Abstract
The pandemic spread of coronavirus (COVID-19) has been reported first at the end of 2019. It continues disturbing various human aspects with multiple pandemic waves showing more fatal novel variants. Now Egypt faces the sixth wave of the pandemic with controlled governmental measures. COVID-19 is an infectious respiratory disease-causing mild to moderate illness that can be progressed into life-threatening complications based on patients- and variant type-related factors. The symptoms vary from dry cough, fever to difficulty in breathing that required urgent hospitalization. Most countries have authorized their national protocols for managing manifested symptoms and thus lowering the rate of patients’ hospitalization and boosting the healthcare systems. These protocols are still in use even with the development and approval of several vaccines. These protocols were instructed to aid home isolation, bed rest, dietary supplements, and additionally the administration of antipyretic, steroids, and antiviral drugs. The current review aimed to highlight the administered protocols in the Middle East, namely in Egypt and the Kingdom of Saudi Arabia demonstrating how these protocols have shown potential effectiveness in treating patients and saving many soles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following the Spanish flu (H1N1) in 1918, Asian flu (H2N2) in 1957, Hong Kong flu (H3N2) in 1968, and Pandemic flu (H1N1) in 2009, the World Health Organization (WHO) considered officially on the 11th of March 2020 that the symptoms associated with the current coronavirus as the fifth viral pandemic disease. The virus was first isolated from a novel human pneumonia case in Wuhan, China as a novel zoonotic disease in Dec. 2019, and hence, named coronavirus disease or COVID-19 (Dong et al. 2020). Its outbreaks have resulted in generalized chaos of human life since this date on Earth and a huge number of deaths all over the world. The WHO estimates the global case fatality rate by 2.2% for COVID-19 (Marois et al. 2020). The WHO confirmed that there was evidence that the novel coronavirus may be transferred from human-to-human, i.e., droplets infection, causing the severe respiratory syndrome, which is a critical danger to public health, and therefore, was named also severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (Zheng 2020). Spreading of COVID-19 in 223 countries with more than 472 million cases, and 6 million deaths, was reported by WHO till March 2022. The recent SARS-CoV-2 variant, Omicron has been reported in 76 countries since November 2021(Cascella et al. 2022).
Previous literature has classified coronaviruses into alpha, beta, gamma, and delta types, i.e., four sub-groupings. SARS-CoV-2 (a beta virus) has some characteristics differentiating it from most of the past decade's pandemics as SARS-CoV-1. First and foremost, its rate of transmission is very high as its basic reproduction number (R0) ranges from 1.9 to 6.5 (Li et al. 2020; Park et al. 2020a, b), i.e., the virus is contagious to a large extent and can spread very widely in a very short period (Peeri et al. 2020). Its high infectivity is mainly attributed to its ability to be transmitted from both asymptomatic and pre-symptomatic cases (Bai et al. 2020). Moreover, the virus has demonstrated further fatal variants via several genomic mutations, including the UK B.1.1.7 strain, P.1 or B.1.1.248, 1.351, B.1.526, and B.1.427/B.1.429. These variants have worsened subsequently the crisis. The virus life cycle and mode of infection have been discussed previously in several publications and the readers can refer to for further information (V’kovski et al. 2021).
Besides, the spectrum of its severity is different. Most of the cases infected with COVID-19 manifest mild or no symptoms (Lake 2020), while in the remaining cases, the disease can worsen into life-threatening bilateral pneumonia, with symptoms varying from dyspnea to complete respiratory failure and death (Day 2020; Odone et al. 2020). Whereas most COVID-19 cases have mild (40%) or moderate (40%) symptoms, about 15% of cases develop severe sickness which needs oxygen support, and the remaining 5% develop critical disease (Surveillances 2020) as shown in Table 1 There are some reported risk factors for severe COVID-19 disease and death including old age, smoking, cancer, cardiac diseases, diabetes, hypertension, and chronic lung diseases (Alqahtani et al. 2020; Wang et al. 2020a, b, c; Organization 2020a, b; Zhou et al. 2020).
To control the COVID-19 infection spread and to get into a normal post-pandemic situation, we need to raise the population's immunity. This can be achieved either naturally or via using vaccines (DeRoo et al. 2020). Using vaccines appears to be the quick fix to stop this pandemic spread, and thus they must be distributed very rapidly and efficiently. Consequently, the optimal method to rapidly control the current global crisis of pandemic COVID-19 is by developing and applying safe and effective vaccines (Shah et al. 2020). For the past few months, different vaccines have been implemented globally, and additional ones are under development, some of them have reached the application stages and others are in advanced clinical trials phases (Hagens et al. 2021). Based on the preliminary results of the current clinical trials of candidate vaccines, it seems that many vaccines can induce an adequate immune response and are generally well-tolerated but may be effective for a short period because of incomplete revealing of the genetic code of SARS-CoV-2 (Le et al. 2020). Thus far, they can protect against the serious symptoms of the disease and can potentially inhibit asymptomatic infections and the subsequent transmission (Jackson et al. 2020; Xia et al. 2020).
The development and implementation of the COVID-19 vaccine have been carried out under huge clinical, economical, and political pressure. Even with the dense need for superfast tracks for vaccine production, the Food and Drug Administration (FDA) took a decision not to authorize any vaccine without fulfilling its standards (Avorn and Kesselheim 2020). This is since any defect in the safety regulations for new vaccine authorization could potentially threaten the confidence and trust in the well-established global vaccination programs (Trogen et al. 2020). Thus, vaccines of COVID-19 have undergone very careful assessments for licensing, registration, and implementation, and this will continue to be applied to the new coming vaccines in 2021. Currently, several vaccines have been introduced to the market, including BioNTech-Pfizer (BNT162b2), Astra Zeneca (AZD1222), Moderna vaccine (mRNA-1273), Johnson & Johnson Vaccine (Ad26.COV2.S), and others, based on various technologies.
The use of treatment protocols has been encouraged since the development of an effective vaccine takes years (Haque and Pant 2020) and when available, the required doses are not enough for the world's countries’ populations. Hence, most of the world's countries have authorized the use of some conventional drugs since the beginning of the pandemic, based mainly on experiences acquired from previous coronavirus diseases, severity, and the response of the diverse patients to drugs.
The present article reviewed the official protocols used in the Middle East, especially in Egypt and the Kingdom of Saudi Arabia, where they are among the most important countries that have highly qualified health care systems and succeeded to face to a great extent the COVID-19 infection waves in the Middle East region (Hassany et al. 2020; Post et al. 2021). This review is a part of our ongoing project (Khalifa et al. 2020a, b) where the aim is to assess the current COVID-19 situation including the managing protocols, specifically the commonly used drugs in both countries as representative examples of the Middle East region. These points affect consequently choosing the best drug combinations and assessing their importance and mechanism of action to decrease and treat the symptoms effectively, in parallel with the world's plan to discover and develop new lines of treatments and vaccines.
The pandemic COVID-19 and its waves
To date, the pandemic COVID-19 has experienced two waves in many countries. Nevertheless, a lot of world condition is suffering now from the third wave. The manifestations of fever, cough, dyspnea, and pneumonia were comparable in both waves, though frequent cases in the second wave presented with gastrointestinal symptoms such as nausea, vomiting, and abdominal pain (Zhang et al. 2020; Fan et al. 2021).
A noticeable difference between the first and second waves is the decrease in the case fatality rate (CFR) relative to the first one. CFR of COVID-19 is calculated by dividing the reported COVID-19 deaths number by the number of total cases. CFR is an important indicator for the quantification of the disease severity and the efficacy of treatment (Fan et al. 2021). There could be many reasons for this decrease in COVID-19 CFR in the second wave. First, a very large number of people in the vulnerable groups (such as the elderly and those with health conditions) probably died in the first wave, particularly in the countries with high infection rates. Second, the healthcare system capacity in many countries might have been better prepared for the second wave (Saito et al. 2020; Fan et al. 2021). Finally, the change of age structure of the infected people is a factor in the decrease of the CFR as the first wave involved mainly the elderly and those with health conditions while the second wave involved mainly children and healthier young individuals. The low compliance of social distancing and thus the increase in the virus transmission in children and young people could be a reason for this change in age structure in the second wave (Zhao et al. 2020). In early 2021 the vaccination campaigns started with a general hope to reach the end of corona tunnel. Lock-down measures were not fully respected in different countries, some relaxation was observed while spreading of more aggressive variants such as British, South-African and Brazilian variants. As a result, the third wave of COVID-19 appeared with increasing infection rates in different countries such as France, Germany as well as India which showed the highest peak in the third wave. USA and UK experienced a successful vaccination strategy (Rothengatter et al. 2021). The fourth and fifth waves were also experienced, which was especially affecting adolescents and young adults due to epidemic exhaustion, wrong information, absence of coordination among different administrative organizations, disagreements in many communities between public health services and the legislative actuality, and the appearance and distribution of the new SARS-CoV-2 variants (García-Basteiro et al. 2020; Han et al. 2020).
Now Egypt faces the sixth wave of the pandemic with controlled governmental measures. The majority of cases could be treated at home, and the severe cases are hospitalized. The World Health Organisation reported that the most likely causes of the recent increase in infections worldwide are two offshoots of the Omicron variant, BA.4 and BA.5 (www.thenationalnews.com).
Demographics of Egypt and Saudi Arabia Kingdom and COVID-19 cases
Demographic studies revealed that Egypt and Saudi Arabia are quietly different. The current population, i.e., till July 17, 2022, of Egypt is 106,432,734. The population density in Egypt is 103 per Km2 (266 people per mi2). The total land area is 995,450 Km2 (384,345 sq. miles) and 43.0% of the population is urban (44,041,052 people in 2020). Furthermore, the percentage of elderly Egyptians (65+ years) is 4.44% of the total population. The median age in Egypt is 24.6 years (Worldometer 2022). While the current population of Saudi Arabia is 35,916,911. The population density in Saudi Arabia is 16 per Km2 (42 people per mi2). The total land area is 2,149,690 Km2 (830,000 sq. miles) and 84.0 % of the population is urban (29,255,576 people in 2020). The percentage of the Saudi elderly (65+ years) is 3.63% of the total Saudi population and the median age in Saudi Arabia is 31.8 years.
WHO has reported till the 17th of July 2022 that the number of reported COVID-19 cases in Egypt is 515,645 (0.48 % of the total population), while in the Kingdom of Saudi Arabia is 803,158 (2,23 % of the total population). The cumulative total death cases in Egypt are 24,613 whereas in the Kingdom of Saudi Arabia are 22,362. These dates are obtained from the WHO Coronavirus (COVID-19) Dashboard, accessed on 17-6-2022 (WHO 2022). In Saudi Arabia, among closed cases that had an outcome 787,599 (98%) recovered and/or discharged and 22,362 (2%) deaths. While among 6,329 active cases (which are currently infected patients), 151 cases are considered critical. In Egypt, 122 cases are critical ones of 48,850 active cases (Worldometer 2022). COVID-19 total recorded cases versus total deaths in Egypt and Saudi Arabia are demonstrated in Figure 1 and Table 2.
Kingdom of Saudi Arabia's COVID-19 preparedness and response: building on the MERS experience
It is noteworthy that the region of the Middle East had previously witnessed outbreaks of the two other coronaviruses, SARS, and the Middle East respiratory syndrome coronavirus (MERS). MERS is an emerging viral respiratory disease caused by the MERS coronavirus, also known as MERS-CoV, which was first reported in Saudi Arabia in 2012. Thus, the affected countries had subsequently learned some lessons about handling outbreaks (Mounts et al. 2013; Sawaya et al. 2020). For instance, the ministry of health in the Kingdom of Saudi Arabia established the command-and-control center and the Saudi center for disease control and prevention shortly after the detection of MERS. Today, these two centers represent the country's frontline response to COVID-19 (Malik and Mahjour 2015). Furthermore, the Saudi Ministry of Health developed the National Health Laboratory (NHL) as a reference laboratory with a focus on advanced diagnostics for infectious diseases in high biocontainment laboratories (Algaissi et al. 2020).
Saudi Arabia's Ministry of Health, military hospitals, and other government-sponsored hospitals offer free healthcare services to the public. There is also a large network of for-profit hospitals in the private sector throughout the world. The number of beds per 1000 people in Saudi Arabia is 2.2. Saudi Arabia is also a signatory to the WHO International Health Regulation (IHR; 2005) and has been reporting on pandemic preparedness and adhering to WHO policies on infection prevention and control (IPC) (Memish et al. 2014; Hashem et al. 2019). To meet the rising demand for health care services in Saudi Arabia, the Saudi Vision 2030 considers fundamental systemic changes in the healthcare sector (Algaissi et al. 2020). Biosafety in diagnostic laboratories has improved significantly, like the implementation of strict IPC systems in all hospitals throughout the world. In addition, the Saudi Ministry of Health has designated more than 25 regional hospitals to isolate and treat MERS patients. These hospitals are now fully equipped to handle COVID-19 patients and have begun to do so (Algaissi et al. 2020).
Development of management protocols
It was a critical issue to prevent and control COVID-19 on the national and global scales owing to the intense increase in COVID-19 cases all over the world. The governments of each country provided guidelines to teach citizens how to protect themselves from COVID-19. Additionally, they provided important guidance for healthcare professionals to help them take steps to reduce the spread of COVID-19 (Yoo et al. 2020). Bearing in mind that each country had various health care systems, capacities, risks, socioeconomic and political challenges, it was predictable that each nation reacted to this global threat with relatively unique measures (Yoo et al. 2020).
The used protocols are mainly based on three main aspects. The first one is to apply transmission-based precautions, including isolation for symptomatic patients for 10 days after the onset of symptoms, plus three days (at least) without symptoms. The second aspect is the treatment of acute co-infections by using antibiotic therapy. The third aspect includes the prevention of complications (Organization 2020a, b).
It was hard to find out an optimal therapeutic compound that consistently resulted in positive results across SARS, MERS, and COVID-19. This might be because there is not a universal “cure” for such viral diseases. The very subtle differences among these three coronaviruses, along with the shortage of objective information from the clinical experiences of the previous SARS and MERS epidemics, might be other reasons (Han et al. 2021).
In addition to the supportive treatment, including bed rest, preserving hydration and electrolyte balance, maintaining a stable environment, closely monitoring vital signs and oxygen saturation, providing drugs with potential antiviral activity (Shang et al. 2020). These drugs differ from the approved protocols by the ministry of health in each country as summarized in Figure 2 and Table 3.
It is believed that two main processes drive COVID-19 pathogenesis. In the early stage of the clinical course, the disease is mainly driven by the replication of SARS-CoV-2. Later, the disease seems to be driven by a dysregulated immune and/or inflammatory response resulting in tissue damage. Thus, antiviral therapies would have the highest positive outcome when the course of the disease is in its early stage, while the immunosuppressive and/or anti-inflammatory treatments are more likely to be useful in the later stages of the clinical course of COVID-19 (Trougakos et al. 2021).
In the following subsections, each authorized drugs would be covered following its pharmacological class, in addition to the importance and mechanism of action, into antiviral, antimicrobial, anti-inflammatory, antioxidant, and miscellaneous.
Antiviral drugs
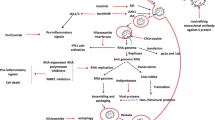
The antiviral drugs act via different modes interfering with either viral entry, viral transcription, or inhibition of proteases that are involved in viral assembly, Figure 3.
Potential mechanisms of the antiviral drugs against the SARS-CoV-2 targeting viral entry, transcription, and assembly. Antivirals as chloroquine and hydroxychloroquine can act on the viral entry, while remdesivir on the transcription step and lopinavir as well as nirmatrelvir on inhibition of viral proteases
Hydroxychloroquine and chloroquine
Chloroquine and hydroxychloroquine (HCQ) were suggested to be beneficial against SARS-COV-2. This is based on the in vitro finding showing that both drugs could cause glycosylation of ACE2 receptors making cells refractory to infection by SARS-COV-2 (Pahan and Pahan 2020) Although, the use of hydroxychloroquine did not result in a substantially reduced or increased risk of intubation or death in COVID-19 patients (Geleris et al. 2020). Moreover, both drugs were revealed to have immunomodulatory effects and thus potentially helpful in the reduction of COVID-19 severity (Ghazy et al. 2020; Schrezenmeier and Dörner 2020). Unfortunately, there are many controlled trials that revealed that both hydroxychloroquine and chloroquine have little or no value for people infected with COVID‐19 especially in decreasing the risk of death and might be with no effect on the progression to mechanical ventilation. These results could mean that these drugs are less likely to be effective in protecting people from COVID-19 infection. Although most of the management protocols have not recommended the use of chloroquine and hydroxychloroquine, it is believed to be effective in patients with resistant fever and to have a synergistic effect with azithromycin. One of the side effects of chloroquine is gastric upsets, so proton pump inhibitors (PPI) are to be described. Chloroquine is contraindicated in pregnant, hepatic, and cardiac patients (Hoffmann et al. 2020; Han et al. 2021).
Oseltamivir
Oseltamivir is a neuraminidase inhibitor that have FDA approval in 1999 (Hayden et al. 1999). Since that time, it has played a vital role in the treatment of both influenza A and influenza B infections (Zhang and Yap 2004) by preventing the release of virions from the infected cells and significantly hindering their spread in the body (Ward et al. 2005). Even though SARS-CoV-2 does not express neuraminidase, Zhang et al. discovered through homology modelling that the Spike 1 (S1) protein of SARS-CoV has an active core like that of neuraminidase. In other words, by focusing on S1 protein activity, neuraminidase inhibitors could prevent SARS-CoV from spreading. (Zhang and Yap 2004). A research group has explored the effect of oseltamivir in silico, suggesting that it is highly effective against SARS-CoV-2 especially if combined with Lopinavir/Ritonavir (Muralidharan et al. 2021). However, other studies have debated these results disapproving the use of oseltamivir in COVID-19 treatment (Rosa and Santos 2020; Tan et al. 2020).
Remdesivir
Remdesivir is an inhibitor of the viral RNA-dependent RNA polymerase leading to pre-mature termination of the viral RNA transcription and subsequently results in RNA synthesis inhibition (Khanal 2020; Saha et al. 2020). It was found to have an in vitro inhibitory activity against SARS and MERS-CoV (Brown et al. 2019; Sheahan et al. 2020). It was early identified as an intriguing candidate for COVID-19 treatment owing to its in vitro capability to inhibit SARS-CoV-2 and this was proved by several researchers who conducted clinical trials and they found that remdesivir resulted in a shorter time to recovery of COVID-19 patients. Monitoring of liver and kidney functions is recommended as when they increase, remdesivir should be stopped (Spinner et al. 2020; Wang et al. 2020a, b, c).
Lopinavir
Lopinavir is a protease inhibitor that is approved to be used for human immunodeficiency virus 1 treatment as it can resemble the peptide linkage and bind to the substrate-binding pockets of the virus enzymes. Thus, inhibiting the enzyme activity and resulting in the formation of immature and non-infectious viral particles (Uzunova et al. 2020). Previous trials and in vitro studies on coronaviruses including SARS and MERS have reported that lopinavir, especially when administered with ritonavir to enhance its plasma half-life, conferred clinical benefits (Li et al. 2020; Yao et al. 2020). This is attributed to its ability to inhibit the main protease of coronaviruses, which is critical for virus replication (Liu and Wang 2020). Thus, many studies have been conducted to evaluate the usefulness of lopinavir in the treatment of COVID-19 patients. One of these studies has found that lopinavir reduced the clinical symptoms in the treated animals with no effect on the virus titers (Park et al. 2020a, b). Other observational studies have reported that lopinavir decreased the duration of virus shedding (Yan et al. 2020) and fever (Ye et al. 2020).
Favipiravir
Favipiravir, an anti-RNA virus synthetic prodrug, has been approved in Japan for the treatment of the emerging pandemic influenza infections in 2014 (Furuta et al. 2013). Within the tissue, it does phosphoribosylation to the active drug that acts as a substrate for the RNA-dependent RNA-polymerase enzyme of the virus, thus decreasing its activity and resulting in the termination of the viral protein synthesis (Madelain et al. 2016). Some clinical studies have been conducted worldwide to evaluate the effectiveness of favipiravir in COVID-19 management as it is considered a promising candidate for the treatment of such pandemic infection due to its ability to inhibit RNA-dependent RNA-polymerase of SARS-CoV-2 (Shannon et al. 2020). These trials revealed that favipiravir has resulted in a shorter length of time of viral clearance (Cai et al. 2020) and enhanced clinical improvement (Agrawal et al. 2020).
Molnupiravir
Molnupiravir is an oral prodrug of β-D-N4-hydroxycytidine (NHC), a ribonucleoside analog that has broad antiviral activity against RNA viruses. NHC uptake by viral RNA-dependent RNA-polymerases results in viral mutations and lethal mutagenesis. It has strong antiviral properties against SARS-CoV-2 (Fischer et al. 2021a; Kabinger et al. 2021; Zhou et al. 2021) with good safety and tolerability profile (Fischer et al. 2021b). It was issued on 23/12/2021 by FDA for treatment of mild to moderate COVID-19. It was approved to be used within 5 days of symptom onset, for patients who lack an alternative to alternate antiviral medications and are at high risk of developing a severe illness. In the MOVe-OUT trial, molnupiravir decreased the rate of hospitalization or death by 30% relative to placebo (Singh et al. 2022). There is a certain concern, theoretically, regarding that molnupiravir might be incorporated into the host DNA cells, resulting in different mutations. Till now, there is no detected risk for genotoxicity depending on the available information introduced to FDA (Singh et al. 2022).
Ritonavir-boosted nirmatrelvir (Paxlovid.®)
Nirmatrelvir is an antiviral drug against all coronaviruses including COVID-19. It inhibits viral protease enzyme (MPRO), It is co-administered with ritonavir as Paxlovid. Ritonavir is a potent CYP 3A4 (cytochrome P450) inhibitor and could boost HIV protease inhibitors. This combination was authorized by FDA on 22/12/2021 for COVID-19 treatment (Pillaiyar et al. 2016; Owen et al. 2021; Saravolatz et al. 2022). It is prescribed for mild to moderate cases of COVID-19 who are at high risk to turn into severe cases. Paxlovid was expected to treat Omicron (B.1.1.529) variant and its BA.2 subvariant (Takashita et al. 2022). 89% reduction of risk of hospitalization or death according to placebo was reported in patients of SARS-CoV-2 (Hammond et al. 2022; Saravolatz et al. 2022)It showed a greater effect than remdesivir (87% relative reduction) (Gottlieb et al. 2022) and molnupiravir (30% relative reduction) (Jayk Bernal et al. 2022).
Antimicrobial drugs
Azithromycin
It has been shown that azithromycin (an antibacterial macrolide compound) has an in vitro and in vivo antiviral activity against a large panel of viruses including Ebola, Zika, influenza H1N1 virus, respiratory syncytial virus, enterovirus, and rhinovirus (repurposing of azithromycin to be used as antiviral agent) (Beigelman et al. 2015; Retallack et al. 2016; Mosquera et al. 2018; Wu et al. 2018; Li et al. 2019; Tran et al. 2019; Du et al. 2020). Also, it has been found that it has a significant antiviral effect against SARS-CoV-2 (Zeng et al. 2019; Andreani et al. 2020; Bleyzac et al. 2020; Gautret et al. 2020). Azithromycin is supposed to decrease the SARS-CoV-2 virus's entrance into human cells (Lythgoe and Middleton 2020; Yuki et al. 2020). Moreover, it can modulate the immune response of the body via suppressing several cytokines that are involved in the respiratory syndrome of COVID-19 patients. It decreases the production of several pro-inflammatory interleukins (IL) including IL-1β, IL-6, IL-8, IL-10, IL-12, and IFN-α (Chen et al. 2020a, b; Huang et al. 2020; McGonagle et al. 2020). Another important property of azithromycin is its antibacterial properties, which would prevent or treat secondary bacterial infections in COVID-19 cases (Bleyzac et al. 2020).
Meropenem
Meropenem is a carbapenem antibacterial compound with a broad spectrum of activity including Gram-positive, Gram-negative, and anaerobic bacteria (Morris and Cerceo 2020). Like all other β-lactams, meropenem inhibits the synthesis of bacterial cell walls via binding to transpeptidases or penicillin-binding proteins (PBPs) leading to their inactivation (Townsend et al. 2020). It is prescribed in COVID-19 to treat both communities acquired, and hospital-acquired pneumonia (Rawson et al. 2020). Further, it is crucial in the treatment of patients suffering from secondary bacterial co-infections that are not linked to their respiratory presentation such as bloodstream or urinary tract infections. Meropenem is recommended in patients with secondary bacterial infections or immunocompromised patients (Hughes et al. 2020; Rawson et al. 2020; Seaton et al. 2020).
Levofloxacin
Levofloxacin belongs to fluoroquinolones which are synthetic broad-spectrum antimicrobial agents (Pham et al. 2019). Remarkably, levofloxacin could exert an antiviral action against many viruses such as vaccinia, cytomegalovirus, herpes simplex, varicella-zoster, hepatitis C, and human immune deficiency viruses (Khan et al. 2012; Karampela and Dalamaga 2020; Scroggs et al. 2021). It could also suppress the replication of SARS-CoV-2 via binding to its main protease. Interestingly, it exhibits an immunomodulatory activity via inhibition of the pro-inflammatory cytokines leading to attenuation of the inflammation response (Karampela and Dalamaga 2020). Notably, levofloxacin is significant in the management of severe community-acquired pneumonia due to its pharmacokinetic properties (Noreddin and Elkhatib 2010), thus it is important in the treatment of pneumonia in COVID-19. Levofloxacin is contraindicated with chloroquine in patients with heart diseases, and its dose must be adjusted in patients with renal diseases (Noreddin and Elkhatib 2010; Khan et al. 2012; Karampela and Dalamaga 2020; Scroggs et al. 2021).
Anti-pyretic and anti-inflammatory drugs
Paracetamol
In March 2020, non-steroidal anti-inflammatory drugs (NSAIDs) were strongly discouraged to be prescribed to COVID-19 cases owned to the alert by several studies (Micallef et al. 2020a, b) on the possible aggravation of the disease and the risk of secondary infections and complications, mainly of the lungs. In addition, ibuprofen especially induces overexpression of angiotensin-converting enzyme 2 (ACE2) which helps the primary entry of SARS-CoV-2. However, paracetamol is considered a safer antipyretic alternative for the early management of both pain and fever in COVID-19 patients having neither anti-inflammatory nor antiplatelet activity (Driver et al. 2020).
Baricitinib
It has been speculated that mitigation of the immune response and prevention of the hyperinflammatory state in COVID-19 patients could improve clinical outcomes. Baricitinib is a selective inhibitor of Janus kinase (JAK) 1 and 2 and it has a therapeutic application in the treatment of inflammatory diseases as rheumatoid arthritis (Schwartz et al. 2017). It was predicted to have potential therapeutic activity against SARS-CoV-2 (Richardson et al. 2020; Stebbing et al. 2020). Baricitinib blocks the cytokine signalling pathway that is activated in COVID-19 severe instance, including IL-2, Il-6, IL10, and interferon-γ, therefore, it effectively suppresses the cytokine storm (Kalil et al. 2021; Sims et al. 2021).
Antioxidant drugs
Ascorbic acid (vitamin C)
Vitamin C has various potentials which make it a beneficial medicinal agent for different respiratory infections such as COVID-19 and influenza. It has a strong antioxidant, anti-inflammatory, and immune-supportive compound (Hoang et al. 2020). Vitamin C has an important role in virus infection via dissipation of the pro-inflammatory response, improvement of the function of the epithelial barrier, enhancement of the alveolar fluid clearance, and inhibition of the sepsis-associated coagulation abnormalities (Bharara et al. 2016). Moreover, vitamin C has a significant impact on the production of type I interferons attenuating the immune response against viruses (Kim et al. 2013) and it can upregulate the activity of natural killer cells and cytotoxic T-lymphocyte both in vitro and in vivo. A common side effect of vitamin C is gastric upset, so PPIs are recommended (Carr and Maggini 2017).
Cyanocobalamin
Cyanocobalamin is a synthetic product of vitamin B12. Vit. B12 has an effective role in the red blood cells and myelin synthesis in addition to its important function in cellular growth. Also, it is a modulator for gut microbiota and its low levels could result in elevation of methylmalonic acid and homocysteine, leading to an increase in inflammation and oxidative stress (Mikkelsen et al. 2017). Nevertheless, SARS-CoV-2 could interfere with the metabolism of vitamin B12 causing symptoms of vitamin B12 deficiency which might lead to diseases of the gastrointestinal, respiratory, and central nervous systems. Besides, vitamin B12 deficiency results in elevated oxidative stress, activation of the coagulation cascade, vasoconstriction, pulmonary and renal vasculopathy (Sabry et al. 2020).
N-acetyl cysteine
N-acetyl cysteine is a precursor of the antioxidant glutathione with a direct effect on many oxidant species. It also exhibits mucolytic activity as it can loosen the thick mucus in the lungs. Moreover, it has a role in boosting the immune system and reduction of inflammation (McCarty and DiNicolantonio 2020). The E protein of SARS-CoV-2 has a triple cysteine motif that interacts with a similar motif in the S protein through disulfide bonds, and N-acetyl cysteine can cleave these bonds thus, it would decrease SARS-CoV-2 infectivity (Jorge-AarÃ3n and Rosa-Ester 2020).
Miscellaneous drugs
Magnesium sulphate
Electrolytes such as sodium, potassium, and magnesium are basic elements for the maintenance of normal cell physiological function (Palmer and Clegg 2016, 2019). Magnesium is mainly concentrated in the mitochondria, and it is an essential element for various biochemical reactions in the body in addition to its participation in certain physiological functions and metabolism (Komiya and Runnels 2015), such as energy metabolism and synthesis of both proteins and nucleic acids (Abiri and Vafa 2020). Magnesium also has anti-inflammatory and antioxidant activity (Han et al. 2018; Abiri and Vafa 2020; Ozen et al. 2020) and it is vital in the regulation of homeostasis of different systems such as the digestive, neurological, and respiratory systems (Tang et al. 2020; Bachnas et al. 2021). Using the aforementioned data, it is proved that magnesium sulfate can be beneficial in the supportive cure of COVID-19 especially in critically ill patients (Tang et al. 2020).
Aminophylline
Aminophylline is a drug made of theophylline and ethylenediamine with a ratio of 2 to 1. It is approved by FDA to be used in relieving symptoms of reversible airway blockage as in asthma or other chronic lung diseases like chronic bronchitis (Armanian et al. 2014). Its mechanism of action is not completely understood but it seems like the smooth muscle relaxation of the lungs and pulmonary way via phosphodiesterase inhibition (Rabe et al. 1995). It is also an adenosine receptor antagonist; thus, it has an indirect role in bronchodilation (Polosa and Blackburn 2009), and theophylline increases the activity of histone deacetylases to the site of active inflammation leading to the anti-inflammatory effect (To et al. 2010; Wei et al. 2019). Aminophylline releases theophylline, after administration, which is responsible for bronchodilation.
Enoxaparin
Enoxaparin is low molecular weight heparin and was first approved for medical use in the treatment of venous thromboembolism in 1993 (Miranda et al. 2017; van Gameren et al. 2018; Xing et al. 2018). It potentiates antithrombin III to form a complex that leads to irreversible inactivation of factor Xa (Nutescu et al. 2016). It is thought that enoxaparin has a potential therapeutic impact in COVID-19 patients. This is because enoxaparin plays a part in preventing COVID-19 infection via decreasing the viral entry to human cells. It can also reduce the release of IL-6 that is associated with cytokine storms. In addition to its ability to prevent the activation of the coagulation cascade, venous thromboembolism, and thrombosis in the small and middle-size vessels of the lung (Drago et al. 2020; Hasan et al. 2020). The use and dose of enoxaparin are decided following the D-dimer test. Anticoagulants in COVID-19 Patients with an elevated D-dimer are recommended. Daily monitoring of PT/INR and PTT is also advised for patients concurrently on enoxaparin and used with precautions in hypertensive patients (Organization 2020a, b; Han et al. 2021).
Corticosteroids
Corticosteroids include glucocorticoids and mineralocorticoids, and they are produced by the adrenal cortex. They do not attack the viruses directly, rather they act through their immunosuppressive and anti-inflammatory activities (Ramamoorthy and Cidlowski 2016). Their anti-inflammatory activity is attributed to the suppression of the pro-inflammatory genes via signal transduction by binding to their steroid receptors (Cruz-Topete and Cidlowski 2015; Budhathoki et al. 2020). Due to their effects, corticosteroids have been used in the past during the outbreaks of SARS-CoV and MERS-CoV (Russell et al. 2020), and their uses in the current days in the pandemic of COVID-19 is based on the genetic homology with both SARS and MERS coronaviruses (Budhathoki et al. 2020). There is a controversy regarding the use of corticosteroids in COVID-19 patients. Wu and his colleagues noticed a reduction in mortality when used in COVID-19 patients with acute respiratory distress (Wu et al. 2020), whereas many other retrospective studies revealed an increase in mortality among the corticosteroid receiving groups. Corticosteroids are not used in the first days of infection unless with hypoxic patients or patients with severe pulmonary abnormalities on chest X-ray. Diabetic patients on corticosteroids are closely monitored, and gradual steroid withdrawal is needed to avoid sudden termination and unwanted consequences (Huang et al. 2020; Wang et al. 2020a, b, c; Zhou et al. 2020).
Proton pump inhibitors
Proton pump inhibitors are drugs for treating gastric acid-related diseases by suppressing the acid secretion in the gastric lumen via inhibiting H+/K+ ATPase. Additionally, it was revealed that the proton pump inhibitors are effective on the elements of the immune system including neutrophils, monocytes, and endothelial cells (Wandall 1992). They can suppress the functions of neutrophils such as chemotaxis and superoxide production (Ubagai et al. 2009). Moreover, they have an anti-inflammatory effect through the reduction of the neutrophil adhesion molecules and free oxygen radicals (Biçakçi et al. 2005). They can also activate heme oxygenase-1 (HO-1) which is an endogenous antioxidant (Taştemur and Ataseven 2020). Based on all these data plus the fact that these drugs are inexpensive, widespread, and immediately available, these drugs are used in the treatment of COVID-19 pandemic infection. In addition, proton pump inhibitors are used to relieve the side effects of other drugs involved in the management protocols.
Furosemide
Furosemide was approved by FDA to be used to treat diseases connected to edoema and volume overload brought on by congestive heart failure, renal failure, or liver failure (Felker et al. 2011). Its mechanism of action depends mainly on inhibition of reabsorption of sodium and chloride ions in the proximal tubules, distal tubules, and thick ascending loop of Henle via inhibition of the sodium-chloride cotransport system leading to excessive excretion of water containing sodium, chloride, calcium, and magnesium (Shankar and Brater 2003). In aged COVID-19 patients having a high proportion of cardiac comorbidities, mild and moderate pneumonia could be accompanied by a certain degree of acute heart failure and ischemia (Sisti et al. 2021). Thus, furosemide is thought to be essential for COVID-19 patients especially, when corticosteroids are administered, to limit corticosteroid-induced retention (Kevorkian et al. 2021).
Intravenous immunoglobulin
Polyclonal immunoglobulin gamma is the main component of intravenous immunoglobulin (IVIG), a blood product that is derived from healthy donors. In several autoimmune and inflammatory illnesses, it has been used as an immunomodulatory treatment. (Galeotti et al. 2017). Remarkable positive results have been perceived by the administration of IVIG to patients with SARS and MERS (Arabi et al. 2014). Bearing in mind the devastating immune response that occurs in many COVID-19 patients (Zhu et al. 2020), in addition to the similarities in pathogenesis between SARS and COVID-19, it seems that IVIG could produce good clinical outcomes in COVID-19 patients (Cao et al. 2020a, b). Several investigations have been carried out to assess its effectiveness in COVID-19 cases and they provided evidence that supported the IVIG injection enhances clinical results in severe COVID-19 cases (Cao et al. 2020a, b; Gharebaghi et al. 2020; Xie et al. 2020).
Tocilizumab (Anti-SARS-CoV-2 monoclonal antibody)
Tocilizumab is an immunosuppressive drug which is indicated in treating rheumatoid arthritis and giant cell arteritis. It is a humanized monoclonal antibody which suppresses the signalling of IL-6. IL-6 is a cytokine involved in the pathogenesis of several diseases such as lymphoproliferative and autoimmune diseases. In June 2021, it was granted for the treatment of COVID-19 as an emergency use authorization by the FDA in hospitalized patients, who are receiving systemic corticosteroids and require supplemental oxygen and non-invasive or invasive mechanical ventilation (Kenny and Mallon 2021). Tocilizumab prevented mortality in patients hospitalized for COVID-19. This effect was observed to a greater extent in patients getting concomitant corticosteroids and administered tocilizumab in the first 10 days from the begining of symptoms onset (Rubio‐Rivas et al. 2021).
Oxygen therapy
ARDS was diagnosed in about 67 percent of patients, with 71 percent requiring artificial ventilation. For patients with SARS-CoV-2 at various stages of the disease, oxygen therapy is recommended. Oxygen saturation must be 92% or more so the need for various oxygen delivery devices is determined by the patient's condition. In mild to moderately symptomatic patients, a nasal cannula and blow-over oxygen at 4–6 L/min can be applied, with the patient's face covered with an N95 or comparable face mask. A high-flow nasal cannula delivers heated humidified oxygen at rates ranging from 10 to 50 L/min where it creates positive pressure at high flows, used especially in conditions such as respiratory distress, preoxygenation, and apneic diffusion of oxygen in airway treatments. Supraglottic devices are still recommended in severe airway situations, especially in apneic patients (https://www.covid19treatmentguidelines.nih.gov/; Lyons and Callaghan 2020; Smit et al. 2020). Common side effects of oxygen therapy are a decrease in lung secretions, dryness, middle ear barotrauma, and retinal detachment especially in pediatric patients (Heyboer et al. 2017; Barrett et al. 2020).
Drugs used in the management protocol, their doses, side effects, and contraindications are summarized in Table 4.
In comparison to the management protocol of the United States of America and the rest of the world
The line of treatment and drugs administered in COVID-19 management protocols are quietly similar worldwide. In the USA, however, mild and moderate cases of COVID-19 should be administered with anti-SARS-CoV-2 antibody-based therapies including bamlanivimab plus etesevimab or casirivimab plus imdevimab. Bamlanivimab is a neutralizing monoclonal antibody that can target the receptor-binding domain (RBD) of the spike (S) protein of SARS-CoV-2. Etesevimab is another neutralizing monoclonal antibody that can bind to a different and overlapping epitope in the S protein RBD of SARS-CoV-2. Both casirivimab and imdevimab are recombinant human monoclonal antibodies that can bind to different and non-overlapping epitopes of the RBD of the S protein of SARS-CoV-2. These combination therapies are used in non-hospitalized mild to moderate COVID-19 cases with laboratory-confirmed SARS-CoV-2 infection to decrease the risk for progression to severe disease and hospitalization (https://www.covid19treatmentguidelines.nih.gov/).
Remdesivir and corticosteroids are recommended to be used in the severe cases of COVID-19 in the USA and this is like the Middle East. In the USA, an additional drug is added to the management protocol used in severe cases to improve the survival among COVID-19 patients exhibiting rapid respiratory decompensation. This drug is tocilizumab which is a recombinant humanized monoclonal antibody against IL-6 receptor (https://www.covid19treatmentguidelines.nih.gov/). IL-6 levels are highly correlated to the severity of COVID-19 suggesting that the immune dysregulation and acute respiratory distress syndrome that occur in severe cases of COVID-19 could be influenced by IL-6(https://www.covid19treatmentguidelines.nih.gov/).
The treatment guidelines for COVID-19 have many similarities among different countries worldwide. Treatment protocols usually include main four medication groups: antiviral, systematic corticosteroids, antimalarial, and antibiotics, which can vary in dosages, and durations. Notably, the cost of the utilized treatment protocols is an important factor as the prices of antimalarial drugs and corticosteroid therapy could be affordable for most of the countries. Nevertheless, the cost of the antiviral drugs and immunomodulators is very high which could hinder their availability to COVID-19 patients especially in low-income countries (https://www.covid19treatmentguidelines.nih.gov/.). The antiviral drugs used worldwide include remdesivir, lopinavir/ritonavir, darunavir/cobicistat, favipiravir, umifenovir, ribavirin, or oseltamivir. Systematic corticosteroids include dexamethasone, methylprednisolone, prednisone, prednisolone, or hydrocortisone. The conventional antimalarial drugs include hydroxychloroquine and chloroquine. Immunomodulators comprise tocilizumab, siltuximab, sarilumab, canakinumab, anakinra, ruxolitinib, or baricitinib. Aside, other medications, such as antibiotics and anticoagulants, are used in the management of COVID-19 to eradicate concurrent bacterial infections or to prevent complications (Jirjees et al. 2021). Table 5 shows a summary of different medications used for the treatment of COVID-19 patients in different countries worldwide.
There are many questions concerning COVID-19 that are still without clear answers owing to the disease’s novelty and the excessive load on healthcare systems. This may explain the relatively low number of publications concerning the effectiveness and safety of the endorsed drugs in the management of COVID-19 patients.
Conclusions
COVID-19 with its spread waves has significantly affected human life since the end of 2019 with high mortality rates. Various lines of treatment have evolved due to the wide array of COVID-19 symptoms. The review evaluated authorized drugs in official protocols that were authorized in the Middle East and administered based on the degree of the disease severity. The use of such protocols has succeeded to decrease the number of hospitalized patients in two medical centers in the Middle East, i.e., Egypt and the Kingdom of Saudi Arabia, despite the absence of official statistical data demonstrating the effectiveness of the used protocols. Similarities between the two countries were observed regarding the administration of corticosteroids, paracetamol, hydroxychloroquine, molnupiravir and monoclonal antibodies. Yet, the other supplementary drugs, including vitamin C and proton pump inhibitors differed in-between.
Recognizing the clinical features of COVID-19 disease is crucial to be targeted by specific therapies. For instance, the anti-viral agents could be needed to target the viral entry and replication, while the immunomodulatory drugs are more likely to have a role in the cytokine storm in patients with a high risk of requiring intensive care to prevent uncontrolled inflammation and subsequent death. Thus, immunomodulatory drugs are recommended to be added to the management protocol used in Egypt as baricitinib, which is used in the management protocol of the Kingdom of Saudi Arabia to control the cytokine storm that occurs in severe cases of COVID-19. Immunomodulatory drugs like IL-6 inhibitors include anti-IL-6 receptor monoclonal antibodies as sarilumab and tocilizumab, and anti-IL-6 monoclonal antibodies as siltuximab (https://www.covid19treatmentguidelines.nih.gov/.). The article may provide the medical staff with the required overview and compare the various used drugs to improve currently used and develop new lines of disease management and treatment, especially that the long-term effectiveness of the developed vaccines has not been confirmed yet.
Data availability
Enquiries about data availability should be directed to the authors.
References
Abdullah M, Jamil RT, Attia FN (2020) Vitamin C (ascorbic acid). StatPearls [Internet]
Abiri B, Vafa M (2020) Effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammatory biomarkers, and SIRT1 in obese women: a study protocol for a double-blind, randomized, placebo-controlled trial. Trials 21(1):1–7
Agrawal U, Raju R, Udwadia ZF (2020) Favipiravir: a new and emerging antiviral option in COVID-19. Med J Armed Forces India. https://doi.org/10.1016/j.mjafi.2020.08.004
Al Amin A, Gupta V (2020) Vitamin B12 (Cobalamin).https://europepmc.org/article/nbk/nbk559132
Algaissi AA, Alharbi NK, Hassanain M, Hashem AM (2020a) Preparedness and response to COVID-19 in Saudi Arabia: building on MERS experience. J Infect Public Health. https://doi.org/10.1016/j.jiph.2020.04.016
Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, Quaderi S, Mandal S, Hurst JR (2020) Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS ONE 15(5):e0233147
Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, Boxberger M, Wurtz N, Rolain J-M, Colson P, La Scola B (2020) In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog 145:104228
Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, Hawa H, Alothman A, Khaldi A, Al Raiy B (2014) Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 160(6):389–397
Armanian AM, Badiee Z, Afghari R, Salehimehr N, Hassanzade A, Sheikhzadeh S, Tehrani MS, Rezvan G (2014) Prophylactic aminophylline for prevention of apnea at higher-risk preterm neonates. Iran Red Crescent Med J. https://doi.org/10.5812/ircmj.12559
Avorn J, Kesselheim A (2020) Regulatory decision-making on COVID-19 vaccines during a public health emergency. JAMA 324(13):1284–1285
Bachnas MA, Akbar MIA, Dachlan EG, Dekker G (2021) The role of magnesium sulfate (MgSO4) in fetal neuroprotection. J Matern Fetal Neonatal Med 34(6):966–978
Bai Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, Wang M (2020) Presumed asymptomatic carrier transmission of COVID-19. JAMA 323(14):1406–1407
Barrett R, Catangui E, Scott R (2020) Acute oxygen therapy: a cross-sectional study of prescribing practices at an english hospital immediately before COVID-19 pandemic. Expert Rev Respir Med. https://doi.org/10.1080/17476348.2021.1826316
Beigelman A, Isaacson-Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, Lyons K, Schweiger TL, Zheng J, Schechtman KB (2015) Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 135(5):1171-1178.e1171
Bharara A, Grossman C, Grinnan D, Syed A, Fisher B, DeWilde C, Natarajan R (2016) Intravenous vitamin C administered as adjunctive therapy for recurrent acute respiratory distress syndrome. Case Rep Crit Care. https://doi.org/10.1155/2016/8560871
Biçakçi Ü, Tander B, Aritürk E, Aydin BK, Aydin O, Rizalar R, Eren Z, Bernay F (2005) Effects of omeprazole and gentamicin on the biochemical and histopathological alterations of the hypoxia/reoxygenation induced intestinal injury in newborn rats. Pediatr Surg Int 21(10):800–805
Bleyzac N, Goutelle S, Bourguignon L, Tod M (2020) Azithromycin for COVID-19: more than just an antimicrobial? Springer
Brown AJ, Won JJ, Graham RL, Dinnon KH III, Sims AC, Feng JY, Cihlar T, Denison MR, Baric RS, Sheahan TP (2019) Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res 169:104541
Budhathoki P, Shrestha DB, Rawal E, Khadka S (2020) Corticosteroids in COVID-19: is it rational? A systematic review and meta-analysis. SN Compr Clin Med 2:1–21
Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y (2020) Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering 6(10):1192–1198
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M (2020a) A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. https://doi.org/10.1056/NEJMoa2001282
Cao W, Liu X, Bai T, Fan H, Hong K, Song H, Han Y, Lin L, Ruan L, Li T (2020b) High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open forum infectious diseases. Oxford University Press US
Carr AC, Maggini S (2017) Vitamin C and immune function. Nutrients 9(11):1211
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R (2022) Features, evaluation, and treatment of coronavirus (COVID-19). Statpearls [internet]
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H (2020a) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig 130(5):2620–2629
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y (2020b) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513
Cruz-Topete D, Cidlowski JA (2015) One hormone, two actions: anti-and pro-inflammatory effects of glucocorticoids. NeuroImmunoModulation 22(1–2):20–32
Day M (2020) Covid-19: four fifths of cases are asymptomatic, China figures indicate. Br Med J Publ Group. https://doi.org/10.1136/bmj.m1375
DeRoo SS, Pudalov NJ, Fu LY (2020) Planning for a COVID-19 vaccination program. JAMA 323(24):2458–2459
Dong E, Du H, Gardner L (2020) An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 20(5):533–534
Drago F, Gozzo L, Li L, Stella A, Cosmi B (2020) Use of enoxaparin to counteract COVID-19 infection and reduce thromboembolic venous complications: a review of the current evidence. Front Pharmacol 11:1469
Driver B, Marks DC, van der Wal DE (2020) Not all (N) SAID and done: effects of nonsteroidal anti-inflammatory drugs and paracetamol intake on platelets. Res Pract Thromb Haemost 4(1):36–45
Du X, Zuo X, Meng F, Wu F, Zhao X, Li C, Cheng G, Qin FX-F (2020) Combinatorial screening of a panel of FDA-approved drugs identifies several candidates with anti-Ebola activities. Biochem Biophys Res Commun 522(4):862–868
Duffy E (2022) Treating COVID-19 with Paxlovid in primary care. https://www.akohiringa.co.nz/education/treating-covid-19-with-paxlovid-in-primary-care
Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP, Grau S (2021) Azithromycin in the treatment of COVID-19: a review. Expert Rev Anti Infect Ther 19(2):147–163
Ershad M, Vearrier D (2019) N acetylcysteine. StatPearls [Internet]
Fan G, Yang Z, Lin Q, Zhao S, Yang L, He D (2021) Decreased case fatality rate of COVID-19 in the second wave: a study in 53 countries or regions. Transbound Emerg Dis 68(2):213–215
Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO (2011) Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 364(9):797–805
Fischer W, Eron JJ, Holman W, Cohen MS, Fang L, Szewczyk LJ, Sheahan TP, Baric R, Mollan KR, Wolfe CR (2021a) Molnupiravir, an oral antiviral treatment for COVID-19. MedRxiv. https://doi.org/10.1101/2021.06.17.21258639
Fischer WA, Eron JJ Jr, Holman W, Cohen MS, Fang L, Szewczyk LJ, Sheahan TP, Baric R, Mollan KR, Wolfe CR (2021b) A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med 14(628):eabl7430
Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL (2013) Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir Res 100(2):446–454
Galeotti C, Kaveri SV, Bayry J (2017) IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol 29(11):491–498
Gandhi RT, Lynch JB, Del Rio C (2020) Mild or moderate covid-19. N Engl J Med 383(18):1757–1766
García-Basteiro AL, Legido-Quigley H, Álvarez-Dardet C, Arenas A, Bengoa R, Borrell C, Del Val M, Franco M, Gea-Sánchez M, Gestal J (2020) Evaluation of the COVID-19 response in Spain: principles and requirements. The Lancet Public Health 5(11):e575
Gautret P, Lagier J-C, Parola P, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56(1):105949
Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG (2020) Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med 382(25):2411–2418
Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi S-R, Hajizadeh R (2020) The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis 20(1):1–8
Ghazy RM, Almaghraby A, Shaaban R, Kamal A, Beshir H, Moursi A, Ramadan A, Taha SHN (2020) A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment. Sci Rep 10(1):1–18
Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, Oguchi G, Ryan P, Nielsen BU, Brown M (2022) Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med 386(4):305–315
Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DS (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720
Hagens A, İnkaya AÇ, Yildirak K, Sancar M, van der Schans J, Acar Sancar A, Ünal S, Postma M, Yeğenoğlu S (2021) COVID-19 vaccination scenarios: a cost-effectiveness analysis for Turkey. Vaccines 9(4):399
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A (2022) Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med 386(15):1397–1408
Han F, Xu L, Huang Y, Chen T, Zhou T, Yang L (2018) Magnesium sulphate can alleviate oxidative stress and reduce inflammatory cytokines in rat placenta of intrahepatic cholestasis of pregnancy model. Arch Gynecol Obstet 298(3):631–638
Han E, Tan MMJ, Turk E, Sridhar D, Leung GM, Shibuya K, Asgari N, Oh J, García-Basteiro AL, Hanefeld J (2020) Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet 396(10261):1525–1534
Han YJ, Lee KH, Yoon S, Nam SW, Ryu S, Seong D, Kim JS, Lee JY, Yang JW, Lee J (2021) Treatment of severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and coronavirus disease 2019 (COVID-19): a systematic review of in vitro, in vivo, and clinical trials. Theranostics 11(3):1207
Haque A, Pant AB (2020) Efforts at COVID-19 vaccine development: challenges and successes. Vaccines 8(4):739
Hasan SS, Radford S, Kow CS, Zaidi STR (2020) Venous thromboembolism in critically ill COVID-19 patients receiving prophylactic or therapeutic anticoagulation: a systematic review and meta-analysis. J Thromb Thrombolysis 50(4):814–821
Hashem AM, Al-Subhi TL, Badroon NA, Hassan AM, Bajrai LHM, Banassir TM, Alquthami KM, Azhar EI (2019) MERS-CoV, influenza and other respiratory viruses among symptomatic pilgrims during 2014 Hajj season. J Med Virol 91(6):911–917
Hassany M, Abdel-Razek W, Asem N, AbdAllah M, Zaid H (2020) Estimation of COVID-19 burden in Egypt. Lancet Infect Dis 20(8):896–897
Hayden FG, Treanor JJ, Fritz RS, Lobo M, Betts RF, Miller M, Kinnersley N, Mills RG, Ward P, Straus SE (1999) Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA 282(13):1240–1246
Heyboer M III, Sharma D, Santiago W, McCulloch N (2017) Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care 6(6):210–224
Hoang BX, Shaw DG, Fang W, Han B (2020b) A possible application of high dose vitamin C in the prevention and therapy for coronavirus infections. J Glob Antimicrob Resist. https://doi.org/10.1016/j.jgar.2020.09.025
Hodgens A, Sharman T (2020) Corticosteroids. StatPearls [Internet]
Hoffmann M, Mösbauer K, Hofmann-Winkler H, Kaul A, Kleine-Weber H, Krüger N, Gassen NC, Müller MA, Drosten C, Pöhlmann S (2020) Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature 585(7826):588–590
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395(10223):497–506
Hughes S, Troise O, Donaldson H, Mughal N, Moore LS (2020) Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 26(10):1395–1399
Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ (2020) An mRNA vaccine against SARS-CoV-2—preliminary report. N Eng J Med. https://doi.org/10.1056/NEJMoa2022483
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML (2022) Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med 386(6):509–520
Jirjees F, Saad AK, Al Hano Z, Hatahet T, Al Obaidi H, Dallal Bashi YH (2021) COVID-19 treatment guidelines: do they really reflect best medical practices to manage the pandemic? Infect Dis Rep 13(2):259–284
Jorge-AarÃn R, Rosa-Ester M (2020) N-acetylcysteine as a potential treatment for novel coronavirus disease 2019. Future Microbiol 15:959–962
Jupalli A, Iqbal AM (2020) Enoxaparin. StatPearls [Internet]
Kabinger F, Stiller C, Schmitzová J, Dienemann C, Kokic G, Hillen HS, Höbartner C, Cramer P (2021) Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol 28(9):740–746
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S (2021) Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med 384(9):795–807
Karampela I, Dalamaga M (2020) Could respiratory fluoroquinolones, levofloxacin and moxifloxacin, prove to be beneficial as an adjunct treatment in COVID-19? Arch Med Res 51(7):741–742
Kenny G, Mallon PW (2021) Tocilizumab for the treatment of non-critical COVID-19 pneumonia: an overview of the rationale and clinical evidence to date. Expert Rev Clin Pharmacol 14(10):1279–1287
Kevorkian J-P, Riveline J-P, Vandiedonck C, Girard D, Galland J, Féron F, Gautier J-F, Mégarbane B (2021) Early short-course corticosteroids and furosemide combination to treat non-critically ill COVID-19 patients: an observational cohort study. J Infect 82(1):e22
Khalifa SAM, Mohamed BS, Elashal MH, Du M, Guo Z, Zhao C, Musharraf SG, Boskabady MH, El-Seedi HHR, Efferth T, El-Seedi HR (2020a) Comprehensive overview on multiple strategies fighting COVID-19. Int J Environ Res Public Health 17(16):5813
Khalifa SAM, Yosri N, El-Mallah MF, Ghonaim R, Guo Z, Musharraf SG, Du M, Khatib A, Xiao J, Saeed A, El-Seedi HHR, Zhao C, Efferth T, El-Seedi HR (2020b) Screening for natural and derived bio-active compounds in preclinical and clinical studies: one of the frontlines of fighting the coronaviruses pandemic. Phytomedicine 85:153311
Khan IA, Siddiqui S, Rehmani S, Kazmi SU, Ali SH (2012) Fluoroquinolones inhibit HCV by targeting its helicase. Antivir Ther 17(3):467
Khan TM, Patel R, Siddiqui AH (2021) Furosemide. StatPearls [Internet]
Khanal P (2020) Remdesivir for COVID-19 treatment: mechanism of action, synthesis, and clinical trials. J Pharm Pharm Sci 9:1062–1068
Kim Y, Kim H, Bae S, Choi J, Lim SY, Lee N, Kong JM, Hwang Y-I, Kang JS, Lee WJ (2013) Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-α/β at the initial stage of influenza A virus (H3N2) infection. Immune Network 13(2):70–74
Komiya Y, Runnels LW (2015) TRPM channels and magnesium in early embryonic development. Int J Dev Biol 59:281
Lake MA (2020) What we know so far: COVID-19 current clinical knowledge and research. Clin Med 20(2):124
Langarizadeh MA, Tavakoli MR, Abiri A, Ghasempour A, Rezaei M, Ameri A (2021) A review on function and side effects of systemic corticosteroids used in high-grade COVID-19 to prevent cytokine storms. Excli J 20:339
Le TT, Andreadakis Z, Kumar A, Román RG, Tollefsen S, Saville M, Mayhew S (2020) The COVID-19 vaccine development landscape. Nat Rev Drug Discov 19(5):305–306
Li C, Zu S, Deng Y-Q, Li D, Parvatiyar K, Quanquin N, Shang J, Sun N, Su J, Liu Z (2019) Azithromycin protects against Zika virus infection by upregulating virus-induced type I and III interferon responses. Antimicrob Agents Chemother 63(12):e00394-e1319
Li H, Liu S-M, Yu X-H, Tang S-L, Tang C-K (2020a) Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents 55(5):105951
Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y, Mo X, Wang J, Wang Y, Peng P (2020bc) An exploratory randomized, controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI). MedRxiv 13:e0195068
Liu X, Wang X-J (2020) Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genom 47(2):119
Lyons C, Callaghan M (2020) The use of high-flow nasal oxygen in COVID-19. Wiley Online Library
Lythgoe MP, Middleton P (2020) Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol Sci 41(6):363–382
Madelain V, Nguyen THT, Olivo A, De Lamballerie X, Guedj J, Taburet A-M, Mentré F (2016) Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet 55(8):907–923
Malik MR, Mahjour J (2015) Closing the knowledge gaps on MERS: three and half years since its detection, what have we learnt and what needs to be done urgently? EMHJ-East Mediterr Health J 22(2):85–86
Marois G, Muttarak R, Scherbov S (2020) Assessing the potential impact of COVID-19 on life expectancy. PLoS ONE 15(9):e0238678
McCarty MF, DiNicolantonio JJ (2020) Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog Cardiovasc Dis 63(3):383
McGonagle D, Sharif K, O’Regan A, Bridgewood C (2020) The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 19(6):102537
Memish ZA, Zumla A, Alhakeem RF, Assiri A, Turkestani A, Al Harby KD, Alyemni M, Dhafar K, Gautret P, Barbeschi M (2014) Hajj: infectious disease surveillance and control. The Lancet 383(9934):2073–2082
Micallef J, Soeiro T, Jonville-Béra A-P (2020a) COVID-19 and NSAIDs: primum non nocere. Therapie 75(5):514
Micallef J, Soeiro T, Jonville-Béra A-P, F. S. of Pharmacology (2020b) Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapies 75(4):355–362
Mikkelsen K, Stojanovska L, Prakash M, Apostolopoulos V (2017) The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas 96:58–71
Miranda S, Le Cam-Duchez V, Benichou J, Donnadieu N, Barbay V, Le Besnerais M, Delmas F-X, Cuvelier A, Lévesque H, Benhamou Y (2017) Adjusted value of thromboprophylaxis in hospitalized obese patients: a comparative study of two regimens of enoxaparin: the ITOHENOX study. Thromb Res 155:1–5
Morris S, Cerceo E (2020) Trends, epidemiology, and management of multi-drug resistant gram-negative bacterial infections in the hospitalized setting. Antibiotics 9(4):196
Mosquera RA, De Jesus-Rojas W, Stark JM, Yadav A, Jon CK, Atkins CL, Samuels CL, Gonzales TR, McBeth KE, Hashmi SS (2018) Role of prophylactic azithromycin to reduce airway inflammation and mortality in a RSV mouse infection model. Pediatr Pulmonol 53(5):567–574
Mounts A, De La Rocque S, Fitzner J, Garcia E, Thomas H, Brown D, Schuster H, Vandemaele K, Esmat H, Eremin S (2013) The early response to a novel coronavirus in the Middle East. EMHJ-East Mediterr Health J 19(1):S19–S25
Muralidharan N, Sakthivel R, Velmurugan D, Gromiha MM (2021) Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. J Biomol Struct Dyn 39(7):2673–2678
Noreddin AM, Elkhatib WF (2010) Levofloxacin in the treatment of community-acquired pneumonia. Expert Rev Anti Infect Ther 8(5):505–514
Noronha L, Arasu A, Crook J (2021) 220 Use of paracetamol for closure of patent ductus arteriosus in premature infants in a level 3 NICU. BMJ Spec J. https://doi.org/10.1136/bmjpo-2021-RCPCH.119
Nutescu EA, Burnett A, Fanikos J, Spinler S, Wittkowsky A (2016) Pharmacology of anticoagulants used in the treatment of venous thromboembolism. J Thromb Thrombolysis 41(1):15–31
Odone A, Delmonte D, Scognamiglio T, Signorelli C (2020) COVID-19 deaths in Lombardy, Italy: data in context. The Lancet Public Health 5(6):e310
Organization W. H. (2020a) Clinical management of COVID-19: interim guidance. World Health Organization
Organization W.H. (2020b) Multisystem inflammatory syndrome in children and adolescents with COVID-19: scientific brief. World Health Organization
Owen DR, Allerton CM, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, Cardin RD, Carlo A, Coffman KJ (2021) An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 374(6575):1586–1593
Ozen M, Xie H, Shin N, Al Yousif G, Clemens J, McLane MW, Lei J, Burd I (2020) Magnesium sulfate inhibits inflammation through P2X7 receptors in human umbilical vein endothelial cells. Pediatr Res 87(3):463–471
Pahan P, Pahan K (2020) Smooth or risky revisit of an old malaria drug for COVID-19? J Neuroimmune Pharmacol 15:174–180
Palmer BF, Clegg DJ (2016) Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. https://doi.org/10.1152/advan.00121.2016
Palmer BF, Clegg DJ (2019) Physiology and pathophysiology of potassium homeostasis: core curriculum 2019. Am J Kidney Dis 74(5):682–695
Park M, Cook AR, Lim JT, Sun Y, Dickens BL (2020a) A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med 9(4):967
Park S-J, Yu K-M, Kim Y-I, Kim S-M, Kim E-H, Kim S-G, Kim EJ, Casel MAB, Rollon R, Jang S-G (2020b) Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 infection in ferrets. Mbio 11(3):e01114-01120
Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, Baghbanzadeh M, Aghamohammadi N, Zhang W, Haque U (2020) The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol 49(3):717–726
Pham TD, Ziora ZM, Blaskovich MA (2019) Quinolone antibiotics. MedChemComm 10(10):1719–1739
Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung S-H (2016) An overview of severe acute respiratory syndrome–coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J Med Chem 59(14):6595–6628
Podder V, Sadiq NM (2019) Levofloxacin. StatPearls, pp 1–2. https://europepmc.org/article/nbk/nbk545180
Polosa R, Blackburn MR (2009) Adenosine receptors as targets for therapeutic intervention in asthma and chronic obstructive pulmonary disease. Trends Pharmacol Sci 30(10):528–535
Post L, Marogi E, Moss CB, Murphy RL, Ison MG, Achenbach CJ, Resnick D, Singh L, White J, Boctor MJ, Welch SB, Oehmke JF (2021) SARS-CoV-2 surveillance in the Middle East and North Africa: longitudinal trend analysis. J Med Internet Res 23(1):e25830
Rabe K, Magnussen H, Dent G (1995) Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants. Eur Respir J 8(4):637–642
Ramamoorthy S, Cidlowski JA (2016) Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin 42(1):15–31
Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, Satta G, Cooke G, Holmes A (2020) Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 71(9):2459–2468
Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, Leon WRM, Krencik R, Ullian EM, Spatazza J (2016) Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci 113(50):14408–14413
Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Rawling M, Savory E, Stebbing J (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (london, England) 395(10223):e30
Rosa SGV, Santos WC (2020) Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica 44:e40
Rothengatter W, Zhang J, Hayashi Y, Nosach A, Wang K, Oum TH (2021) Pandemic waves and the time after covid-19–consequences for the transport sector. Transp Policy 110:225–237
Rubio-Rivas M, Forero CG, Mora-Luján JM, Montero A, Formiga F, Homs NA, Albà-Albalate J, Sánchez L, Rello J, Corbella X (2021) Beneficial and harmful outcomes of tocilizumab in severe COVID-19: a systematic review and meta-analysis. Pharmacother J Hum Pharmacol Drug Ther 41(11):884–906
Russell CD, Millar JE, Baillie JK (2020) Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet 395(10223):473–475
Sabry W, Elemary M, Burnouf T, Seghatchian J, Goubran H (2020) Vitamin B12 deficiency and metabolism-mediated thrombotic microangiopathy (MM-TMA). Transfus Apheres Sci 59(1):102717
Saha A, Sharma AR, Bhattacharya M, Sharma G, Lee S-S, Chakraborty C (2020) Probable molecular mechanism of remdesivir for the treatment of COVID-19: need to know more. Arch Med Res 51(6):585–586
Saito S, Asai Y, Matsunaga N, Hayakawa K, Terada M, Ohtsu H, Tsuzuki S, Ohmagari N (2020) First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics: comparison of the two COVID-19 waves in Japan. J Infect 82(4):84–123
Saravolatz LD, Depcinski S, Sharma M (2022) Molnupiravir and nirmatrelvir-ritonavir: oral COVID antiviral drugs. Clin Infect Dis. https://doi.org/10.1093/cid/ciac180
Sawaya T, Ballouz T, Zaraket H, Rizk N (2020) Coronavirus disease (COVID-19) in the Middle East: a call for a unified response. Front Public Health 8:209
Schrezenmeier E, Dörner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 16(3):155–166
Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ (2017) JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 16(12):843–862
Scroggs SL, Offerdahl DK, Flather DP, Morris CN, Kendall BL, Broeckel RM, Beare PA, Bloom ME (2021) Fluoroquinolone antibiotics exhibit low antiviral activity against SARS-CoV-2 and MERS-CoV. Viruses 13(1):8
Seaton RA, Gibbons CL, Cooper L, Malcolm W, McKinney R, Dundas S, Griffith D, Jeffreys D, Hamilton K, Choo-Kang B (2020) Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect 81(6):952–960
Shah A, Marks PW, Hahn SM (2020) Unwavering regulatory safeguards for COVID-19 vaccines. JAMA 324(10):931–932
Shang Y, Pan C, Yang X, Zhong M, Shang X, Wu Z, Yu Z, Zhang W, Zhong Q, Zheng X (2020) Management of critically ill patients with COVID-19 in ICU: statement from front-line intensive care experts in Wuhan, China. Ann Intens Care 10(1):1–24
Shankar SS, Brater DC (2003) Loop diuretics: from the Na-K-2Cl transporter to clinical use. Am J Physiol-Renal Physiol 284(1):F11–F21
Shannon A, Selisko B, Le N, Huchting J, Touret F, Piorkowski G, Fattorini V, Ferron F, Decroly E, Meier C (2020d) Favipiravir strikes the SARS-CoV-2 at its Achilles heel, the RNA polymerase. BioRxiv 91:e00487
Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO (2020) Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 11(1):1–14
Sims JT, Krishnan V, Chang C-Y, Engle SM, Casalini G, Rodgers GH, Bivi N, Nickoloff BJ, Konrad RJ, de Bono S (2021) Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J Allergy Clin Immunol 147(1):107–111
Singh AK, Singh A, Singh R, Misra A (2022) An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19. Diabetes Metab Syndr 16(2):102396
Sisti N, Valente S, Mandoli GE, Santoro C, Sciaccaluga C, Franchi F, Cameli P, Mondillo S, Cameli M (2021) COVID-19 in patients with heart failure: the new and the old epidemic. Postgrad Med J 97(1145):175–179
Smit F, Oelofse A, Linegar A, Hanekom H, Botes L, Turton E (2020) Supplemental oxygen therapy in COVID-19. SA Heart 17(3):324–328
Spinner CD, Gottlieb RL, Criner GJ, López JRA, Cattelan AM, Viladomiu AS, Ogbuagu O, Malhotra P, Mullane KM, Castagna A (2020) Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 324(11):1048–1057
Srinivasa A, Tosounidou S, Gordon C (2017) Increased incidence of gastrointestinal side effects in patients taking hydroxychloroquine: a brand-related issue? J Rheumatol 44(3):398–398
Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P (2020) COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 20(4):400–402
Steffens NA, Zimmermann ES, Nichelle SM, Brucker N (2021) Meropenem use and therapeutic drug monitoring in clinical practice: a literature review. J Clin Pharm Ther. https://doi.org/10.1111/jcpt.13369
Surveillances V (2020) The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly 2(8):113–122
Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, Iwatsuki-Horimoto K, Halfmann P, Watanabe S, Maeda K (2022) Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA. 2. N Engl J Med 386(15):1475–1477
Tan Q, Duan L, Ma Y, Wu F, Huang Q, Mao K, Xiao W, Xia H, Zhang S, Zhou E (2020) Is oseltamivir suitable for fighting against COVID-19: In silico assessment, in vitro and retrospective study. Bioorg Chem 104:104257
Tang C-F, Ding H, Jiao R-Q, Wu X-X, Kong L-D (2020) Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur J Pharmacol 886:173546
Taştemur Ş, Ataseven H (2020) Is it possible to use proton pump inhibitors in COVID-19 treatment and prophylaxis? Med Hypotheses 143:110018
To Y, Ito K, Kizawa Y, Failla M, Ito M, Kusama T, Elliott WM, Hogg JC, Adcock IM, Barnes PJ (2010) Targeting phosphoinositide-3-kinase-δ with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182(7):897–904
Townsend L, Hughes G, Kerr C, Kelly M, O’Connor R, Sweeney E, Doyle C, O’Riordan R, Martin-Loeches I, Bergin C (2020) Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC-Antimicrob Resist 2(3):071
Tran DH, Sugamata R, Hirose T, Suzuki S, Noguchi Y, Sugawara A, Ito F, Yamamoto T, Kawachi S, Akagawa KS (2019) Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A (H1N1) pdm09 virus infection by interfering with virus internalization process. J Antibiot 72(10):759–768
Trogen B, Oshinsky D, Caplan A (2020) Adverse consequences of rushing a SARS-CoV-2 vaccine: implications for public trust. JAMA 323(24):2460–2461
Trougakos IP, Stamatelopoulos K, Terpos E, Tsitsilonis OE, Aivalioti E, Paraskevis D, Kastritis E, Pavlakis GN, Dimopoulos MA (2021) Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J Biomed Sci 28(1):1–18
Tullu M (2009) Oseltamivir. J Postgrad Med 55(3):225
Ubagai T, Koshibu Y, Koshio O, Ono Y, Nakaki T (2009) Downregulation of immunomodulator gene expression in LPS-stimulated human polymorphonuclear leukocytes by the proton pump inhibitor lansoprazole. J Infect Chemother 15(6):374–379
Uzunova K, Filipova E, Pavlova V, Vekov T (2020) Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed Pharmacother 131:110668
V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V (2021) Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 19(3):155–170
van Gameren M, Lemmert M, Wilschut J, Daemen J, De Jaegere P, Zijlstra F, Van Mieghem N, Diletti R (2018) An update on the use of anticoagulant therapy in ST-segment elevation myocardial infarction. Expert Opin Pharmacother 19(13):1441–1450
Wandall J (1992) Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut 33(5):617–621
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y (2020a) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069
Wang D, Yin Y, Hu C, Liu X, Zhang X, Zhou S, Jian M, Xu H, Prowle J, Hu B (2020b) Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care 24(1):1–9
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q (2020c) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395(10236):1569–1578
Ward P, Small I, Smith J, Suter P, Dutkowski R (2005) Oseltamivir (Tamiflu®) and its potential for use in the event of an influenza pandemic. J Antimicrob Chemother 55(suppl_1):5–21
Wei G, Sun R, Xu T, Kong S, Zhang S (2019) Aminophylline promotes mitochondrial biogenesis in human pulmonary bronchial epithelial cells. Biochem Biophys Res Commun 515(1):31–36
WHO (2022) WHO coronavirus (COVID-19) dashboard. Retrieved 17 Jul 2022
Worldometer/Saudi Arabia United Nations (2022) Department of Economic and Social Affairs, Population Division. Retrieved 17 July 2022
Wu Y-H, Tseng C-K, Lin C-K, Wei C-K, Lee J-C, Young K-C (2018) ICR suckling mouse model of Zika virus infection for disease modeling and drug validation. PLoS Negl Trop Dis 12(10):e0006848
Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180(7):934–943
Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang Y, Zhang W (2020) Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 324(10):951–960
Xie Y, Cao S, Dong H, Li Q, Chen E, Zhang W, Yang L, Fu S, Wang R (2020) Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect 81(2):318
Xing J, Yin X, Chen D (2018) Rivaroxaban versus enoxaparin for the prevention of recurrent venous thromboembolism in patients with cancer: a meta-analysis. Medicine 97(31):e11384
Yan D, Liu X-Y, Zhu Y-N, Huang L, Dan B-T, Zhang G-J, Gao Y-H (2020) Factors associated with prolonged viral shedding and impact of lopinavir/ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. Eur Respir J 56(1):2000799
Yao TT, Qian JD, Zhu WY, Wang Y, Wang GQ (2020) A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—a possible reference for coronavirus disease-19 treatment option. J Med Virol 92(6):556–563
Ye X, Luo Y, Xia S, Sun Q, Ding J, Zhou Y, Chen W, Wang X, Zhang W, Du W (2020) Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur Rev Med Pharmacol Sci 24(6):3390–3396
Yoo JY, Dutra SVO, Fanfan D, Sniffen S, Wang H, Siddiqui J, Song H-S, Bang SH, Kim DE, Kim S (2020) Comparative analysis of COVID-19 guidelines from six countries: a qualitative study on the US, China, South Korea, the UK, Brazil, and Haiti. BMC Public Health 20(1):1–16
Yuki K, Fujiogi M, Koutsogiannaki S (2020) COVID-19 pathophysiology: a review. Clin Immunol 215:108427
Zafar Gondal A, Zulfiqar H (2019) Aminophylline. StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK545175
Zaim S, Chong JH, Sankaranarayanan V, Harky A (2020) COVID-19 and multi-organ response. Curr Prob Cardiol 15:100618
Zeng S, Meng X, Huang Q, Lei N, Zeng L, Jiang X, Guo X (2019) Spiramycin and azithromycin, safe for administration to children, exert antiviral activity against enterovirus A71 in vitro and in vivo. Int J Antimicrob Agents 53(4):362–369
Zhang XW, Yap YL (2004) The 3D structure analysis of SARS-CoV S1 protein reveals a link to influenza virus neuraminidase and implications for drug and antibody discovery. J Mol Struct (thoechem) 681(1–3):137–141
Zhang T, Liu D, Tian D, Xia L (2020e) The roles of nausea and vomiting in COVID-19: did we miss something? J Microbiol Immunol Infect. https://doi.org/10.1016/j.jmii.2020.10.005
Zhao SZ, Wong JYH, Wu Y, Choi EPH, Wang MP, Lam TH (2020) Social distancing compliance under COVID-19 pandemic and mental health impacts: a population-based study. Int J Environ Res Public Health 17(18):6692
Zheng J (2020) SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci 16(10):1678
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062
Zhou S, Hill CS, Sarkar S, Tse LV, Woodburn BM, Schinazi RF, Sheahan TP, Baric RS, Heise MT, Swanstrom R (2021) β-d-N 4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells. J Infect Dis 224(3):415–419
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R (2020f) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. https://doi.org/10.1056/NEJMoa2001017
Acknowledgements
Authors would like to thank Dr. Ahmed Abd Elmaula Shaffik and Dr. Aliaa Negm for providing us all necessary data about the treatments and protocols.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Writing-original draft preparation, EE, WAN, SAE-S, and AZ; writing-review and editing EE, WAN, SAE-S, and AZ; Data collection and analysis: EE, and WAN All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elekhnawy, E., Negm, W.A., El-Sherbeni, S.A. et al. Assessment of drugs administered in the Middle East as part of the COVID-19 management protocols. Inflammopharmacol 30, 1935–1954 (2022). https://doi.org/10.1007/s10787-022-01050-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01050-7