Abstract
The present work reports experimental data on the thermal conductivity of the four hydrocarbons cyclohexane, n-decane, n-hexadecane, and squalane in the liquid state at ambient pressure up to temperatures of 353.15 K. Absolute measurements were performed with a steady-state guarded parallel-plate instrument (GPPI) with an average expanded (coverage factor k = 2) measurement uncertainty of 2 %. For the linear alkanes n-decane and n-hexadecane as well as the cyclic compound cyclohexane, the measured thermal conductivities agree with reference correlations in the literature, indicating the reliability of the technique used for the study of fluids with relatively low thermal conductivities and weak absorption of radiation. For the first time, experimental data are determined for the long-branched alkane squalane between (278 and 353) K, which cannot be accurately represented with estimation methods commonly used in the literature. In summary, the present measurement results confirm the existing database for representative linear and cyclic hydrocarbons and provide first experimental thermal conductivities for squalane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hydrocarbons represent a fluid class that is among the most relevant ones in process and energy technology. Liquid hydrocarbons can be found as bulk chemicals and working fluids in various technical applications, such as their synthesis in the Fischer–Tropsch process [1] or the use as phase change media for energy storage [2, 3], fuels in combustion processes [4,5,6], and lubricating oils in machineries [7, 8]. For the modeling of heat transfer in such processes, one key property is the thermal conductivity of the involved fluids.
In general, there exists a relatively large experimental database for the thermal conductivity of liquid hydrocarbons in the literature. This holds especially true for linear n-alkanes with a carbon number between 5 and 16 as well as for the cyclic aliphatic and aromatic C6-based compounds cyclohexane and benzene including related derivatives. For these substances, corresponding reference correlations for the thermal conductivity as a function of temperature and pressure were developed by considering reliable experimental data sources and are recommended in the NIST reference database REFPROP [9]. These correlations are associated with typical uncertainties in the liquid state between (2 and 4) %. A considerably smaller experimental database is given for longer-chained and/or branched hydrocarbons such as the C30-based 2, 6, 10, 15, 19, 23-hexamethyltetracosane, commonly known as squalane. The latter compound represents also a potential high-viscosity reference fluid [10], but experimental information on its thermal conductivity is lacking to the best of the authors’ knowledge.
The aim of the present study is the accurate experimental determination of the thermal conductivity of four liquid hydrocarbons at ambient pressure as a function of temperature. In addition to validating and extending the existing database for the thermal conductivity of selected hydrocarbons, this study contributes to a recently started collaborative research project addressing the flow in oil-lubricated rotary displacement compressors, where the oil is in contact with the refrigerant. The efficiency of such compressor types depends strongly on the unavoidable gap flow between the rotating contour and the static housing that is influenced by the thermophysical properties of the oil–refrigerant mixtures [11]. Therefore, one main objective of this project is to improve the modeling of the thermophysical properties of such highly asymmetric mixtures based on the data of their pure components. This should be achieved by gradually increasing the asymmetry of binary model mixtures. In combination with the so-called natural refrigerants propane and carbon dioxide which are alternatives to classical refrigerants such as hydrofluorocarbons, the compounds n-decane (n-C10H22), n-hexadecane (n-C16H34), and squalane (C30H62) serve as model representatives with increasing molecular size on the oil side. For these hydrocarbons, the thermal conductivity data presented here serve as a reference for the corresponding mixture data to be measured later on. Cyclohexane (C6H12) was additionally studied here as a model hydrocarbon with a cyclic structure, which is relevant as, e.g., feedstock for the synthesis of plastic materials [12, 13] or as medium for the production of metallic oxide nanoparticles with dimensions of only a few nanometers [14]. In the present work, absolute measurements on the thermal conductivity of the oil-based compounds were performed by using a steady-state guarded parallel-plate instrument, GPPI [15] in the temperature range from (278 to 353) K at ambient pressure.
In the following, first the sample preparation and a concise description of the GPPI are given. Thereafter, the results of the thermal conductivity measurements of the investigated hydrocarbons are presented and discussed by comparison with existing reference correlations. In the case of squalane, the measurement results are correlated as function of temperature and used to test simple estimation schemes reported in the literature.
2 Experimental Section
2.1 Materials and Sample Preparation
Table 1 summarizes the relevant information about the used hydrocarbon chemicals studied in this work. It lists the molar mass M as well as the source and the specified purity of the samples. The certificates of analysis are given in the Supplementary Information. All samples were used as provided by the manufacturer. The water content of the samples with an expanded (coverage factor k = 2) uncertainty of 10 % was determined via coulometric Karl-Fischer titration (Metrohm 899) before and after the thermal conductivity measurements. In all cases, the average values of the water mass fractions listed in Table 1 are smaller than 10−4 which correspond to values smaller than 100 parts per million (ppm).
2.2 Guarded Parallel-Plate Instrument (GPPI): Thermal Conductivity
For the absolute measurement of the thermal conductivity λ of the four hydrocarbons at ambient pressure as a function of temperature T, a steady-state guarded parallel-plate instrument (GPPI) was used. Details of this instrument [15] and its application to the investigation of different fluids [16,17,18] can be found in the references provided. In this section, only the main features of the instrument and the experimental procedure relevant to the present study are described.
The working principle of the GPPI is to represent the ideal one-dimensional form of Fourier’s law of heat conduction for a planar sample as accurately as possible in the experiment. Here, the sample is placed between two parallel circular copper plates at a defined layer thickness. By maintaining a temperature difference ΔT between the two outer surfaces of the sample, a heat flux is transferred from the upper hot plate through the sample to the cold bottom plate as a result of the electrical power dissipated in the upper plate of known heat transfer area. To determine λ in an accurate way, advective heat transfer through the sample, parasitic heat leakages to the surrounding, and radiative heat transfer between the two plate surfaces have to be minimized or considered. Contributions from advection and heat leakages are suppressed to a minimum with the help of the design of the GPPI, cf. Refs. [15, 19].
Radiative heat transfer is reduced by the use of polished plate surfaces covered by a thin chrome layer with an emission coefficient of about 0.04. Since organic compounds are weakly absorbing fluids, the radiation contribution λr needs to be considered in order to accurately determine λ. For this, λr is subtracted from the thermal conductivity value λeff (= λ + λr) measured effectively in the experiment. Following the procedure from our previous works [16,17,18,19,20], λr was estimated by the approach proposed by Braun et al. [21] that is detailed in Refs. [19, 22]. As input parameters, the refractive index and the transmission spectra of the samples in the infrared wavelength range are required. Infrared refractive-index data n for the studied liquids are not available in the literature and are not easy to measure. As an approximation, the refractive index at the sodium vapor line (nD = 589.3 nm) and at the mean temperatures of all λ measurements were used, as collected from Refs. [18, 23]. The transmissions D of the four liquids were measured at ambient conditions with a Fourier transform infrared spectrometer (JASCO FT/IR-4600) for wave numbers ν ranging from (700 to 4000) cm−1 at an assumed layer thickness of 1 µm and were employed consistently for all temperatures studied in the thermal conductivity experiments. The raw data for the transmission spectra are provided in the Supplementary Information. For the investigated systems, the contribution of λr to the measured λeff values was found to be between (0.80 and 2.2) %.
In all thermal conductivity measurements, the sample layer thickness remained constant at (2.06 ± 0.04) mm (k = 2). Details to the procedure for sample filling can be found in Ref. [15]. To exchange the samples between two measurement series, approximately 250 ml of ethanol were circulated through the sample layer. Thereafter, remaining contaminations from the sample layer were removed by increasing T of the GPPI to 343 K and applying a vacuum for at least 0.5 h. Following this drying procedure, the sample layer was flushed with 50 ml of the sample to be investigated prior to the filling. Initially, n-decane was investigated, followed by cyclohexane, n-hexadecane, and squalane. For three ΔT = (2.0, 2.5, and 3.0) K, the thermal conductivity was measured from the lowest to the highest temperature at different mean T, corresponding to the averages between the surface temperatures of the hot and cold plates. While the lowest T investigated was set to 278.15 K for n-decane and squalane, somewhat larger values of 283.15 K for cyclohexane and 298.15 K for n-hexadecane were selected considering the melting points. The upper T investigated was adjusted to be 333.15 K for cyclohexane and 353.15 K for the other three liquids. For all liquids, repetition measurements conducted at (293.15 or 298.15) K after reaching the largest T were found to match the initial λ results clearly within the single experimental uncertainties. At the end, an independent repetition measurement with freshly filled n-decane was conducted to verify if there were changes in the sample thickness, formation of deposits on the plate surfaces, and/or cross-contaminations from previous fillings. The results for λ obtained from the repetition at (293.15, 313.15, and 333.15) K at ΔT = (2.0, 2.5, 3.0) K show excellent agreement with the data from the first measurement set with relative deviations within 0.3 %.
3 Results and Discussion
In this section, the thermal conductivity results for pure cyclohexane, n-decane, n-hexadecane, and squalane at ambient pressure are presented and discussed first. This includes a comparison of the results from this work with existing reference correlations for cyclohexane, n-decane, and n-hexadecane. Thereafter, the measured thermal conductivities of squalane are correlated as a function of T between (278 and 353) K and are compared against selected estimation models.
3.1 Thermal Conductivity of Investigated Hydrocarbons
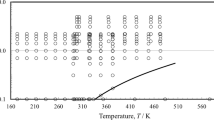
The individual results for the thermal conductivity of pure cyclohexane, squalane, n-decane and n-hexadecane obtained at ΔT = (2.0, 2.5, and 3.0) K are summarized in the Supplementary Information. Since the individual results of same uncertainties agree very well with each other and clearly within their measurement uncertainties for each liquid and state point, an unweighted averaging of the thermal conductivities was performed. These final measurement results for the thermal conductivity with a total expanded (k = 2) measurement uncertainty of 2 % are listed in Table 2 and are illustrated in Fig. 1 as a function of temperature. For the average temperatures T, an expanded (k = 2) uncertainty of 11 mK can be stated based on the procedure discussed in Ref. [15]. In Fig. 1, the reference correlations λref of cyclohexane [24], n-decane [25], n-hexadecane [26] with stated estimated uncertainties of (2.5, 3.0, and 4.0) %, respectively, are also included for comparison.
Thermal conductivity λ of cyclohexane ( ), n-decane (
), n-decane ( ), n-hexadecane (
), n-hexadecane ( ), and squalane (■) at atmospheric pressure as a function of temperature obtained by GPPI and listed in Table 2. The error bars represent the expanded (k = 2) uncertainties of the measurement results of 2 % which are exemplarily shown for each measurement series. The solid lines indicate the reference correlations for λ of cyclohexane (
), and squalane (■) at atmospheric pressure as a function of temperature obtained by GPPI and listed in Table 2. The error bars represent the expanded (k = 2) uncertainties of the measurement results of 2 % which are exemplarily shown for each measurement series. The solid lines indicate the reference correlations for λ of cyclohexane ( ) [24], n-decane (
) [24], n-decane ( ) [25], and n-hexadecane (
) [25], and n-hexadecane ( ) [26]. The dashed line (– –) serves as a guide for the eye for representing the thermal conductivities of squalane
) [26]. The dashed line (– –) serves as a guide for the eye for representing the thermal conductivities of squalane
Figure 1 shows that the thermal conductivities of the four investigated hydrocarbons cover relatively low values in a total range between about (110 and 145) mW·m−1·K−1 and decrease linearly with increasing T. Moreover, λ increases with increasing carbon number, with the exception of the branched alkane squalane. The latter compound shows thermal conductivities between those of n-decane and n-hexadecane. It has been reported in literature [27, 28] that the thermal conductivity of branched alkanes is lower than that of their linear isomers, indicating the reduced capability of branched compounds to transport thermal energy. It is worth to mention that the experimental λ results for squalane exhibit the weakest temperature-dependent dependency, while the other three linear and cyclic hydrocarbons show steeper and very similar trends.
For cyclohexane, n-decane, n-hexadecane, a very good agreement between the present measurement results and the reference correlations [24,25,26] is observed, cf. Figure 1. As visualized in more detail in Fig. 2, the relative percentage deviations of all measured data from λref are in a range between (− 0.9 and + 1.3) %, i.e., clearly within the experimental uncertainties. In addition, the temperature-dependent trend of the reference correlations is reproduced by the measurement results. A further comparison with available experimental data for the thermal conductivity of the three relevant substances is not in the scope of the present work since all the considered reference correlations have been developed based on critically selected, reliable experimental data sets. Overall, the agreement of the present measurement results with reference data once again demonstrates the reliability of the used GPPI for the absolute determination of the thermal conductivity of fluids.
Relative deviations of the thermal conductivities of cyclohexane ( ), n-decane (
), n-decane ( ), and n-hexadecane (
), and n-hexadecane ( ) at atmospheric pressure measured in this work from the corresponding reference correlations [24,25,26] as a function of temperature. The dashed lines represent the expanded (k = 2) uncertainties of λref given by 2.5 % for cyclohexane (
) at atmospheric pressure measured in this work from the corresponding reference correlations [24,25,26] as a function of temperature. The dashed lines represent the expanded (k = 2) uncertainties of λref given by 2.5 % for cyclohexane ( ), 3.0 % for n-decane (
), 3.0 % for n-decane ( ) and 4.0 % for n-hexadecane (
) and 4.0 % for n-hexadecane ( )
)
3.2 Thermal Conductivity of Squalane
For squalane, the first experimental λ data reported in literature so far are listed in Table 2. Figure 3 depicts the experimental data for λ at ambient pressure as a function of T, together with a simple correlation for the thermal conductivity of squalane. This correlation represents a fit of the measurement results in the form of a polynomial of second-order fit with respect to temperature T in Kelvin between (278 and 353) K according to
Thermal conductivity λ of squalane at atmospheric pressure measured in this work (■) and correlated via Eq. 1 (—) in comparison with calculated values obtained from the Latini model [29, 30] (– –) and the Sastri model [31] (– ▪ –) as a function of temperature. The error bars represent the expanded (k = 2) uncertainties of the measurement results of 2 % which are exemplarily shown
In the fitting, the experimental data were considered with equal statistical weight. Table 3 summarizes the fit coefficients of Eq. 1 and their statistical uncertainties (k = 2). All measured thermal conductivities deviate from λcor within ± 0.28 %, with an average absolute relative deviation (AARD) of 0.12 %.
To check whether common estimation schemes for the thermal conductivity of liquids can represent the experimental behavior, we applied the original version of the Latini model [29, 30] and the Sastri model [31], as described in Ref. [32]. In the latter reference, it is stated that these two empirical models can estimate the thermal conductivity of organic liquids reasonably well with typical deviations from experimental data below 15 %. As input for both models, the required information on the normal boiling temperature at 0.1 MPa, Tb = 695.2 K [33], and the critical temperature Tc = 795.9 K [34] were taken from measurement results for squalane reported in the literature. Furthermore, the molar mass M provided in Table 1 is included in the Latini model.
As can be seen from Fig. 3, the Latini model [29, 30] overestimates the experimental λ values of squalane by around +6.9 % at 278.15 K and +2.8 % at 353.15 K, while the Sastri model [31] gives underestimations by −5.4 % at 278.15 K and −7.1 % at 353.15 K. In both cases, the T-dependency of the thermal conductivity is somewhat too large in comparison to the experimental behavior. The values for λ calculated from both models are also very sensitive to minor variations in Tb and Tc. This demonstrates that accurate experimental data are indispensable for testing and further developing prediction or, better to say, estimation models for the thermal conductivity of liquids, in particular for relatively long-chained hydrocarbons such as squalane.
4 Conclusions
The present work provides measurement results for the thermal conductivity of liquid cyclohexane, n-decane, n-hexadecane, and squalane at atmospheric pressure as a function of temperature up to 353 K with an expanded (k = 2) uncertainty of 2 %. The comparison of the measured thermal conductivities of cyclohexane, n-decane, and n-hexadecane with existing reference correlations revealed very good agreement with a maximum deviation of 1.3 %. This validates the performance of the used steady-state guarded parallel-plate instrument for the study of liquids with relatively low thermal conductivity and weak absorption of radiation, where thermal radiation effects need to be considered. In the case of squalane between (278 and 353) K, a new experimental database for the thermal conductivity was established and tested against common estimation schemes that fail in predicting the behavior quantitatively. Thus, the results from this work can serve as a reference not only for testing and improving corresponding correlation schemes, but also for future investigations on the thermal conductivity of binary mixtures with so-called natural refrigerants such as carbon dioxide and propane.
5 Supplementary Information
Raw data related to the thermal conductivity measurements and infrared spectra are available in two Microsoft Excel files. The file with the name “1_Raw_data_thermal_conductivity_hydrocarbons.xlsx” contains the raw data for the thermal conductivity measurement of pure cyclohexane, n-decane, n-hexadecane, and squalane, while the raw data for the infrared spectra are given in the file “2_Raw_data_infrared_spectrum_hydrocarbons.xlsx”.
References
P.M. Maitlis, A. de Klerk (eds.), Greener Fischer-Tropsch Processes for Fuels and Feedstocks, 1st edn. (Wiley, Hoboken, 2013). https://doi.org/10.1002/9783527656837
C. Vélez, M. Khayet, J.M. Ortiz de Zárate, Temperature-dependent thermal properties of solid/liquid phase change even-numbered n-alkanes: n-hexadecane, n-octadecane and n-eicosane. Appl. Energy 143, 383–394 (2015). https://doi.org/10.1016/j.apenergy.2015.01.054
S. Kashani, A.A. Ranjbar, M.M. Madani, M. Mastiani, H. Jalaly, Numerical study of solidification of a nano-enhanced phase change material (NEPCM) in a thermal storage system. J. Appl. Mech. Tech. Phys. 54, 702–712 (2013). https://doi.org/10.1134/S0021894413050027
X. Hui, C. Zhang, M. Xia, C.-J. Sung, Effects of hydrogen addition on combustion characteristics of n-decane/air mixtures. Combust. Flame 161, 2252–2262 (2014). https://doi.org/10.1016/j.combustflame.2014.03.007
C. Xu, Q. Wang, Y. Song, K. Liu, X. Li, Explosion characteristics of n-decane/hydrogen/air mixtures. Int. J. Hydrog. Energy 47, 38837–38848 (2022). https://doi.org/10.1016/j.ijhydene.2022.09.048
F. Oppong, X. Li, C. Xu, Y. Li, Q. Wang, Y. Liu, L. Qian, Investigation on n-decane–hydrogen laminar combustion characteristics using the constant volume combustion method. Int. J. Hydrog. Energy 53, 1350–1360 (2024). https://doi.org/10.1016/j.ijhydene.2023.11.361
I.S.Y. Ku, T. Reddyhoff, R. Wayte, J.H. Choo, A.S. Holmes, H.A. Spikes, Lubrication of microelectromechanical devices using liquids of different viscosities. J. Tribol. (2012). https://doi.org/10.1115/1.4005819
M. Diaby, M. Sablier, A. Le Negrate, M. El Fassi, Kinetic study of the thermo-oxidative degradation of squalane (C30H62) modeling the base oil of engine lubricants. J. Eng. Gas Turbines Power (2009). https://doi.org/10.1115/1.3155797
E.W. Lemmon, M.L. Huber, M.O. McLinden, NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties (REFPROP), Version 100; National Institute of Standards and Technology, Standard Reference Data Program (NIST, Gaithersburg, 2018)
J. Fernandez, M.J. Assael, R.M. Enick, J.P.M. Trusler, International standard for viscosity at temperatures up to 473 K and pressures below 200 MPa (IUPAC Technical Report). Pure Appl. Chem. 91, 161–172 (2019). https://doi.org/10.1515/pac-2018-0202
K. Kauder, R. Deipenwisch, Non-Newtonian oil in screw type compressors. VDI Berichte 1391, 45–59 (1998)
P. Wu, P. Bai, Z. Yan, G.X.S. Zhao, Gold nanoparticles supported on mesoporous silica: origin of high activity and role of Au NPs in selective oxidation of cyclohexane. Sci. Rep. 6, 18817 (2016). https://doi.org/10.1038/srep18817
U. Schuchardt, D. Cardoso, R. Sercheli, R. Pereira, R.S. Da Cruz, M.C. Guerreiro, D. Mandelli, E.V. Spinacé, E.L. Pires, Cyclohexane oxidation continues to be a challenge. Appl. Catal. A 211, 1–17 (2001). https://doi.org/10.1016/S0926-860X(01)00472-0
M.Z. Hossain, D. Hojo, A. Yoko, G. Seong, N. Aoki, T. Tomai, S. Takami, T. Adschiri, Dispersion and rheology of nanofluids with various concentrations of organic modified nanoparticles: modifier and solvent effects. Colloids Surf. A 583, 123876 (2019). https://doi.org/10.1016/j.colsurfa.2019.123876
F.E. Berger Bioucas, M.H. Rausch, T.M. Koller, A.P. Fröba, Guarded parallel-plate instrument for the determination of the thermal conductivity of gases, liquids, solids, and heterogeneous systems. Int. J. Heat Mass Transf. 212, 124283 (2023). https://doi.org/10.1016/j.ijheatmasstransfer.2023.124283
F.E.B. Bioucas, T.M. Koller, A.P. Fröba, Thermal conductivity of glycerol at atmospheric pressure between 268 K and 363 K by using a steady-state parallel-plate instrument. Int. J. Thermophys. 45, 52 (2024). https://doi.org/10.1007/s10765-024-03347-x
F.E. Berger Bioucas, M.H. Rausch, J. Schmidt, A. Bück, T.M. Koller, A.P. Fröba, Effective thermal conductivity of nanofluids: measurement and prediction. Int. J. Thermophys. 41, 55 (2020). https://doi.org/10.1007/s10765-020-2621-2
F.E. Berger Bioucas, T.M. Koller, A.P. Fröba, Effective thermal conductivity of microemulsions consisting of water micelles in n-decane. Int. J. Heat Mass Transf. 200, 123526 (2023). https://doi.org/10.1016/j.ijheatmasstransfer.2022.123526
M.H. Rausch, K. Krzeminski, A. Leipertz, A.P. Fröba, A new guarded parallel-plate instrument for the measurement of the thermal conductivity of fluids and solids. Int. J. Heat Mass Transf. 58, 610–618 (2013). https://doi.org/10.1016/j.ijheatmasstransfer.2012.11.069
F.E. Berger Bioucas, M. Piszko, M. Kerscher, P. Preuster, M.H. Rausch, T.M. Koller, P. Wasserscheid, A.P. Fröba, Thermal conductivity of hydrocarbon liquid organic hydrogen carrier systems: measurement and prediction. J. Chem. Eng. Data 65, 5003–5017 (2020). https://doi.org/10.1021/acs.jced.0c00613
R. Braun, S. Fischer, A. Schaber, Elimination of the radiant component of measured liquid thermal conductivities. Wärme Stoffübertrag 17, 121–124 (1983). https://doi.org/10.1007/BF01007228
A.P. Fröba, M.H. Rausch, K. Krzeminski, D. Assenbaum, P. Wasserscheid, A. Leipertz, Thermal conductivity of ionic liquids: measurement and prediction. Int. J. Thermophys. 31, 2059–2077 (2010). https://doi.org/10.1007/s10765-010-0889-3
W. O’Reilly, Numerical data and functional relationships in science and technology. Phys. Earth Planet. Interiors 45, 304–305 (1987). https://doi.org/10.1016/0031-9201(87)90019-7
A. Koutian, M.J. Assael, M.L. Huber, R.A. Perkins, Reference correlation of the thermal conductivity of cyclohexane from the triple point to 640 K and up to 175 MPa. J. Phys. Chem. Ref. Data 46, 013102 (2017). https://doi.org/10.1063/1.4974325
M.L. Huber, R.A. Perkins, Thermal conductivity correlations for minor constituent fluids in natural gas: n-octane, n-nonane and n-decane. Fluid Phase Equilib. 227, 47–55 (2005). https://doi.org/10.1016/j.fluid.2004.10.031
S.A. Monogenidou, M.J. Assael, M.L. Huber, Reference correlation for the thermal conductivity of n-hexadecane from the triple point to 700 K and up to 50 MPa. J. Phys. Chem. Ref. Data 47, 013103 (2018). https://doi.org/10.1063/1.5021459
J.C.G. Calado, J.M.N.A. Fareleira, C.A. Nieto de Castro, W.A. Wakeham, Thermal conductivity of five hydrocarbons along the saturation line. Int. J. Thermophys. 4, 193–208 (1983). https://doi.org/10.1007/BF00502352
H. Watanabe, Thermal conductivity and thermal diffusivity of sixteen isomers of alkanes: CnH2n+2 (n = 6 to 8). J. Chem. Eng. Data 48, 124–136 (2003). https://doi.org/10.1021/je020125e
C. Baroncini, P. Di Filippo, G. Latini, M. Pacetti, An improved correlation for the calculation of liquid thermal conductivity. Int. J. Thermophys. 1, 159–175 (1980). https://doi.org/10.1007/BF00504518
G. Latini, M. Pacetti, The thermal conductivity of liquids—a critical survey, in thermal conductivity, vol. 15, ed. by V.V. Mirkovich (Springer, Boston, 1978), pp.245–253. https://doi.org/10.1007/978-1-4615-9083-5_31
S.R.S. Sastri, K.K. Rao, A new temperature–thermal conductivity relationship for predicting saturated liquid thermal conductivity. Chem. Eng. J. 74, 161–169 (1999). https://doi.org/10.1016/S1385-8947(99)00046-7
B.E. Poling, J.M. Prausnitz, J.P. O’Connell, The Properties of Gases and Liquids, 5th edn. (McGraw-Hill, New York, 2001)
W.H. Gourdin, Estimate of the vapor pressure of squalane at approximately 293 K using a Knudsen cell method. J. Chem. Eng. Data 66, 1630–1639 (2021). https://doi.org/10.1021/acs.jced.0c00914
D.M. VonNiederhausern, G.M. Wilson, N.F. Giles, Critical point and vapor pressure measurements at high temperatures by means of a new apparatus with ultralow residence times. J. Chem. Eng. Data 45, 157–160 (2000). https://doi.org/10.1021/je990232h
Acknowledgements
The authors gratefully acknowledge funding of the Erlangen Graduate School in Advanced Optical Technologies (SAOT) by the Bavarian State Ministry for Science and Art.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) via Project Grant FR1709/28-1 within the Research Unit FOR 5595 Archimedes (Oil-refrigerant multiphase flows in gaps with moving boundaries—Novel microscopic and macroscopic approaches for experiment, modeling, and simulation)—Project Number 510921053.
Author information
Authors and Affiliations
Contributions
Experimental investigations and evaluations were performed by Francisco E. Berger Bioucas. The first draft of the manuscript was written by Francisco E. Berger Bioucas. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berger Bioucas, F.E., Rausch, M.H., Koller, T.M. et al. Thermal Conductivity of Liquid Cyclohexane, n-Decane, n-Hexadecane, and Squalane at Atmospheric Pressure up to 353 K Determined with a Steady-State Parallel-Plate Instrument. Int J Thermophys 45, 93 (2024). https://doi.org/10.1007/s10765-024-03383-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-024-03383-7