Abstract
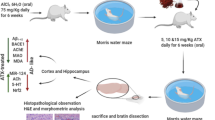
STZ is a glucosamine-nitrosourea compound, causes dysfunctioning of insulin receptors in the brain and disrupts glucose metabolism, produces cognitive decline and AD-like symptoms. ICV injection of STZ causes accumulation of Aβ and cognitive dysfunctions. Andrographolide (ANDRO) is a major bioactive constituent of Andrographis paniculata, has various biological activities such as antioxidant, anti-inflammatory, anti-cholinesterase, and neuroprotective properties. The study aimed to evaluate the neuroprotective effect of ANDRO against ICV-STZ induced AD-like symptoms in rats. To conduct the study, the Wistar rat received two injections of STZ (3 mg/kg) through the ICV route. Rats were treated with three different doses of ANDRO (15, 30, and 60 mg/kg, p.o.) and donepezil (5 mg/kg, p.o.) for 14 days. The behavioral impairments were analyzed on weekly basis. Subsequently, rats were sacrificed for the assessment of biochemical (MDA, Nitrite, GSH, SOD, Catalase and AChE), neuroinflammatory markers (IL-1β, IL-16, and TNF-α), neurotransmitters (glutamate and GABA), level of Aβ1–42 and p tau in the hippocampus on day 21st. Our result indicated that ANDRO treatment provided a protective effect against STZ induced behavioral deficits and changes in the biochemical, neuroinflammatory mediators, and neurotransmitters of the hippocampus. Further, ANDRO also reduced the level of Aβ1–42 and p tau in the rat hippocampus. These findings suggested that the antioxidant, anti-inflammatory, anti-cholinesterase potential of ANDRO contributed to its neuroprotective effect as well as promising therapeutic candidate for the treatment of cognitive impairment and AD-like symptoms.









Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- STZ:
-
Streptozotocin
- ANDRO:
-
Andrographolide
- ICV:
-
Intracerebroventricular
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahn Y, Seo J, Park J, Won J, Yeo H-G, Kim K, Jeon C-Y, Huh J-W, Lee S-R, Lee D-S (2020) Synaptic loss and amyloid beta alterations in the rodent hippocampus induced by streptozotocin injection into the cisterna magna. Lab Anim Res 36(1):1–6
Arora R, Deshmukh R (2017) Embelin attenuates intracerebroventricular streptozotocin-induced behavioral, biochemical, and neurochemical abnormalities in rats. Mol Neurobiol 54(9):6670–6680
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20(3):148–160
Cui SY, Song JZ, Cui XY, Hu X, Ma YN, Shi YT, Luo Y, Ge YR, Ding H, Ye H (2018) Intracerebroventricular streptozotocin-induced Alzheimer’s disease-like sleep disorders in rats: role of the GABAergic system in the parabrachial complex. CNS Neurosci Ther 24(12):1241–1252
Del MR, McDonald W (1987) Distribution of superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Mechan Ageing Dev 41(1–2):29–38
Donzanti BA, Yamamoto BK (1988) An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci 43(11):913–922
El Sayed NS, Ghoneum MH (2020) Antia, a natural antioxidant product, attenuates cognitive dysfunction in streptozotocin-induced mouse model of sporadic Alzheimer’s disease by targeting the amyloidogenic, inflammatory, autophagy, and oxidative stress pathways. Oxid Med Cell Longev 2020:1–14
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95
Galasko D, Montine TJ (2010) Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark Med 4(1):27–36
Gerzson MF, Pacheco SM, Soares MS, Bona NP, Oliveira PS, Azambuja JH, da Costa P, Gutierres JM, Carvalho FB, Morsch VM (2019) Effects of tannic acid in streptozotocin-induced sporadic Alzheimer’s disease: insights into memory, redox status, Na+, K+-ATPase and acetylcholinesterase activity. Arch Physiol Biochem 125:1–8
Gong P, Chen Y-q, Lin A-h, Zhang H-b, Zhang Y, Richard DY, Yu Y (2020) p47 phox deficiency improves cognitive impairment and attenuates tau hyperphosphorylation in mouse models of AD. Alzheimer’s Res Ther 12(1):1–18
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126(1):131–138
Gutierres JM, Carvalho FB, Schetinger MRC, Marisco P, Agostinho P, Rodrigues M, Rubin MA, Schmatz R, da Silva CR, Cognato GdP (2014) Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci 96(1–2):7–17
Han XJ, Hu YY, Yang ZJ, Jiang LP, Shi SL, Li YR, Guo MY, Wu HL, Wan YY (2017) Amyloid β-42 induces neuronal apoptosis by targeting mitochondria. Mol Med Rep 16(4):4521–4528
Hira S, Saleem U, Anwar F, Sohail MF, Raza Z, Ahmad B (2019) β-Carotene: a natural compound improves cognitive impairment and oxidative stress in a mouse model of streptozotocin-induced Alzheimer’s disease. Biomolecules 9(9):441
Huang D, Liu D, Yin J, Qian T, Shrestha S, Ni H (2017) Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur Radiol 27(7):2698–2705
Jiang X, Wang R, Zhai S, Zhang Y, Wang D (2020) Forsythoside B attenuates memory impairment and neuroinflammation via inhibition on NF-κB signaling in Alzheimer’s disease. J Neuroinflammation 17(1):1–15
Kakuda N, Miyasaka T, Iwasaki N, Nirasawa T, Wada-Kakuda S, Takahashi-Fujigasaki J, Murayama S, Ihara Y, Ikegawa M (2017) Distinct deposition of amyloid-β species in brains with Alzheimer’s disease pathology visualized with MALDI imaging mass spectrometry. Acta Neuropathol Commun 5(1):1–8
Kandimalla R, Reddy PH (2017) Therapeutics of neurotransmitters in Alzheimer’s disease. J Alzheimers Dis 57(4):1049–1069
Lanzillotta C, Di Domenico F, Perluigi M, Butterfield DA (2019) Targeting mitochondria in Alzheimer disease: rationale and perspectives. CNS Drugs 33(10):957–969
Lei M, Xu H, Li Z, Wang Z, O'Malley TT, Zhang D, Walsh DM, Xu P, Selkoe DJ, Li S (2016) Soluble Aβ oligomers impair hippocampal LTP by disrupting glutamatergic/GABAergic balance. Neurobiol Dis 85:111–121
Li X, Wang T, Zhang D, Li H, Shen H, Ding X, Chen G (2018) Andrographolide ameliorates intracerebral hemorrhage induced secondary brain injury by inhibiting neuroinflammation induction. Neuropharmacology 141:305–315
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mathuranath P, George A, Ranjith N, Justus S, Kumar MS, Menon R, Sarma PS, Verghese J (2012) Incidence of Alzheimer’s disease in India: a 10 years follow-up study. Neurol India 60(6):625
Murtishaw AS, Heaney CF, Bolton MM, Belmonte KCD, Langhardt MA, Kinney JW (2018) Intermittent streptozotocin administration induces behavioral and pathological features relevant to Alzheimer’s disease and vascular dementia. Neuropharmacology 137:164–177
Mushtaq N, Schmatz R, Pereira LB, Ahmad M, Stefanello N, Vieira JM, Abdalla F, Rodrigues MV, Baldissarelli J, Pelinson LP (2014) Rosmarinic acid prevents lipid peroxidation and increase in acetylcholinesterase activity in brain of streptozotocin-induced diabetic rats. Cell Biochem Funct 32(3):287–293
Naderi S, Habibi A, Kesmati M, Rezaie A, Ghanbarzadeh M (2018) The effects of six weeks high intensity interval training on amyloid beta1–42 peptide in hippocampus of rat model of Alzheimer’s disease induced with STZ. J Clin Res Paramed Sci 7(2):86866–86870
Nichols E, Szoeke CE, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, Aichour MTE, Akinyemi RO, Alahdab F, Asgedom SW (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(1):88–106
Ortí-Casañ N, Wu Y, Naudé PJ, De Deyn PP, Zuhorn IS, Eisel UL (2019) Targeting TNFR2 as a novel therapeutic strategy for Alzheimer’s disease. Front Neurosci 13:49
Picca A, Calvani R, Coelho-Junior HJ, Landi F, Bernabei R, Marzetti E (2020) Mitochondrial dysfunction, oxidative stress, and neuroinflammation: intertwined roads to neurodegeneration. Antioxidants 9(8):647
Ravelli KG, dos Anjos RB, Camarini R, Hernandes MS, Britto LR (2017) Intracerebroventricular streptozotocin as a model of Alzheimer’s disease: neurochemical and behavioral characterization in mice. Neurotox Res 31(3):327–333
Rimpi A, Rahul D (2020) Chebulinic acid negated the development of streptozotocin-induced experimental dementia in rats. Int J Pharm Invest 10(1):24–31
Sagbakken M, Spilker RS, Nielsen TR (2018) Dementia and immigrant groups: a qualitative study of challenges related to identifying, assessing, and diagnosing dementia. BMC Health Serv Res 18(1):1–14
Sharma V, Bala A, Deshmukh R, Bedi K, Sharma P (2012) Neuroprotective effect of RO-20-1724-a phosphodiesterase4 inhibitor against intracerebroventricular streptozotocin induced cognitive deficit and oxidative stress in rats. Pharmacol Biochem Behav 101(2):239–245
Wang H, Zhang H (2018) Reconsideration of anticholinesterase therapeutic strategies against Alzheimer’s disease. ACS Chem Neurosci 10(2):852–862
Wang D, Liu L, Li S, Wang C (2018) Effects of paeoniflorin on neurobehavior, oxidative stress, brain insulin signaling, and synaptic alterations in intracerebroventricular streptozotocin-induced cognitive impairment in mice. Physiol Behav 191:12–20
Wang C, Cai Z, Wang W, Wei M, Kou D, Li T, Yang Z, Guo H, Le W, Li S (2019a) Piperine attenuates cognitive impairment in an experimental mouse model of sporadic Alzheimer’s disease. J Nutr Biochem 70:147–155
Wang D-P, Yin H, Lin Q, Fang S-P, Shen J-H, Wu Y-F, Su S-H, Hai J (2019b) Andrographolide enhances hippocampal BDNF signaling and suppresses neuronal apoptosis, astroglial activation, neuroinflammation, and spatial memory deficits in a rat model of chronic cerebral hypoperfusion. Naunyn Schmiedebergs Arch Pharmacol 392(10):1277–1284
Wei J, Yang F, Gong C, Shi X, Wang G (2019) Protective effect of daidzein against streptozotocin-induced Alzheimer’s disease via improving cognitive dysfunction and oxidative stress in rat model. J Biochem Mol Toxicol 33(6):22319
Wills E (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99(3):667–676
Xu Z-P, Gan G-S, Liu Y-M, Xiao J-S, Liu H-X, Mei B, Zhang J-J (2018) Adiponectin attenuates streptozotocin-induced tau hyperphosphorylation and cognitive deficits by rescuing PI3K/Akt/GSK-3β pathway. Neurochem Res 43(2):316–323
Yahaya M, Zolkiffly S, Moklas M, Hamid HA, Stanslas J, Zainol M, Mehat M (2020) Possible epigenetic role of vitexin in regulating neuroinflammation in Alzheimer’s disease. J Immunol Res 2020:1–7
Acknowledgements
The authors are highly thankful to Mr. Parveen Garg, Chairman, ISFCP for providing an excellent research platform.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, R., Kaur, K. & Singh, S. Protective effect of andrographolide against STZ induced Alzheimer’s disease in experimental rats: possible neuromodulation and Aβ(1–42) analysis. Inflammopharmacol 29, 1157–1168 (2021). https://doi.org/10.1007/s10787-021-00843-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-021-00843-6