Abstract
Background
Sarcoidosis has often been termed an “immune paradox” as there is peripheral anergy to common recall antigens despite pronounced TH1-dominant inflammation at disease sites, such as the lung, with up-regulation of interferon γ, IL-27 and transcription factors. Peripheral blood may reflect the anergic state, while exhaled breath condensate (EBC) analysis may offer insights into the lung disease.
Methods
A cross-sectional study was conducted to investigate the expression of TH1 cytokines and transcription factors (IFNγ, IL-27 and T-bet) in the peripheral blood and/or EBC of sarcoidosis patients and healthy controls. Whole blood and EBC were collected from sarcoidosis patients and healthy controls. TH1 cytokine expression levels were then measured in peripheral blood mononuclear cells (PBMCs) and/or plasma and EBC using quantitative real-time PCR, ELISA and via Western blotting.
Results
Compared to healthy controls, PBMC IL-27 mRNA was higher in patients (p = 0.0019). There were no significant differences in plasma IL-27 protein between patients and controls (p = 0.20). T-bet mRNA and protein were lower (p = 0.010 and p = 0.0043, respectively) in patients compared to controls. There were no significant differences in PBMC IFNγ mRNA and protein expression (p = 0.68 and p = 0.74, respectively) nor in EBC IL-27 levels.
Conclusions
Our data indicate that depressed T-bet mRNA and protein expression could contribute to the peripheral anergy in sarcoidosis and that IL-27 mRNA levels are elevated in the PBMC from those with sarcoidosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a multi-system granulomatous disorder of undetermined aetiology (Loke et al. 2013). Lymphocytes in sarcoid granulomas are strongly TH1 polarised and are characterised by an amplified T-bet expression and IFNγ production (Gharaee-Kermani et al. 2009). Sarcoidosis is also often termed an “immune paradox” as there is peripheral anergy shown by a diminished delayed-type hypersensitivity to common recall antigens in the skin despite exaggerated inflammation at disease sites (Miyara et al. 2006; Morgenthau and Iannuzzi 2011). It has been suggested that underpinning this phenomenon is a disequilibrium between effector and regulatory lymphocytes (Treg cells), more specifically CD4+CD25brightFoxP3+ cells (Mathew et al. 2008), which congregate in the peripheral blood and exercise anti-proliferative effects on naïve T-cells. Others suggest that the exaggerated immune response at disease sites results in the accumulation of activated T-cells at these sites and consequently, peripheral lymphopenia (Hunninghake et al. 1979). It is also possible that in chronic sarcoidosis, immunosuppressive CD8+ T-cells become more plentiful peripherally, thus effecting an anergic response (Planck et al. 2003). Despite intensive research upon pro-inflammatory mechanisms, there is a paucity of studies investigating the roles of immunosuppressive cytokines in the regulation of inflammation in sarcoidosis.
IL-27 is a heterodimeric cytokine structurally related to IL-12 (Villarino et al. 2004). It has recently been increasingly labelled as an attenuator of inflammatory responses. Mice that have been depleted of the IL-27 receptor WSX-1 produced more pro-inflammatory cytokines when infected with Trypanosoma cruzi and T. gondii (Villarino et al. 2003). IL-27 was also shown to repress cytokine production from activated T lymphocytes in vitro (Yoshimura et al. 2006) and promote IL-10 production (Stumhofer et al. 2007). Other mechanisms include inhibiting the differentiation of TH17 cells (Stumhofer et al. 2006) and activating the programmed cell death proteins, PD-1–PD-L1 (Topalian et al. 2012). Although Larousserie et al. (2004) have demonstrated the presence of Epstein-Barr virus-induced gene 3 and p28 (subunits of IL-27) in sarcoidosis lymph node biopsies using immunohistochemistry, there are, to date, no studies quantifying the expression of IL-27 in sarcoidosis.
Sarcoidosis remains a diagnosis of exclusion best confirmed by histological evidence showing non-caseating granulomas in the absence of granulomatous agents (Baughman et al. 2011). This necessitates a tissue biopsy which is invasive. Exhaled breath condensate (EBC) offers a non-invasive approach to sampling the fluid lining the respiratory tract and (Kazani and Israel 2012) our group has detected significantly elevated markers of macrophage activity (i.e. TNF-α, TGF-β1 and neopterin) (Ahmadzai et al. 2013) and IFNγ (Huang et al. 2013) in sarcoidosis EBC compared to healthy control subjects. There are, to our knowledge, no studies on IL-27 in peripheral blood mononuclear cells (PBMCs) and EBC of sarcoidosis patients.
In sarcoidosis, the expressions of IFNγ and Tbx21 in CD4+ T-cells are up-regulated post-T-cell activation. Together with STAT-4, interleukin-12 (IL-12) up-regulates IFNγ expression. IFNγ then stimulates STAT-1 which intensifies the expression of T-bet by the Tbx21 gene. T-bet enhances the transcriptional competence of the IFNγ gene and increases IFNγ production. T-bet also antagonises Gata3, therefore, down-regulating TH2 cytokines in the acute phase of sarcoidosis. Given that T-bet consigns T cells into the TH1 subset and the ability of IL-27 to mitigate inflammatory responses, we studied the expression of the above-mentioned TH1 modulators in the peripheral blood of sarcoidosis patients with the goal of elucidating whether they were associated with sarcoidosis peripheral anergy.
Methods
Study design
This was an observational, cross-sectional study which was approved by the Human Research Ethics Committees of St. Vincent’s Hospital and Prince of Wales Hospital, Sydney Australia (ethics approval number: 10/005). Informed, written consent was obtained from all participants. Sarcoidosis patients visiting the Prince of Wales Hospital Chest and Sarcoidosis Clinics and healthy controls from both the community and Prince of Wales Hospital were invited to participate. Patient inclusion criteria necessitated a diagnosis of sarcoidosis established prior to participation, based on the guidelines from the World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG), the American Thoracic Society and the European Respiratory Society (Hunninghake et al. 1999). Patients on immunosuppressive medication were not excluded. Control subject criteria required a FEV1/FVC ratio of >80 % on spirometry and no significant medical history of atopic, granulomatous or autoimmune disorders.
EBC collection and storage
EBC samples were collected using the EcoScreen condenser (Erich Jaeger GmbH, Hochberg, Germany). Subjects were asked to breathe for 15 min (tidal breathing) into a mouthpiece attached to a one-way valve. The EBC samples were stored in Protein LoBind tubes (Eppendorf, Sydney, Australia) in 120 µl aliquots, de-aerated with argon gas to remove CO2 and stored at −80 °C until analysis.
Blood processing: plasma and PBMC isolation and cryopreservation
Approximately, 50 ml of blood was obtained in acid citrate dextrose-containing Vacutainer tubes (BD Biosciences, Sydney, Australia). Whole blood was centrifuged at 700×g for 10 min. The supernatant, plasma (10 ml), was removed, separated into 1 ml aliquots and stored at −80 °C until further analysis. PBMCs were then isolated by density gradient centrifugation using Ficoll-Hypaque Lymphoprep (STEMCELL Technologies, Melbourne, Australia) (Böyum 1968) and were kept in vapour-phase nitrogen at −190 °C until further analysis.
RNA extraction from PBMC and EBC
Prior to cryopreservation, 5 million PBMCs were separated, lysed with TRIzol (Life Technologies, Melbourne, Australia) and stored at −80 °C until total RNA extraction according to the manufacturer’s protocol. The extracted RNA was resuspended in 20 µl of diethylpyrocarbonate-treated water. RNA concentration and purity were assessed using the NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Melbourne, Australia).
cDNA synthesis and qRT-PCR analysis
RNA (≥1.0 µg) from PBMC was used for first-strand cDNA synthesis using a Superscript III first-strand synthesis kit (Invitrogen, CA, USA), following the manufacturer’s protocols. Quantitative real-time PCR for mRNA detection was performed using Sensimix SYBR (Bioline, London, UK) on the Light Cycler 480 (Roche, Mannheim, Germany) using standard conditions with hypoxanthine–guanine phosphoribosyltransferase (HPRT) as the reference control.
Primers
Custom primers were designed using Primer3 (http://primer3.ut.ee/) and were purchased from either Invitrogen or Integrated DNA technologies (Singapore) (see Table 1 for primer sequences).
Protein extraction from PBMC
Cryovials containing PBMCs were thawed in a 37 °C water bath. PBMCs were centrifuged at 400×g for 10 min at 4 °C and washed twice with DPBS. The supernatant was discarded and total protein was extracted using cell lysis buffer (Cell Signalling Technology, MA, USA) containing phenylmethylsulfonyl fluoride (Sigma-Aldrich, Missouri, USA). Samples were then vortexed and centrifuged at 4000×g for 40 min at 4 °C. The protein lysate was collected and kept at −80 °C until assayed.
Quantification of cytokine production in plasma, EBC and PBMC cell lysates via ELISA
Cytokine expression was quantified in plasma (100 µl), which had undergone a two-fold dilution and undiluted EBC (100 µl). Protein concentrations in PBMC cell lysates were determined via the Bradford method (Bradford 1976). IFNγ protein levels were measured in 17 µg of total protein from PBMC cell lysates of patients and controls. IFNγ, IL-27 (R&D Systems, MN, USA) and IL-27p28 (BioScientific, GeneWorks, SA, Australia) levels were determined by specific ELISAs with detection limits of 15.6, 156.3 and 75 pg/ml, respectively. Assays were performed according to the manufacturer’s instructions. Absorbance was determined by spectrophotometry (SpectraMaxPlus Plate Reader, Molecular Devices, Surrey, UK) at manufacturer-specific wavelengths and the concentration in sample was determined by linear interpolation.
Western blotting for T-bet
Equal amounts of PBMC cell lysate protein (4 µg) from both patient and healthy controls were loaded, subjected to 10 % SDS–polyacrylamide gel electrophoresis and were electrophoretically transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Massachusetts, USA). Non-specific sites were blocked with 5 % BSA in TBST (Tris-buffered saline and 0.1 % Tween-20) for 1 h at room temperature. The membrane was washed five times for 5 min with PBST (phosphate-buffered saline and 0.05 % Tween-20) and incubated overnight at 4 °C with a polyclonal rabbit anti-human T-bet specific primary antibody (1:1000 final dilution) (Cell Signalling Technologies, MA, USA). After five washes with PBST, the membrane was incubated with goat anti-rabbit IgG HRP-conjugated secondary antibody (1:3000 final dilution, Cell Signalling Technologies). Protein bands were visualised with an enhanced chemiluminescence kit (PerkinElmer, MA, USA) using the ImageQuant LAS4000 (GE Healthcare Life Sciences, New Jersey, USA). Bands were analysed semi-quantitatively using ImageJ software (http://rsbweb.nih.gov/ij/) for T-bet quantification. To adjust for differences in image densities between membranes, the image densities of all bands were normalised to a standard sample which was repeated on all gels.
Statistics
GraphPad Prism 6.0 software (La Jolla, CA, USA) was used for data analysis. The D’Agostino & Pearson omnibus normality test was used to test data for normality. Subject to data set normality, the data were analysed using unpaired t tests or Mann–Whitney U tests as appropriate. p < 0.05 was considered to be statistically significant. Data are expressed as mean ± SD or median ± range.
Results
Subject characteristics
Sarcoidosis patients (n = 18) and healthy controls (n = 21) were recruited (see Table 2 for full subject characteristics). Sarcoidosis patients had an elevated mean serum ACE level (48.9 U/L, NR < 42 U/L) and, compared to controls, demonstrated peripheral lymphopenia (p = 0.0004) typical of sarcoidosis (Iannuzzi and Fontana 2011).
TH1 cytokine mRNA expression in PBMCs
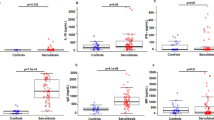
There were no statistical differences in PBMC IFNγ mRNA levels between controls and sarcoidosis patients (p = 0.68, Fig. 1a). Compared to healthy controls, T-bet mRNA levels were lower in sarcoidosis patients (p = 0.010, Fig. 1b), while IL-27 mRNA levels were higher in sarcoidosis patients (p = 0.019, Fig. 1c).
To analyse the ability of TH1 cytokine mRNA levels to predict disease severity, sarcoidosis patients were further divided according to radiological stages. The mRNA expression levels of all cytokines did not vary significantly between the radiological stages (IFNγ: p = 0.63; T-bet: p = 0.10; IL-27: p = 0.086) (Online Resources 1 and 2). When patients were grouped according to treatment status, there were no significant differences between the groups for all TH1 cytokines studied (IFNγ: p = 0.71; T-bet: p = 0.31; IL-27: p = 0.26) (Online Resources 1 and 2).
TH1 cytokine protein expression in plasma and/or PBMC cell lysate
Comparative analysis demonstrated no significant differences in IFNγ in PBMC cell lysates or total IL-27 protein levels in the plasma of controls and sarcoidosis patients (IFNγ: p = 0.74 Fig. 1d; IL-27: p = 0.94 Fig. 1f). The more sensitive IL-27p28 assay showed six sarcoid subjects versus one control subject had detectable levels (mean 79 ± 110 vs. 29 ± 25 pg/ml, respectively, p = 0.026). T-bet protein was detected as bands of 65 kDa (Fig. 2). Densitometric analysis demonstrated that, compared to controls, T-bet protein expression in PBMCs was lower in sarcoidosis patients (p = 0.0043, Fig. 1e).
When analysed according to radiological stage and treatment status, there were no significant differences between controls and sarcoidosis patients for all TH1 cytokines (radiological staging: IFNγ: p = 0.59, T-bet: p = 0.14, IL-27: p = 0.85; treatment status: IFNγ: p = 0.43, T-bet: p = 0.27, IL-27: p = 0.97) (Online Resource 1 and 2).
IL-27 expression in EBC
Samples that had undetectable IL-27 were arbitrary assigned a value equivalent to half of the lowest level detected by the kit (Edmé et al. 2008). IL-27 was detected in 6/20 controls and 6/18 sarcoidosis patients. The mean IL-27 concentrations were 3.1 ± 4.2 pg/ml (mean ± SD) in controls and 1.8 ± 3.0 pg/ml in sarcoidosis patients. Nonetheless, this difference was not statistically significant (p = 0.57) (Online Resource 3).
Discussion
This study aimed to quantify the expression of TH1 cytokines in the peripheral blood of sarcoidosis patients (Loke et al. 2013), but because post-transcriptional regulation has been known to make mRNA levels poor approximations for protein levels (Hack 2004), both mRNA and protein levels of TH1 cytokines in the peripheral blood of sarcoidosis patients were studied.
T-bet has been shown to orchestrate TH1 responses especially in the transcription of IFNγ (Djuretic et al. 2007). In comparison with healthy controls, T-bet mRNA and protein expression were lower in the sarcoidosis patients in keeping with our hypothesis, suggesting T-bet involvement in the immunopathogenesis of sarcoidosis. To date, only Kriegova et al. (2011) have examined the role of T-bet in sarcoidosis and demonstrated that T-bet mRNA expression levels were increased in BAL lymphocytes. While they did not examine T-bet protein expression levels, one could surmise that T-bet expression levels in the periphery are decreased at these sites of anergy but increased at sites of disease suggesting T-bet involvement in the peripheral anergy underpinning sarcoidosis. This inference is further reinforced by our findings which showed consistency between mRNA and protein expression levels and other findings of Kriegova et al. (in the same study), where T-bet mRNA levels were found to correlate with IFNγ and other chemokines associated with TH1 immunity, reinforcing its role as a key modulator of TH1 immunity.
This study is the first to demonstrate increased PBMC IL-27 mRNA expression in sarcoidosis patients compared to healthy controls. There was a significant trend to elevated IL27p28 levels in the plasma from sarcoidosis patients but not in the IL-27 assay, suggesting that the levels are low and very sensitive assays are required. The low levels could be attributed to the short in vivo half-life of IL-27 (Do et al. 2012; Pot et al. 2010) or differences in the half-life of IL-27 between patients and controls, and possibly translational repression.
There were no significant differences between PBMC IFNγ mRNA expression levels in sarcoidosis patients and controls in keeping with previous findings (Swider et al. 1995). Intracellular PBMC IFNγ protein expression levels were measured using ELISA in PBMC cell lysates. To our knowledge, this was the first time this experimental approach was used to quantify intracellular IFNγ protein in sarcoidosis patients. Mirroring mRNA data and data from another study that analysed intracellular IFNγ protein expression using flow cytometry (Hill et al. 2008), IFNγ protein levels were not elevated in PBMCs of sarcoidosis patients, suggesting that mechanisms within PBMCs may be implicated for the suppression of IFNγ. Small, 20–25 nucleotide, non-coding RNAs, known as microRNAs (miRNAs) have been shown to be natural repressors of gene translation (Jung et al. 2010). Aberrant miRNA expressions have been observed in sarcoidosis patients (Crouser et al. 2012). Additionally, members of the miR-29 family have been known to target IFNγ (Ma et al. 2011) and T-bet (Steiner et al. 2011) thereby influencing TH1 immunity. The low levels of T-bet mRNA and protein observed in this study suggest that miR-29a could have a role in modulating the TH1 profile within PBMCs by suppressing IFNγ and T-bet.
This is the first study to detect IL-27 in EBC of sarcoidosis patients. EBC IL-27 levels were not significantly different between sarcoidosis controls and patients. Although one may conclude that IL-27 may not be implicated in the immunopathogenesis of pulmonary sarcoidosis, all 6/18 sarcoidosis patients who had detectable IL-27 EBC levels had pulmonary sarcoidosis. In view of the low IL-27 levels, this observation begets further investigation using higher sensitivity assays.
To assess the ability of the aforementioned immunomodulators to delineate clinical phenotype and assess their expression levels in relation to treatment status, we analysed immunomodulator expression according to the radiological stage and treatment status of sarcoidosis patients. In these small subgroups, radiological staging and immunosuppressive treatment was not associated with the expression of these modulators in the peripheral blood and EBC. The expression of these modulators is not influenced by radiological staging or immunosuppression in this study, but these findings are limited by small residual sample sizes indicating the need to confirm these results in a larger cohort of sarcoidosis patients.
Conclusion
This study is the first to document depressed T-bet mRNA and protein expression in the peripheral blood of sarcoidosis patients compared to controls, suggesting a role for T-bet in modulating the TH1 immunological response and a possible role of T-bet in the sarcoidosis peripheral anergy. We also documented elevated IL-27 mRNA and IL-27p28 levels in PBMCs of sarcoidosis patients compared to controls. Given that this same trend of IL-27 expression was not observed in IL-27 protein levels in the plasma and that there were no differences in EBC IL-27 protein expression between patients and controls, IL-27 may have limited involvement in sarcoidosis. Larger scale studies are required to validate the reproducibility of these findings.
References
Ahmadzai H, Cameron B, Chui JJY et al (2013) Measurement of neopterin, TGF-β1 and ACE in the exhaled breath condensate of patients with sarcoidosis. J Breath Res 7:046003
Baughman RP, Culver DA, Judson MA (2011) A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med 183(5):573–581
Böyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest 97:77–89
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Crouser ED, Julian MW, Crawford M et al (2012) Differential expression of microRNA and predicted targets in pulmonary sarcoidosis. Biochem Biophys Res Commun 417:886–891
Djuretic IM, Levanon D, Negreanu V et al (2007) Erratum: transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol 8:145–153
Do JS, Visperas A, Oh K et al (2012) Memory CD4 T cells induce selective expression of IL-27 in CD8+ dendritic cells and regulate homeostatic naive T cell proliferation. J Immunol 188:230–237
Edmé JL, Tellart AS, Launay D et al (2008) Cytokine concentrations in exhaled breath condensates in systemic sclerosis. Inflamm Res 57:151–156
Gharaee-Kermani M, Hu B, Phan SH et al (2009) Recent advances in molecular targets and treatment of Idiopathic Pulmonary Fibrosis: focus on TGFβ signaling and the myofibroblast. Curr Med Chem 16:1400–1417
Hack CJ (2004) Integrated transcriptome and proteome data: the challenges ahead. Brief Funct Genomics Proteomics 3:212–219
Hill TA, Lightman S, Pantelidis P et al (2008) Intracellular cytokine profiles and T cell activation in pulmonary sarcoidosis. Cytokine 42:289–292
Huang S, Herbert C, Keoshkerian E et al (2013) IFN-γ levels in exhaled breath condensate and peripheral blood of sarcoidosis patients reflect an immune paradox. Respirology 18:48
Hunninghake GW, Fulmer JD, Young RC Jr et al (1979) Localization of the immune response in sarcoidosis. Am Rev Respir Dis 120:49–57
Hunninghake GW, Costabel U, Ando M et al (1999) Statement on sarcoidosis. Am J Respir Crit Care Med 160:736–755
Iannuzzi MC, Fontana JR (2011) Sarcoidosis: clinical presentation, immunopathogenesis, and therapeutics. J Am Med Assoc (JAMA) 305:391–399
Jung M, Schaefer A, Steiner I et al (2010) Robust MicroRNA stability in degraded RNA preparations from human tissue and cell samples. Clin Chem 56:998–1006
Kazani S, Israel E (2012) Utility of exhaled breath condensates across respiratory diseases. Am J Respir Crit Care Med 185:791–792
Kriegova E, Fillerova R, Tomankova T et al (2011) T-helper cell type-1 transcription factor T-bet is upregulated in pulmonary sarcoidosis. Eur Respir J 38:1136–1144
Larousserie F, Pflanz S, Coulomb-L’Herminé A et al (2004) Expression of IL-27 in human Th1-associated granulomatous diseases. J Pathol 202:164–171
Loke WSJ, Herbert C, Thomas PS (2013) Sarcoidosis: immunopathogenesis and immunological markers. Int J Chronic Dis. doi:10.1155/2013/928601
Ma F, Xu S, Liu X et al (2011) The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol 2011:861–869
Mathew S, Bauer KL, Fischoeder A et al (2008) The anergic state in sarcoidosis is associated with diminished dendritic cell function. J Immunol 181:746–755
Miyara M, Amoura Z, Parizot C et al (2006) The immune paradox of sarcoidosis and regulatory T cells. J Exp Med 203:359–370
Morgenthau AS, Iannuzzi MC (2011) Recent advances in sarcoidosis. Chest 139:174–182
Planck A, Katchar K, Eklund A et al (2003) T-lymphocyte activity in HLA-DR17 positive patients with active and clinically recovered sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 20:110–117
Pot C, Apetoh L, Awasthi A et al (2010) Molecular pathways in the induction of interleukin-27-driven regulatory type 1 cells. J Interferon Cytokine Res 30:381–388
Scadding JG (1961) Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years observation. Br Med J 2:1165–1172
Steiner D, Thomas M, Hu J et al (2011) MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity 35:169–181
Stumhofer JS, Laurence A, Wilson EH et al (2006) Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 7:937–945
Stumhofer JS, Silver JS, Laurence A et al (2007) Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 8:1363–1371
Swider C et al (1995) Presence of mRNA for interferon-gamma (IFN-γ) in blood mononuclear cells is associated with an active stage I sarcoidosis. Clin Exp Immunol 100:401–405
Topalian SL, Drake CG, Pardoll DM (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24:207–212
Villarino A, Hibbert L, Lieberman L et al (2003) The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19:645–655
Villarino AV, Huang E, Hunter CA (2004) Understanding the pro- and anti-inflammatory properties of IL-27. J Immunol 173:715–720
Yoshimura T, Takeda A, Hamano S et al (2006) Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol 177:5377–5385
Acknowledgments
We acknowledge the kind assistance of the staff at the Department of Respiratory Medicine, Prince of Wales Hospital, for their assistance with recruitment, and to the participants for donating their time and samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study complied with the ethical standards of the institution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Loke, W.S.J., Freeman, A., Garthwaite, L. et al. T-bet and interleukin-27: possible TH1 immunomodulators of sarcoidosis. Inflammopharmacol 23, 283–290 (2015). https://doi.org/10.1007/s10787-015-0247-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-015-0247-y