Abstract
The chronic respiratory non-communicable diseases, asthma and chronic obstructive pulmonary disease (COPD) are among the leading causes of global mortality and morbidity. Individuals suffering from these diseases are particularly susceptible to respiratory infections caused by bacterial and/or viral pathogens, which frequently result in exacerbation of symptoms, lung function decline, frequent hospital emergency visits and increased socioeconomic burden. Human rhinoviruses (HRV) remain the major viral pathogen group implicated in exacerbations of both asthma and COPD. The rhinoviral entry into the host lung epithelium is facilitated primarily by the adhesion site (“receptor”) intercellular adhesion molecule-1 (ICAM-1), coincidentally expressed on the respiratory epithelium in these conditions. Multiple observations of increased airway ICAM-1 protein in asthmatics, smokers and smoking-related COPD have been recorded in the literature. However, the lack of robust therapies for COPD in particular has triggered a renewed interest in assessing receptor antagonism-based anti-viral strategies for treatment of intercurrent viral infections in those with pre-existing chronic lung diseases. Given the crucial role ICAM-1 plays in facilitating HRV adhesion and, thus, transmissibility to the host respiratory system, as well as the up-regulation of ICAM-1 by smoking, we summarize the role of HRV in smoking-induced COPD and especially highlight the role of ICAM-1 in epithelial viral adhesion and chronic lung disease progression. Further, the review also sheds light specifically on evolving precision therapeutic strategies in blocking ICAM-1 for preventing viral adhesion and exacerbations of COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The overall prevalence and clinico-social burden of non-communicable chronic lung diseases (CLDs), primarily asthma and chronic obstructive pulmonary disease (COPD), is increasing constantly from an already high base. Notably, these CLDs are amongst the top five causes of morbidity and mortality worldwide, affecting more than half a billion individuals and accounting for over 3 million deaths annually (WHO 2014). Asthma is a complex and heterogeneous lung disease, predominantly characterized by airway inflammation, smooth muscle hyperplasia and airway hyperreactivity (Papi et al. 2018). Allergic asthma is the most typical asthma type that exacerbates in response to non-specific environmental triggers such as microbial infections, non-infectious exposures (mould, dust mite, pollen etc.) and host genetic makeup (Papi et al. 2018). Similarly, COPD is also a complex and heterogenous lung disease caused by long-term exposure to various toxic substances, especially cigarette smoke and biomass smoke. This repeated and chronic exposure of the airways and lungs to the deleterious constituents of noxious particles and gases results in mainly small airway remodeling and destruction, leading to the development of poorly reversible airflow obstruction (Global Strategy for the Diagnosis 2015).
Although both asthma and COPD are non-communicable diseases, there is growing evidence of an association between both asthma and COPD clinical course and several major communicable respiratory illnesses, especially COPD patients' vulnerability towards frequent viral/bacterial infections. Patients who have asthma and COPD often exhibit acute exacerbations (AE), which are described as a sudden worsening of symptoms frequently requiring specialist consultations, prescription changes, emergency GP or hospital visits and hospitalization. These AEs are significantly associated with several complications such as pneumonia, increased risk of morbidity/mortality, the triggering of a rapid decline in the lung function parameters, all of which elevate healthcare costs and compromise lifestyle (Qureshi et al. 2014). AEs occur in more than half of CLD patients despite adherence to prescribed disease management strategies.
The majority of AEs are caused by infective triggers, either bacterial or viral or both (together or sequentially). `Non-infective triggers (e.g., environmental pollutants) are also implicated, but may account for a fraction of these exacerbations. For instance, the polymerase chain reaction (PCR) based methodologies have confirmed viral pathogens in up to 77% of asthma (Johnston et al. 2005) and 60% of COPD exacerbations (Sykes et al. 2007). Importantly, Human Rhinovirus (HRV/RV) is the primary trigger for exacerbations of both asthma and COPD (Wedzicha 2004), as well as major viral pathogen implicated in childhood respiratory infections (Taylor et al. 2017). Other viruses such as coronavirus, influenza and parainfluenza viruses, and Respiratory Syncytial Virus (RSV) play a part but have a relatively minor role. Indeed, the prevalence of HRV and its viral load were higher in patients with COPD exacerbations than those with stable disease (Seemungal et al. 2001; Wedzicha 2004; George et al. 2014). Viral-induced AE episodes are also associated with more severe exacerbations and accompanying symptoms as well as greater likelihood of hospital admissions and longer recovery time (Seemungal et al. 2000). Moreover, in a clinical study involving experimental airway HRV infection in COPD patients, the HRV load correlated with sputum neutrophil counts and interleukin (IL)-8 production (Ledford et al. 2004). This indicates the activation of innate immune responses which are known to be associated with clinical presentation of HRV-induced AEs. The attempts to type HRV isolated from the airways in exacerbations of COPD or asthma have only commenced quite recently. One study reported that HRV-C was strongly associated with more severe exacerbations in asthma, including those requiring hospitalization (Cox et al. 2013). Another study identified HRV-A and HRV-C as the HRVs most predominantly isolated from exacerbating COPD or asthma patients, although HRV-A had a greater number of genotypes in both COPD and asthma (Ko et al. 2019). Moreover, there was no association observed between the various types of HRVs in either AECOPD or asthma exacerbations with hospital LOS, readmission or mortality (Ko et al. 2019). Further research is warranted to profile the cadherin-related family member 3 (CDHR3) receptor adhesion site for HRV-C in lung tissues from chronic lung diseases to devise further preventive anti-viral strategies.
A few proposed mechanisms increase viral susceptibility in patients with CRDs including immune dysregulation, mucociliary dysfunction, and notably, activated epithelial exhibiting increased expression of viral adhesion receptors. All these mechanisms combined potentially lead to more frequent episodes of “acute bronchitis” in patients with CRDs than in healthy individuals (Mallia et al. 2011). Notably, the up-regulation and modulation of surface-expressed intercellular adhesion molecule -1 (ICAM-1), either by the external stimuli (i.e., cigarette smoke) or by the HRV itself, is perhaps the predominant mechanism for HRV adherence (at least for > 60% of the HRV major group) to airway/lung epithelia. Crucially, ICAM-1 has also been shown to regulate inflammatory cell recruitment and activation, most notably macrophages and lymphocytes. Paradoxically, increased expression of ICAM-1 also seems to enhance the susceptibility to infection that is primarily viral. However, several bacterial and parasitic pathogens also utilize ICAM-1 for adhesion and invasion, such as Haemophilus influenzae and Plasmodium falciparum. Taken together, it is evident that HRV infection leads to AEs in chronic lung diseases, especially COPD as an exemplar perhaps for other CRDs, and the lack of effective preventive and/or treatment approaches for AEs currently means that more research aimed directly towards producing therapeutic blockade of this pathway is urgently warranted. Specifically, blocking viral adhesion to respiratory epithelia holds great promise to mitigate viral-induced AEs in CRDs. We will summarize the crucial role of HRV in inducing AEs in CRDs, along with the key role of ICAM-1 in facilitating the adhesion and invasion of HRV in respiratory epithelia. We will then summarize the current understanding of ICAM-1 in smokers, as well as those with established CRDs. Finally, we will briefly discuss the role of ICAM-1 in other diseases, e.g., malaria and COVID-19, and the potential of anti-ICAM-1 therapies as illustrated in mitigating microbial infections in pre-clinical models.
Human rhinovirus (HRV)
HRV, initially isolated from individuals expressing common cold symptoms in the early 1950s, represents a serologically diverse group of pathogenic picornaviruses (> 160 serotypes) (Bochkov and Gern 2016). Depending upon the phylogenetic relationships and sequence divergence, HRVs were classified into three serotypes: HRV-A, HRV-B, and HRV-C (McIntyre et al. 2013; Kuroda et al. 2015).
HRVs are RNA viruses, containing positive-sense, non-enveloped, single-stranded RNA (ssRNA), and placed under the Picornaviridae family. Genetic makeup constitutes approximately 7200 base pairs, ranging up to 27 nm in diameter. The viral genome consists of a single functional gene, whose translated protein is cleaved by virally encoded proteases to produce 11 viral proteins (VP). Among these four proteins, VP1, VP2, VP3, and VP4 form the outer capsid that encases the genetic material. Other nonstructural proteins are involved in viral genome replication assembly. The antigenic diversity of the virus is accounted for by the proteins VP1, VP2, and VP3, while VP4 anchors the RNA core to the capsid. Each of the four capsid proteins has 60 copies, giving the virion an icosahedral structure with a canyon in VP1 that serves as a site of attachment to cell surface receptors/adhesion sites.
As previously stated, the “major group” of known HRV serotypes, i.e. > 60% of known HRV strains, utilize the cell surface receptor ICAM-1, while other serotypes use low-density lipoprotein receptor (LDLR) as a point of entry. Studies also show that some HRVs belonging to major groups use heparan sulfate as an additional receptor (Palmenberg et al. 2010). HRV-C, in particular, uses cadherin-related family member 3 (CDHR3) to achieve cellular entry (Staunton et al. 1989; Bochkov et al. 2015) (Fig. 1).
Major adhesion receptors for human rhinovirus. intercellular adhesion molecule-1 (ICAM-1) acts as the primary receptor for the major sub-group of HRV (HRV-A and HRV-B), while low-density lipoprotein receptor (LDLR) is utilized by the minor sub-group of HRV (HRV-A and HRV-B). HRV-C serotypes primarily bind to the CDHR3 receptors
Respiratory infections by HRV
HRVs, especially HRV-A and HRV-C, chiefly cause upper respiratory tract (URT) infections but may also infect the lower respiratory tract (LRT), in both children and adults (Hayden 2004; Winther 2011). Several studies using molecular techniques (RT-PCR) to detect HRVs have established respiratory viral infections as a major risk factor for acute exacerbations of asthma and COPD in adults and children, frequently leading to hospital admission. HRV is also associated with wheezing illnesses in children, cardiopulmonary disease, and fatal pneumonia in immune-compromised patients. While other respiratory viruses, such as influenza virus and respiratory syncytial virus (RSV), destroy airway epithelial cells, HRV is not associated with the destruction of the epithelial lining in nasal biopsies from subjects with natural ‘colds’ (n = 29), determined by light or scanning electron microscopy (Winther et al. 1984).
Malmstrom et al. observed that HRV was frequently found in the lower airways in infants (n = 201; age 3–26 months) with recurrent respiratory symptoms. The presence of HRV was associated with increased resistance to airflow, which predicted subsequent asthma in later life (Malmstrom et al. 2006). HRV was detected by both immunohistochemistry and the indirect in-situ RT-PCR (n = 20) in more bronchial biopsies of asthmatics (73%) than in non-asthmatic subjects (22%). Again, the presence of HRV was associated with more airway obstruction, higher numbers of blood eosinophils and leukocytes, and eosinophilia in bronchial mucosa (Wos et al. 2008). Experimental HRV infection of human participants (n = 19) reported the presence of virus in cells from nasal lavage (all subjects), sputum (all subjects), bronchoalveolar lavage (26%), bronchial brushings (28%), and biopsy specimens (36%) (Mosser et al. 2005).
RNA-sequencing (RNA-seq) analysis of HRV-infected air–liquid interface differentiated human airway epithelial cell cultures from 6 asthmatic and six healthy donors identified specific sets of host gene transcriptions associated with increased inflammatory pathways, and changes in epithelial structure (remodeling) and cilium assembly and function (involving CCL5, CXCL10, CX3CL1, MUC5AC, CDHR3, and CCRL1) (Bai et al. 2015).
As stated, HRVs (HRV-A and HRV-C) are also important in triggering acute exacerbations in COPD (AECOPD). Two prospective studies of patients hospitalized with AECOPD found that picornaviruses were the most common viral infection detected by RT-PCR in nasal lavage fluid, nasopharyngeal swab, or induced sputum specimens, occurring in 36–50% of cases (Rohde et al. 2003; Kherad et al. 2010). HRV prevalence and load at exacerbation were significantly higher than in the stable state (53.3% versus 17.2%). COPD patients detected with HRV at exacerbation also had a higher exacerbation frequency per year than patients without HRV. However, secondary bacterial infection was common at about 14 days post-HRV infection (George et al. 2014). Johnston et al. assessed the importance of RV in AECOPD and reported that “cold-like” symptoms could reliably predict exacerbations in more than 80% of patients, despite less than half of patients apparently being infected. Nevertheless, patients with viral detection did present with more severe symptoms than other groups (Johnston et al. 2017).
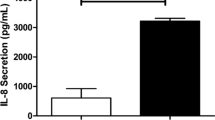
Mallia et al. experimentally infected 13 COPD patients and 13 non-obstructed smoker controls with HRV. They reported significant reductions in post-bronchodilator peak expiratory flow and carbon monoxide diffusion capacity from baseline to HRV infection in COPD patients but no change in controls. The authors also found increased neutrophil elastase and IL-8 levels in sputum supernatants and IL-6 levels in bronchoalveolar lavage (BAL) in COPD patients infected with HRV. Although alveolar macrophages from BAL fluid have demonstrated inadequate IFN-β responses in COPD patients compared to controls, there was only a non-significant trend toward reduced IFN-α and IFN-γ production levels. Interestingly, there was no change in TNF-α levels in either group (Mallia et al. 2011). HRV also impairs the phagocytic ability of macrophage in COPD (Finney et al. 2017). Crucially, alveolar macrophages obtained from bronchoalveolar lavage fluid of COPD patients and pre-treated with HRV (P56) showed significantly diminished ability to phagocytose fluorescently labeled heat-killed Haemophilus influenzae but not Streptococcus pneumoniae. However, this was not observed in case of alveolar macrophages obtained from control participants (Finney et al. 2017).
Importantly, it is crucial to investigate and validate important biomarkers indicative of elevated risk of HRV infections in COPD, which could inform clinicians about ‘at risk’ individuals who may need personalized management strategies. For instance, Quint et al. examined serum interferon gamma-induced protein (IP)-10 as a potential biomarker of HRV infection and reported that COPD patients (n = 136) had higher serum IP-10 levels than age-matched controls without COPD (n = 70), which also correlated with sputum HRV viral load. In the same study, post 2 years of follow-up, the authors reported that serum IP-10 levels increased significantly from baseline in HRV-positive AECOPD. However, no change was observed during HRV-negative exacerbations (Quint et al. 2010). Collectively, the evidence strongly indicate the substantial diversity of HRVs and their ability to utilize host receptors such as ICAM-1 for invasion and infection.
Intercellular adhesion molecule-1 (ICAM-1)
Human ICAM-1 (CD54; a transmembrane glycoprotein) is a member of the immunoglobulin (Ig) superfamily that contains five Ig-like domains, a transmembrane domain, and a short cytoplasmic tail (Springer 1990). It is thought to be expressed constitutively on a wide variety of cells (including leukocytes, endothelial cells and respiratory epithelial cells) (Roebuck and Finnegan 1999), but it is also further inducible by the inflammatory mediators TNF-α, IL-1β and IFN-γ, and inhibited by glucocorticoids (Hubbard and Rothlein 2000). Membrane-bound integrin receptors such as LFA-1 and Mac-1 on leukocytes, CD43, soluble fibrinogen and hyaluronan, matrix factor hyaluronan, some HRVs (~ 60% HRV-A), and Plasmodium falciparum malaria-infected erythrocytes serve as ligands for ICAM-1.
Physiologically, ICAM-1 plays several key roles. ICAM-1 plays a pivotal role in maintaining cell–cell interactions and facilitates leukocyte per-endothelial transmigration from blood into inflamed tissues, possibly leading to initiation of antigen-specific immune responses (van de Stolpe and van der Saag 1996; Lehmann et al. 2003). ICAM-1 functions in the T-cell-mediated host defense system, and also as a co-stimulatory molecule on antigen-presenting cells to activate MHC class II restricted T cells, and on other cell types in association with MHC class I to activate cytotoxic T cells.
The regulation of ICAM-1 at the transcriptional level has been reviewed (van de Stolpe and van der Saag 1996). It has been suggested that the expression of ICAM-1 is transcriptionally regulated through one of four pathways: NF-kB, gamma interferon-Janus kinase/signal transduction-activating transcription (JAK/STAT), mitogen-activated protein (MAP) kinase/activator protein 1 (AP1), and, indirectly, protein kinase C (PKC) (Roebuck and Finnegan 1999). The ICAM-1 promoter contains several enhancer elements, but notably nuclear factor-kappa B (NF-κB), which mediates the effects of 12-O-tetradecanoylphorbol-13-acetate (TPA), IL-1, lipopolysaccharide (LPS), TNF-α, and glucocorticoids. Because of this complexity, the regulation of ICAM-1 expression is cell-specific and depends upon the availability of cytokine/hormone receptors, signal transduction pathways, transcription factors, and post-transcriptional modification (Jahnke et al. 1995). Increased nasal ICAM-1 expression has been reported on the respiratory epithelium of allergic subjects (Gorska-Ciebiada et al. 2006). Results from in vitro primary cell culture models confirm that HRV infects lower airway tissues and suggested viral replication in lower airway epithelial cells (Schroth et al. 1999), and a significant role for ICAM-1 in the infective process (Terajima et al. 1997).
Human bronchial epithelial cells exposed to airway secretions from subjects with bronchiectasis showed increased ICAM-1 mRNA and protein levels in TNF-α-dependent manner (Chan et al. 2008). Moreover, ICAM-1 is subsequently shed by cells and is detected in plasma as soluble (s)ICAM-1, and is found to be increased in many pathological conditions, including malignancies (e.g., melanoma and lymphomas), many inflammatory disorders (e.g., asthma and autoimmune disorders), atherosclerosis, ischemia, certain neurological disorders, and allogeneic organ transplantation (van de Stolpe and van der Saag 1996).
In addition to all of the above, there is strong evidence that ICAM-1 serves as an essential receptor for a major group of human rhinoviruses (HRV) (Greve et al. 1989), for some coxsackieviruses (Marlin et al. 1990), and several bacterial pathogens including the major respiratory opportunistic pathogen, non-typeable Haemophilus influenzae via type IV pilus (Novotny and Bakaletz 2016). In summary, ICAM-1 is one of the most crucial conserved human receptors that is required for a number of key physiological processes, and several microbes have evolved to utilize ICAM-1 as well as other host epithelial receptors to adhere, invade and infect the host.
Effect of cigarette smoke (CS) on ICAM-1 expression
CS exposure is one of the most important risk factors for COPD. Related to increased levels of specific cytokines and subsequent up-regulation of ICAM-1, there is a predisposition in smokers and COPD patients to frequent HRV infection and so AEs in COPD patents. Thus, small-airway epithelial cells from physiologically normal smokers (n = 22) showed significantly higher levels of IL-8 and ICAM-1 mRNA than the non-smokers (n = 17), suggesting that CS smoke is likely to increase epithelial ICAM-1 expression (Takizawa et al. 2000). Clinical studies have demonstrated an elevated level of serum soluble (s)ICAM-1 in COPD-smokers (n = 142) compared to non-COPD active smokers (n = 55) (Lopez-Campos et al. 2012). An in vitro study using primary human bronchial epithelial cells (HBEC) from COPD-smokers and non-smoking control participants reported a significantly increased release of sICAM-1 and IL-1β in cells exposed to CS, compared to control air-exposed cells. In addition, exposure to CS increased permeability of cell culture layers from currently smoking participants (Rusznak et al. 2000). As stated in earlier sections, IL-1β further increases ICAM-1 expression on both airway epithelial cells and endothelial cells.
HRV itself upregulates membrane-bound ICAM-1 expression via a NF-κβ-dependent mechanism in both primary bronchial epithelial cells (12-fold) and A549 cloned respiratory epithelial cells (threefold) (Papi and Johnston 1999). Anti-ICAM-1 antibody (14C11) administered topically or systemically, has been shown to inhibit major group HRV replication in vitro, as well as HRV-induced inflammation, pro-inflammatory cytokine induction and lung virus RNA levels in a mice model (Traub et al. 2013), confirming a potentially crucial role for ICAM-1 as a specific viral facilitator. However, interestingly and perhaps disappointingly, anti-ICAM-1 antibody (14C11) did not prevent cell adhesion via human ICAM-1/LFA-1 interactions in vitro, suggesting the epitope targeted by 14C11 was specific for viral entry.
Although most attention has been on HRV, evidence indicates that ICAM-1 also serves as an adhesion molecule for Haemophilus influenzae (NTHi, via bacterial P5 fimbriae), which is the main bacterial pathogen in COPD, again especially in AECOPD (Sethi and Murphy 2008). Thus, ICAM-1 is an attractive target to block not only for virus-receptor binding but also to check ICAM-1-mediated NTHi adhesion to respiratory cells.
The role of ICAM-1 in asthma
The role of ICAM-1 in asthma has been extensively studied since the 1990s. Wegner and colleagues first discovered the contribution of ICAM-1 to eosinophil migration and increased airway responsiveness (Wegner et al. 1990). Several subsequent studies further validated the potential link between ICAM-1 expression and asthma pathogenesis (Ciprandi et al. 1994; Stanciu and Djukanovic 1998; Lin et al. 2003). ICAM-1 and LFA-1 protein are constitutively expressed on the membrane of various human cells, including T cells and bronchial endothelial cells (Bentley et al. 1993). The expression of ICAM-1 is known to be elevated in asthma, and this favors the binding of ICAM-1-positive cells to LFA-expressing cells (Drake et al. 2018). The adherence of ICAM-1 and LFA-1 proteins then results in uropod formation; these contain regrouped adhesion molecules that facilitate T-cell capture and transmigration into the vascular lumen, resulting in increased T-cell activation and inflammation (Drake et al. 2018). The transmigration of T cells then results in shedding of soluble(s)-ICAM-1 proteins into the blood circulation, which serves as negative feedback for the ICAM-1/LFA-1 binding process and is reported to inhibit further aggregation and transmigration of T cells into the vasculature (Drake et al. 2018). On the contrary, several studies have questioned the potential role of sICAM-1 as an antagonist for ICAM-1/LFA-1-induced inflammation in asthma, primarily because sICAM-1 is also known for its pro-inflammatory properties which play a significant role in diseases like systemic inflammatory response syndrome and gram-negative pneumonia (Mendez et al. 2011; de Pablo et al. 2013). In addition, one study has previously demonstrated that sICAM-1 facilitates the secretion of eosinophil cationic protein and worsens inflammation in asthma, which is contradictory to its proposed antagonist effect on asthma exacerbations (Chihara et al. 1995).
A transmembrane protein known as orosomucoid-like (ORMDL) 3 has gained significant interest in reinforcing ICAM-1-associated asthmatic inflammation. ORMDL3 is encoded by the ORMDL gene family which is strongly correlated with asthma in genome-wide association studies (Galanter et al. 2008). Importantly, ORMDL3 is expressed on lung, pancreas and kidney (Miller et al. 2012), and notably, one study reported that ORMDL3 enhanced the replication of HRV in cultured human lung epithelial cells, suggesting a potential role in viral-induced asthma AEs (Liu et al. 2020). A possible direct link between ORMDL3 and ICAM-1 may also exist. For instance, Zhang et al. recently reported that ORMDL3 knock-down in cultured lung epithelial cells reduced the expression of ICAM-1(Zhang et al. 2019). Besides ICAM-1, the levels of other inflammatory markers, including IL-1β, were found to be downregulated after ORMDL3 knock-down, indicating its potential role in facilitating ICAM-1-induced asthmatic inflammation (Zhang et al. 2019). Further studies are warranted to validate these findings especially in vivo and ex vivo settings. Nevertheless, the potential role of ICAM-1 in asthma is now widely accepted, so the development of therapeutic approaches targeting these proteins may well be beneficial to reduced inflammation, AHR and asthma exacerbations.
ICAM-1 expression in COPD
Despite the likely importance of ICAM-1 in AECOPD, the expression pattern of this viral adhesin has only recently been elucidated in human COPD airways (Shukla et al. 2017a, b). Briefly, the protein expression of ICAM-1 was upregulated throughout the respiratory tract (both large and small airway epithelium) in smokers but was especially marked in subjects with COPD, even when mild (Shukla et al. 2017a, b). Notably, increased ICAM-1 expression was also found in goblet cells and submucosal glands in the large airway of smokers, but especially so in COPD patients (Shukla et al. 2017a, b), which showed greater staining intensity. Moreover, ICAM-1 expression, both at the mRNA and protein level, was upregulated in cultured bronchial epithelial cells (BEAS-2B) exposed to cigarette smoke extract (Shukla et al. 2017a, b).
These findings taken as a whole may be crucial for understanding the vulnerability of smokers and especially COPD patients to airway viral infections, specifically with major group HRVs (group A and B), but also potentially with NTHi. Another study reported the key role of ICAM-1 in the clearance of NTHi from lungs in an elastase-induced experimental model of COPD/emphysema. Mice treated with elastase demonstrated diminished epithelial ICAM-1 protein levels when compared to control mice, presumably a direct effect of the proteinase, accompanied by increased aggregation of NTHi in elastase affected areas (Pang et al. 2008).
The role of ICAM-1 in other diseases
Remarkably, ICAM-1 also plays a vital role in various other infectious diseases, including tuberculosis and malaria. For instance, the addition of M5 protein that adheres explicitly to ICAM-1 and ICAM-4, or siRNA targeting ICAM-1 decreased infection of cultured THP-1 (a human monocytic leukemia cell line) and murine peritoneal macrophages by Mycobacterium tuberculosis (Bhalla et al. 2015). In the same study, the authors also showed that M5 protein blocked the parasite translocation of red blood cells by Plasmodium falciparum, irrespective of whether the strain is drug-susceptible or resistant (Bhalla et al. 2015). Quite recently, ICAM-1/LFA-1 complex has been shown to play an important role in transmission of Murine Leukemia virus between leucocytes, thus, exploiting the host proteins involved in intercellular interactions and barrier function (Engels et al. 2022). Collectively, it is evident that various microbes exploit host ICAM-1/LFA-1 in cell-to-cell transmission and infection, thus, providing a robust pathway to mitigate microbial infection and persistence. These mechanism, however, should be amenable to novel therapeutic interventions.
ICAM-1 as a potential anti-adhesion therapeutic target
The crucial role of ICAM-1 in facilitating some HRV adhesion has led to investigations in blocking this interaction in the context of HRV infections in in vitro cell culture and pre-clinical animal models (Shukla et al. 2017a, b). One study reported a series of novel small compounds, especially “compound 6”, which inhibited viral multiplication (HRV-A/HRV-B). Furthermore, in silico analyses suggest that the activity of compound 6 was indeed similar to that of pleconaril (Kim et al. 2017). Another compound referred to as 3 k was highly efficacious against multiple strains of HRV in in vitro models (Kim et al. 2018).
Another notable anti-adhesion therapy is highly specific monoclonal antibody (mAB) that could block the adhesion interaction between HRV and ICAM-1. For instance, although intranasal administration of rhinovirus receptor murine monoclonal antibody (RRMA, or mAb 1A6) at a dose of 135 μg/subject in human participants did not reduce the rate of infection, a higher dose (1 mg/subject) was associated with delayed shedding of virus and the onset of “cold-like” symptoms. The higher dosage of RRMA was also associated with a reduction in viral titers (Hayden et al. 1988). In addition, mAb 1A6 was shown to prevent HRV infection both in tissue culture and in an animal model of HRV infection (Colonno 1992).
Current research is focused on effective compounds that could inhibit HRV infectivity in pre-clinical cell culture models. For instance, a novel compound, (E)-(S)-4-((S)-2-pent-4-ynoylamino)-5-((S)-2-oxo-pyrrolidin-3-yl)-pent-2-enoic acid ethyl ester, irreversibly inhibited the HRV 3C protease (Patick et al. 2005). More importantly, this compound was found to be active against a wide range of HRV serotypes (35), as well as HRV clinical isolates (5). The inhibitory activity extended to other picornaviruses (8) (mean EC50: 50–75 nM). The compound was also found safe and non-toxic post a single oral dose (~ 2000 mg) administration in healthy participants (Patick et al. 2005).
Although the investigation of novel anti-ICAM-1 therapies are challenging, it is now essential in order to better manage chronic infection in the airways of CRD patients, as well as individuals with COPD experiencing frequent AEs.
Pharmacological properties of anti-ICAM-1 therapies
The results from pre-clinical models have indicated a strong potential for ICAM-1 blockage of HRV infections. Notably, the pharmacological parameters of these anti-ICAM-1 compounds seem to be excellent. For instance, one compound, referred in the previous section as 3 k, exhibited low systemic clearance (0.158 Lh−1 kg−1) and acceptable bioavailability in the oral cavity (27.8%) (Kim et al. 2018). In an in vivo murine model of RV infection, the intravenous dosing of anti-ICAM-1 monoclonal antibody (14C11) mitigated HRC-16 induced inflammation (total cells, neutrophils, lymphocytes and macrophages in the bronchoalveolar lavage fluid) in the lung and cytokine release (Traub et al. 2013). Remarkably, these reductions in inflammatory markers were observed up to seven days post administration of a single dose of anti-ICAM-1 monoclonal antibody (Traub et al. 2013). The clinical and anti-viral potential of such adhesion receptor blocking compounds could lead to novel therapies for managing HRV infections in humans, especially in those with underlying chronic lung diseases or a compromised immune system. This would have the added advantage of prophylactic, long-term protection against the HRV, in particular preceding vulnerable seasonal changes.
Clinical implications of blocking ICAM-1
The clinical utility of ICAM-1 in modulating HRV-induced lung inflammation and associated symptoms has been analyzed in a handful of clinical trials. Turner and colleagues (Turner et al. 1999) evaluated the efficacy of recombinant, soluble ICAM-1 (tremacamra or BIRR 4; 367 µg of tremacamra per nostril per dose for a total of 4.4 mg/day administered as inhaled solution/powder) in a randomized, double-blind, placebo controlled clinical trial. The authors found significant reductions in clinical symptoms (symptom score, clinical colds and nasal mucus) in the individuals who were experimentally infected with rhinovirus type 39 and received tremacamra, either pre- or post-inoculation of viral pathogen (100–300 median tissue culture infective dose administered as 2 inocula of 250 µL per nostril) (Turner et al. 1999). Another approach could be the utilization of gene therapy with downregulating the expression of ICAM-1 on respiratory epithelial cells (Othumpangat et al. 2016). Although not studied clinically and not in the context of HRVs, the downregulation of ICAM-1 by ICAM-1 siRNA on human bronchial epithelial cells resulted in alteration of influenza virus copy numbers, suggesting the crucial role of ICAM-1 in this viral infection (Othumpangat et al. 2016). Both anti-ICAM-1 monoclonal antibodies and/or soluble ICAM-1 protein could offer effective anti-viral approaches to protect against HRV in vulnerable populations. Once the complete pharmacological profiling is complete, this approach could offer a new avenue for prophylactically managing HRV infections in humans, and especially in patients with underlying lung conditions.
Summary and concluding remarks
HRV is major microbial pathogen implicated etiologically in virally-induced acute exacerbations (AEs) in chronic lung diseases, especially COPD and asthma, but probably also in other chronic respiratory diseases such as Idiopathic Pulmonary Fibrosis (IPF). This poses huge healthcare and economic burden on most countries globally. Crucially, there are as yet no effective prophylactic treatments for preventing HRV infections in these highly susceptible populations, as well as no definitive means to mitigate the primary infection or secondary bacterial infections in high risk patient cohorts. ICAM-1, which acts as a primary adhesion site for the HRV, could be effectively blocked to prevent viral-binding to host cells, thus preventing viral-induced infections and associated pathology in patients with COPD/asthma (as well as IPF etc.). There is also potential to combine anti-ICAM-1 therapies with anti-PAFr therapies, that could theoretically then prevent both viral and secondary bacterial infections in highly susceptible lung disease patients. Further research should also focus on characterizing the adhesion receptors for minor group HRVs, such as LDLRs, and other potential receptors, particularly in smokers and patients with COPD and asthma. A combination of ICAM-1 blockers and inhibitors of viral replication could be ideally suited for preventing viral exacerbations in patients with chronic respiratory disease, including smoking-related COPD.
Data availability
Enquiries about data availability should be directed to the authors.
Change history
09 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10787-022-01018-7
References
Bai J, Smock SL, Jackson GR Jr, MacIsaac KD, Huang Y, Mankus C, Oldach J, Roberts B, Ma YL, Klappenbach JA, Crackower MA, Alves SE, Hayden PJ (2015) Phenotypic responses of differentiated asthmatic human airway epithelial cultures to rhinovirus. PLoS ONE 10(2):e0118286
Bentley AM, Durham SR, Robinson DS, Menz G, Storz C, Cromwell O, Kay AB, Wardlaw AJ (1993) Expression of endothelial and leukocyte adhesion molecules interacellular adhesion molecule-1, E-selectin, and vascular cell adhesion molecule-1 in the bronchial mucosa in steady-state and allergen-induced asthma. J Allergy Clin Immunol 92(6):857–868
Bhalla K, Chugh M, Mehrotra S, Rathore S, Tousif S, Prakash Dwivedi V, Prakash P, Kumar Samuchiwal S, Kumar S, Kumar Singh D, Ghanwat S, Kumar D, Das G, Mohmmed A, Malhotra P, Ranganathan A (2015) Host ICAMs play a role in cell invasion by Mycobacterium tuberculosis and Plasmodium falciparum. Nat Commun 6:6049
Bochkov YA, Gern JE (2016) Rhinoviruses and their receptors: implications for allergic disease. Curr Allergy Asthma Rep 16(4):30
Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, Palmenberg AC, Gern JE (2015) Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci USA 112(17):5485–5490
Chan SCH, Shum DKY, Tipoe GL, Mak JCW, Leung ETM, Ip MSM (2008) Upregulation of ICAM-1 expression in bronchial epithelial cells by airway secretions in bronchiectasis. Respir Med 102(2):287–298
Chihara J, Yamamoto T, Kurachi D, Kakazu T, Higashimoto I, Nakajima S (1995) Possible release of eosinophil granule proteins in response to signaling from intercellular adhesion molecule-1 and its ligands. Int Arch Allergy Immunol 108(Suppl 1):52–54
Ciprandi G, Pronzato C, Ricca V, Passalacqua G, Bagnasco M, Canonica GW (1994) Allergen-specific challenge induces intercellular adhesion molecule 1 (ICAM-1 or CD54) on nasal epithelial cells in allergic subjects. Relationships with early and late inflammatory phenomena. Am J Respir Crit Care Med 150(6 Pt 1):1653–1659
Colonno RJ (1992) Virus receptors: the achilles’ heel of human rhinoviruses. In: Block TM, Jungkind D, Crowell RL, Denison M, Walsh LR (eds) Innovations in antiviral development and the detection of virus infections. Springer US, Boston, pp 61–70
Cox DW, Bizzintino J, Ferrari G, Khoo SK, Zhang G, Whelan S, Lee WM, Bochkov YA, Geelhoed GC, Goldblatt J, Gern JE, Laing IA, Le Souëf PN (2013) Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med 188(11):1358–1364
de Pablo R, Monserrat J, Reyes E, Díaz D, Rodríguez-Zapata M, de la Hera A, Prieto A, Álvarez-Mon M (2013) Circulating sICAM-1 and sE-Selectin as biomarker of infection and prognosis in patients with systemic inflammatory response syndrome. Eur J Intern Med 24(2):132–138
Drake MG, Lebold KM, Roth-Carter QR, Pincus AB, Blum ED, Proskocil BJ, Jacoby DB, Fryer AD, Nie Z (2018) Eosinophil and airway nerve interactions in asthma. J Leukoc Biol 104(1):61–67
Engels R, Falk L, Albanese M, Keppler OT, Sewald X (2022) LFA1 and ICAM1 are critical for fusion and spread of murine leukemia virus in vivo. Cell Rep 38(3):110279
Finney L, Belchamber K, Kemp S, Donaldson G, Mallia P, Johnston S, Wedzicha J (2017) P56 Human rhinovirus impairs phagocytosis of haemophilus influenzae in alveolar macrophages in chronic obstructive pulmonary disease. Thorax 72(Suppl 3):A112–A112
Galanter J, Choudhry S, Eng C, Nazario S, Rodríguez-Santana JR, Casal J, Torres-Palacios A, Salas J, Chapela R, Watson HG, Meade K, LeNoir M, Rodríguez-Cintrón W, Avila PC, Burchard EG (2008) ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am J Respir Crit Care Med 177(11):1194–1200
George SN, Garcha DS, Mackay AJ, Patel AR, Singh R, Sapsford RJ, Donaldson GC, Wedzicha JA (2014) Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J 44(1):87–96
Global Strategy for the Diagnosis M. a. P. o. C (2015) Global initiative for chronic obstructive lung disease (GOLD). News & events from around the world
Gorska-Ciebiada M, Ciebiada M, Gorska MM, Gorski P, Grzelewska-Rzymowska I (2006) Intercellular adhesion molecule 1 and tumor necrosis factor alpha in asthma and persistent allergic rhinitis: relationship with disease severity. Ann Allergy Asthma Immunol 97(1):66–72
Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, Kamarck ME, McClelland A (1989) The major human rhinovirus receptor is ICAM-1. Cell 56(5):839–847
Hayden FG (2004) Rhinovirus and the lower respiratory tract. Rev Med Virol 14(1):17–31
Hayden FG, Gwaltney JM Jr, Colonno RJ (1988) Modification of experimental rhinovirus colds by receptor blockade. Antivir Res 9(4):233–247
Hubbard AK, Rothlein R (2000) Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med 28(9):1379–1386
Jahnke A, Van de Stolpe A, Caldenhoven E, Johnson JP (1995) Constitutive expression of human intercellular adhesion molecule-1 (ICAM-1) is regulated by differentially active enhancing and silencing elements. Eur J Biochem 228(2):439–446
Johnston NW, Johnston SL, Duncan JM, Greene JM, Kebadze T, Keith PK, Roy M, Waserman S, Sears MR (2005) The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol 115(1):132–138
Johnston NW, Olsson M, Edsbacker S, Gerhardsson de Verdier M, Gustafson P, McCrae C, Coyle PV, McIvor RA (2017) Colds as predictors of the onset and severity of COPD exacerbations. Int J Chron Obstruct Pulmon Dis 12:839–848
Kherad O, Kaiser L, Bridevaux PO, Sarasin F, Thomas Y, Janssens JP, Rutschmann OT (2010) Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest 138(4):896–904
Kim J, Jung YK, Kim C, Shin JS, Scheers E, Lee JY, Han SB, Lee CK, Neyts J, Ha JD, Jung YS (2017) A novel series of highly potent small molecule inhibitors of rhinovirus replication. J Med Chem 60(13):5472–5492
Kim J, Shin JS, Ahn S, Han SB, Jung YS (2018) 3-Aryl-1,2,4-oxadiazole derivatives active against human rhinovirus. ACS Med Chem Lett 9(7):667–672
Ko FW, Chan PK, Chan RWY, Chan KP, Ip A, Kwok A, Ngai JC, Ng SS, On CT, Hui DS (2019) Molecular detection of respiratory pathogens and typing of human rhinovirus of adults hospitalized for exacerbation of asthma and chronic obstructive pulmonary disease. Respir Res 20(1):210
Kuroda M, Niwa S, Sekizuka T, Tsukagoshi H, Yokoyama M, Ryo A, Sato H, Kiyota N, Noda M, Kozawa K, Shirabe K, Kusaka T, Shimojo N, Hasegawa S, Sugai K, Obuchi M, Tashiro M, Oishi K, Ishii H, Kimura H (2015) Molecular evolution of the VP1, VP2, and VP3 genes in human rhinovirus species C. Sci Rep 5:1–10
Ledford RM, Patel NR, Demenczuk TM, Watanyar A, Herbertz T, Collett MS, Pevear DC (2004) VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J Virol 78(7):3663–3674
Lehmann JCU, Jablonski-Westrich D, Haubold U, Gutierrez-Ramos J-C, Springer T, Hamann A (2003) Overlapping and selective roles of endothelial intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in lymphocyte trafficking. J Immunol 171(5):2588–2593
Lin SJ, Chang LY, Yan DC, Huang YJ, Lin TJ, Lin TY (2003) Decreased intercellular adhesion molecule-1 (CD54) and L-selectin (CD62L) expression on peripheral blood natural killer cells in asthmatic children with acute exacerbation. Allergy 58(1):67–71
Liu Y, Bochkov YA, Eickhoff JC, Hu T, Zumwalde NA, Tan JW, Lopez C, Fichtinger PS, Reddy TR, Overmyer KA, Gumperz JE, Coon J, Mathur SK, Gern JE, Smith JA (2020) Orosomucoid-like 3 supports rhinovirus replication in human epithelial cells. Am J Respir Cell Mol Biol 62(6):783–792
Lopez-Campos JL, Calero C, Arellano-Orden E, Marquez-Martin E, Cejudo-Ramos P, Ortega Ruiz F, Montes-Worboys A (2012) Increased levels of soluble ICAM-1 in chronic obstructive pulmonary disease and resistant smokers are related to active smoking. Biomark Med 6(6):805–811
Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, Papi A, Stanciu LA, Kon OM, Johnson M, Johnston SL (2011) Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 183(6):734–742
Malmstrom K, Pitkaranta A, Carpen O, Pelkonen A, Malmberg LP, Turpeinen M, Kajosaari M, Sarna S, Lindahl H, Haahtela T, Makela MJ (2006) Human rhinovirus in bronchial epithelium of infants with recurrent respiratory symptoms. J Allergy Clin Immunol 118(3):591–596
Marlin SD, Staunton DE, Springer TA, Stratowa C, Sommergruber W, Merluzzi VJ (1990) A soluble form of intercellular adhesion molecule-1 inhibits rhinovirus infection. Nature 344(6261):70–72
McIntyre CL, Savolainen-Kopra C, Hovi T, Simmonds P (2013) Recombination in the evolution of human rhinovirus genomes. Arch Virol 158(7):1497–1515
Mendez MP, Monroy YK, Du M, Preston AM, Tolle L, Lin Y, VanDussen KL, Samuelson LC, Standiford TJ, Curtis JL, Beck JM, Christensen PJ, Paine R 3rd (2011) Overexpression of sICAM-1 in the alveolar epithelial space results in an exaggerated inflammatory response and early death in Gram negative pneumonia. Respir Res 12(1):12
Miller M, Tam AB, Cho JY, Doherty TA, Pham A, Khorram N, Rosenthal P, Mueller JL, Hoffman HM, Suzukawa M, Niwa M, Broide DH (2012) ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc Natl Acad Sci USA 109(41):16648–16653
Mosser AG, Vrtis R, Burchell L, Lee WM, Dick CR, Weisshaar E, Bock D, Swenson CA, Cornwell RD, Meyer KC, Jarjour NN, Busse WW, Gern JE (2005) Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med 171(6):645–651
Novotny LA, Bakaletz LO (2016) Intercellular adhesion molecule 1 serves as a primary cognate receptor for the Type IV pilus of nontypeable Haemophilus influenzae. Cell Microbiol 18(8):1043–1055
Othumpangat S, Noti JD, McMillen CM, Beezhold DH (2016) ICAM-1 regulates the survival of influenza virus in lung epithelial cells during the early stages of infection. Virology 487:85–94
Palmenberg AC, Rathe JA, Liggett SB (2010) Analysis of the complete genome sequences of human rhinovirus. J Allergy Clin Immunol 125(6):1190–1199 (quiz 1191–1200)
Pang B, Hong W, West-Barnette SL, Kock ND, Swords WE (2008) Diminished ICAM-1 expression and impaired pulmonary clearance of nontypeable Haemophilus influenzae in a mouse model of chronic obstructive pulmonary disease/emphysema. Infect Immun 76(11):4959–4967
Papi A, Johnston SL (1999) Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem 274(14):9707–9720
Papi A, Brightling C, Pedersen SE, Reddel HK (2018) Asthma. Lancet (london, England) 391(10122):783–800
Patick AK, Brothers MA, Maldonado F, Binford S, Maldonado O, Fuhrman S, Petersen A, Smith GJ 3rd, Zalman LS, Burns-Naas LA, Tran JQ (2005) In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother 49(6):2267–2275
Quint JK, Donaldson GC, Goldring JJ, Baghai-Ravary R, Hurst JR, Wedzicha JA (2010) Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. Chest 137(4):812–822
Qureshi H, Sharafkhaneh A, Hanania NA (2014) Chronic obstructive pulmonary disease exacerbations: latest evidence and clinical implications. Ther Adv Chronic Dis 5(5):212–227
Roebuck KA, Finnegan A (1999) Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol 66(6):876–888
Rohde G, Wiethege A, Borg I, Kauth M, Bauer T, Gillissen A, Bufe A, Schultze-Werningh G (2003) Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax 58(1):37–42
Rusznak C, Mills PR, Devalia JL, Sapsford RJ, Davies RJ, Lozewicz S (2000) Effect of cigarette smoke on the permeability and IL-1beta and sICAM-1 release from cultured human bronchial epithelial cells of never-smokers, smokers, and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 23(4):530–536
Schroth MK, Grimm E, Frindt P, Galagan DM, Konno SI, Love R, Gern JE (1999) Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol 20(6):1220–1228
Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA (2000) Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161(5):1608–1613
Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, Wedzicha JA (2001) Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164(9):1618–1623
Sethi S, Murphy TF (2008) Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 359(22):2355–2365
Shukla SD, Hansbro PM, Walters EH (2017a) Blocking rhinoviral adhesion molecule (ICAM-1): potential to prevent COPD exacerbations. Int J Chron Obstruct Pulmon Dis 12:1413–1414
Shukla SD, Mahmood MQ, Weston S, Latham R, Muller HK, Sohal SS, Walters EH (2017b) The main rhinovirus respiratory tract adhesion site (ICAM-1) is upregulated in smokers and patients with chronic airflow limitation (CAL). Respir Res 18(1):6
Springer TA (1990) Adhesion receptors of the immune system. Nature 346(6283):425–434
Stanciu LA, Djukanovic R (1998) The role of ICAM-1 on T-cells in the pathogenesis of asthma. Eur Respir J 11(4):949–957
Staunton DE, Merluzzi VJ, Rothlein R, Barton R, Marlin SD, Springer TA (1989) A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell 56(5):849–853
Sykes A, Mallia P, Johnston SL (2007) Diagnosis of pathogens in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 4(8):642–646
Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Umeda A (2000) Increased expression of inflammatory mediators in small-airway epithelium from tobacco smokers. Am J Physiol Lung Cell Mol Physiol 278(5):L906-913
Taylor S, Lopez P, Weckx L, Borja-Tabora C, Ulloa-Gutierrez R, Lazcano-Ponce E, Kerdpanich A, Weber MAR, de Los Santos AM, Tinoco JC, Safadi MA, Lim FS, de Mezerville MH, Faingezicht I, Cruz-Valdez A, Feng Y, Li P, Durviaux S, Haars G, Roy-Ghanta S, Vaughn DW, Nolan T (2017) Respiratory viruses and influenza-like illness: epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J Infect 74(1):29–41
Terajima M, Yamaya M, Sekizawa K, Okinaga S, Suzuki T, Yamada N, Nakayama K, Ohrui T, Oshima T, Numazaki Y, Sasaki H (1997) Rhinovirus infection of primary cultures of human tracheal epithelium: role of ICAM-1 and IL-1beta. Am J Physiol 273(4 Pt 1):L749-759
Traub S, Nikonova A, Carruthers A, Dunmore R, Vousden KA, Gogsadze L, Hao W, Zhu Q, Bernard K, Zhu J, Dymond M, McLean GR, Walton RP, Glanville N, Humbles A, Khaitov M, Wells T, Kolbeck R, Leishman AJ, Sleeman MA, Bartlett NW, Johnston SL (2013) An anti-human ICAM-1 antibody inhibits rhinovirus-induced exacerbations of lung inflammation. PLoS Pathog 9(8):e1003520
Turner RB, Wecker MT, Pohl G, Witek TJ, McNally E, St R, George BW, Hayden FG (1999) Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infectiona randomized clinical trial. JAMA 281(19):1797–1804
van de Stolpe A, van der Saag PT (1996) Intercellular adhesion molecule-1. J Mol Med (berl) 74(1):13–33
Wedzicha JA (2004) Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 1(2):115–120
Wegner CD, Gundel RH, Reilly P, Haynes N, Letts LG, Rothlein R (1990) Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science 247(4941):456–459
WHO (2014) The top 10 causes of death
Winther B (2011) Rhinovirus infections in the upper airway. Proc Am Thorac Soc 8(1):79–89
Winther B, Brofeldt S, Christensen B, Mygind N (1984) Light and scanning electron microscopy of nasal biopsy material from patients with naturally acquired common colds. Acta Otolaryngol 97(3–4):309–318
Wos M, Sanak M, Soja J, Olechnowicz H, Busse WW, Szczeklik A (2008) The presence of rhinovirus in lower airways of patients with bronchial asthma. Am J Respir Crit Care Med 177(10):1082–1089
Zhang Y, Willis-Owen SAG, Spiegel S, Lloyd CM, Moffatt MF, Cookson W (2019) The ORMDL3 asthma gene regulates ICAM1 and has multiple effects on cellular inflammation. Am J Respir Crit Care Med 199(4):478–488
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SDS and EHW conceived the review. All authors contributed to the writing and revision of the manuscript. SDS, EHW designed the figure. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shukla, S.D., Shastri, M.D., Vanka, S.K. et al. Targeting intercellular adhesion molecule-1 (ICAM-1) to reduce rhinovirus-induced acute exacerbations in chronic respiratory diseases. Inflammopharmacol 30, 725–735 (2022). https://doi.org/10.1007/s10787-022-00968-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-00968-2