CA, Alkazmi L, Udoidung NI, Chikezie FM, Bassey BE (2021) Batiha GES (2021) prevalence, risk factors, and coinfection of urogenital schistosomiasis and soil-transmitted helminthiasis among primary school children in biase. Southern Nigeria, J Parasitol Res
Pacheco-Herrero M, Soto-Rojas LO, Harrington CR, Flores-Martinez YM, Villegas-Rojas MM, León-Aguilar AM et al (2021) Elucidating the neuropathologic mechanisms of SARS-CoV-2 infection. Front Neurol 12:444
Rodrigues Ferreira F, Lyra A, Santiago Moisés RC, De Noronha RM, Calliari LE (2021) The potential role of intermittent continuous glucose monitoring in a successful outpatient transition from insulin to glibenclamide in a patient with transient neonatal diabetes in the context of the COVID-19 pandemic. Pediatr Diabetes 22:143–144
Shah A (2020) Novel coronavirus-induced NLRP3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front Immunol 11:1021
Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V (2012) Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab 32(9):1699–1717
Soydan N, Bretzel RG, Fischer B, Wagenlehner F, Pilatz A, Linn T (2013) Reduced capacity of heart rate regulation in response to mild hypoglycemia induced by glibenclamide and physical exercise in type 2 diabetes. Metabolism 62(5):717–724
Tosun C, Kurland DB, Mehta R, Castellani RJ, Dejong JL, Kwon MS et al (2013) Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke 44(12):3522–3528
Vargas G, Geraldo LH, Salomão N, Paes MV, Lima FR, Gomes FC (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and glial cells: Insights and perspectives. Brain Behav Immun 7:100127
Welcome MO, Mastorakis NE (2021) Neuropathophysiology of coronavirus disease 2019: Neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection. Inflammopharmacology 29(4):939–963
Xu F, Shen G, Su Z, He Z, Yuan L (2019) Glibenclamide ameliorates the disrupted blood–brain barrier in experimental intracerebral hemorrhage by inhibiting the activation of NLRP3 inflammasome. Brain Behav 9(4):e01254
Yang X, Wang Z, Jia X (2019) Neuroprotection of glibenclamide against brain injury after cardiac arrest via modulation of NLRP3 inflammasome. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) Jul 23 (pp. 4209–4212). IEEE.
Yaqoob MU, Abd El-Hack ME, Hassan F, El-Saadony MT, Khafaga AF, Batiha GE et al (2021) The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult Sci 100(7):101143
Zhang G, Lin X, Zhang S, Xiu H, Pan C, Cui W (2017) A protective role of glibenclamide in inflammation-associated injury. Mediat Inflamm. 2017:1
Zhao N, Di B, Xu LL (2021) The NLRP3 inflammasome and COVID-19: activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev 61:2–15
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors equally participated in the development of the manuscript and provided their final approval of all content and submission for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Batiha, G.ES., Al-kuraishy, H.M., Al-Gareeb, A.I. et al. Targeting of neuroinflammation by glibenclamide in Covid-19: old weapon from arsenal. Inflammopharmacol 31, 1–7 (2023). https://doi.org/10.1007/s10787-022-01087-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01087-8