Abstract
The present work reports experimental data for the thermal conductivity of glycerol which is an important fluid in many technical applications. Measurements were performed in an absolute way at ambient pressure using a steady-state guarded parallel-plate instrument (GPPI) with an average expanded (k = 2) measurement uncertainty of 2.3%. For data representation over a temperature range from (268.15 to 363.15) K in steps of 5 K, the thermal conductivities are averaged from measurements at three different temperature gradients for each temperature. The present results indicate an almost constant thermal conductivity of glycerol over the studied temperature range and agree with the sparse experimental data available in the literature. Based on the experimental database including the results from this work, a simple correlation for the thermal conductivity of glycerol at 0.1 MPa as a function of temperature between (268 and 413) K is suggested. The additional study on the influence of water as possible contamination up to water mass fractions of 0.02 on the thermal conductivity of glycerol reveals negligible changes. Overall, the experimental results from this work contribute to an improved data situation for the thermal conductivity of glycerol, particularly in the subcooled liquid region at temperatures below 283 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Glycerol is a highly viscous liquid at ambient conditions and has as a very low toxicity [1]. It shows a relatively broad liquid range between about (291 and 563) K at 0.1 MPa [2]. Furthermore, it is well known that glycerol which forms hydrogen bonds can represent a very stable supercooled liquid at temperatures below its melting point Tm of about 291 K down to about 200 K [2,3,4]. Considering these characteristics, glycerol represents a suitable heat transfer fluid for processes involving food, hygienic, and pharmaceutical products [5] as well as in biotechnological and cryopreservation applications [6]. Moreover, glycerol is commonly used in the preparation of nanofluids, where solid nanoparticles of relatively large thermal conductivity are added to liquids [7]. These nanofluids are of potential interest for heat transfer applications [8] but also for process technology serving as cutting [9] or lubrication [10] fluids. Due to its high viscosity and relatively low cost, glycerol is also proposed as a reference fluid for the calibration of transient instruments for thermal conductivity measurements [11, 12].
Despite the numerous applications of glycerol in many fields of heat transfer, the knowledge of the thermal conductivity λ is very limited for this fluid. Until today, only three experimental temperature-dependent studies [13,14,15] dating back to years between 1927 and 1951 are available to the best of the authors’ knowledge. The measurements were performed at ambient pressure for a range of temperatures T between (283.15 and 413.15) K using steady-state parallel-plate instruments of unspecified experimental uncertainties. Although the datasets agree quantitively well, slightly different T-dependent trends were observed. Kaye et al. [13] determined λ of various liquids within a broad T range from (293.15 to 413.15) K. For glycerol, the thermal conductivity was found to increase linearly with increasing T. Bates [14] studied the thermal conductivity of binary mixtures of glycerol and water from (283.14 to 353.12) K. Their results indicate that λ of pure glycerol is constant over the studied T range. Furthermore, the measurements results obtained by Riedel [15] between (293.14 and 373.12) K show an increasing thermal conductivity with increasing T.
In the present work, the thermal conductivity of glycerol was measured at ambient pressure with an absolute method in the form of a guarded parallel-plate instrument. This was motivated not only by a check and extension of the existing database for the thermal conductivity of glycerol but also by an ongoing research project at the Institute of Advanced Optical Technologies – Thermophysical Properties (AOT-TP). The main objective of this project is to contribute to a fundamental understanding of the effective thermal conductivity of dispersions with a liquid continuous phase in the form of nanofluids [9, 16, 17] and microemulsions [18] by experimental and theoretical investigations. Here, the proposed nanofluid systems also include glycerol as base fluid. In order to predict the effective thermal conductivity of these nanofluids, the accurate knowledge of the thermal conductivity of glycerol is required. For the newly designed GPPI instrument used in the present work, its reliability for accurate thermal conductivity measurements of fluids and solids with expanded (k = 2) uncertainties down to about 2% has been discussed in a recent study [17]. The current experiments on pure glycerol were conducted from (268 to 363) K in steps of 5 K, i.e., crossing the transition from the supercooled liquid to the liquid state. To address the hygroscopic nature of glycerol, the effect of water contaminations on λ of glycerol was studied additionally up to a water mass fraction of 0.02 between (283 and 358) K.
In the following, the experimental section is given which includes the sample preparation and a brief introduction to the used GPPI. Thereafter, the measurement results for the thermal conductivity of pure glycerol are presented and discussed by comparison with the available literature data. All experimental datasets have been used to develop a correlation for the thermal conductivity of glycerol at atmospheric pressure within the temperature range of (268 and 413) K. Finally, the influence of water on the thermal conductivity of glycerol is analyzed.
2 Experimental Section
2.1 Materials and Sample Preparation
Glycerol (propane-1,2,3-triol; CAS: 56-81-5; Cat. Number: 036646.K2) with a stated purity of 99.99% by gas chromatography was obtained from Thermo Fisher Scientific. The glycerol was used as provided by the manufacturer for the measurement of the thermal conductivity. The water content of the original glycerol sample was determined via coulometric Karl Fischer titration (Metrohm 899) before and after the thermal conductivity measurements, resulting in values of (0.141 ± 0.004) wt% and (0.337 ± 0.013) wt%, respectively. Here, the uncertainty represents the double standard deviation (k = 2) of five independent measurements. The slight increase in the water content, which is on average 0.239 wt%, is attributed to the hygroscopic nature of glycerol. The results obtained from this measurement refer to the “pure” glycerol sample.
To vary the water content of the used glycerol sample, the latter underwent a drying under reduced pressure at 358 K over 4 h. For this degassed sample, the water content before and after the thermal conductivity measurements were (0.045 ± 0.005) wt% and (0.162 ± 0.022) wt%, i.e., an average water content of 0.104 wt% which is only slightly lower than prior to the drying. Furthermore, two additional samples with a slightly larger average water contents of (1.02 and 2.01) wt% were prepared by weighting using a Sartorius Entris balance (type 224I-1S) with an estimated expanded (k = 2) uncertainty of 1 mg. Here, deionized water with a specific resistance larger than 10 MΩ·cm was used.
2.2 Guarded Parallel-Plate Instrument (GPPI): Thermal Conductivity
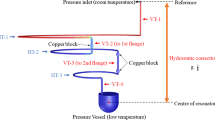
The thermal conductivity of glycerol and its mixtures with water was measured at ambient pressure with a steady-state guarded parallel-plate instrument (GPPI). A comprehensive description of this new self-developed and fully-automated apparatus can be found in Ref. [17]. Here, the performance of this absolute measurement instrument was validated by studying various liquids, gases, and solids within the T range of (283.15 to 358.15) K, corresponding to a broad range of thermal conductivities from about (0.02 up to 20) W·m−1·K−1. The applicability of the GPPI was also been shown for the determination of the effective thermal conductivity of, e.g., ionic liquids [19] and heterogeneous systems, including nanofluids [9, 16, 17] and microemulsions [18]. In the following, only a brief description of the working principle of the used GPPI is given.
The GPPI relies on the application of the ideal one-dimensional form of the Fourier law of heat conduction for a planar sample. The sample with defined thickness s is confined between two parallelly arranged, circular copper plates, i.e., an upper hot plate and a lower cold plate. By applying a temperature difference ΔT between the two outer surfaces of the sample, a heat flux provided by electrical heating to the top plate of a given heat transfer area is transferred through the sample. Based on the measured temperature gradient and heat flux density, the thermal conductivity λ of the sample of interest can be determined. Contributions from heat leakages and convection are considered to be negligible due to the design of the GPPI, as elaborated in Refs. [17, 20]. Since glycerol and water strongly absorb radiation, its contribution to the measured overall heat flux is insignificantly small [17].
The procedure for sample filling is described in detailed in Ref. [17]. Due to the high viscosity of glycerol, the samples were preheated to 343 K to reduce its viscosity and sonicated in an ultrasonic bath (Type RK 103H from Bandelin) to remove air incrustations. The temperature of the GPPI was also adjusted to 343 K. Then, the glycerol-based samples were slowly circulated using two syringes until no air bubbles were observed. For pure glycerol having an average water content of 0.239 wt%, the thermal conductivity was measured at mean temperatures T, i.e., the averages between the surface temperatures of the hot and cold plates, between (268.15 and 363.15) K. Here, s = 1.81 mm and three ΔT of (3.0, 3.5, and 4.0) K were employed. For the three measurements on the water-glycerol mixtures with average water contents of (0.104, 1.02, and 2.01) wt%, parameters of s = 1.29 mm and ΔT = 3.5 K were adjusted at mean T from (283.14 to 358.14) K. Here, the sample thickness remained fixed, while the water concentration was systematically increased from the lowest to the highest value. In between the fillings, the sample layer was flushed with approximately 250 ml of water, ethanol, and acetone. Subsequently, the sample layer was dried by increasing T of the GPPI to 343 K and applying vacuum for at least 30 min.
3 Results and Discussion
This section discusses first the thermal conductivity results for pure glycerol. After presenting the results from this work, a data comparison with the available literature data is carried out. As a result, a correlation for the thermal conductivity of glycerol at atmospheric pressure is developed within the temperature range between (268 and 413) K. As add-on, the present measurement results for the influence of water on λ of glycerol are summarized.
3.1 Thermal Conductivity of Glycerol
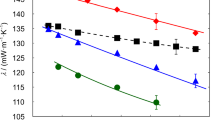
The results for the thermal conductivity of pure glycerol with an average water content of 0.239 wt% obtained at ΔT = (3.0, 3.5, and 4.0) K are summarized in Table 1 and illustrated in Fig. 1 as a function of temperature between (268.15 and 363.15) K. Besides the individual ΔT-dependent results for λ and their expanded (k = 2) uncertainties, also the final thermal conductivities and uncertainties obtained by unweighted averaging are included. A total measurement uncertainty between (2.24 and 2.28)% can be specified for the averaged λ results for glycerol. For the average temperatures T, an expanded uncertainty (k = 2) of 11 mK can be specified in accordance with the procedure detailed in Ref. [17]. As can be seen from Fig. 1, the λ data obtained for the three different ΔT agree very well with each other and clearly within the measurement uncertainty of the single data. Therefore, an averaging of the thermal conductivities can be justified.
Thermal conductivity of pure glycerol with an average water content of 0.239 wt% at atmospheric pressure as a function of temperature obtained by GPPI for s = 1.81 mm:  , ΔT = 3.0 K;
, ΔT = 3.0 K;  , ΔT = 3.5 K;
, ΔT = 3.5 K;  , ΔT = 4.0 K;
, ΔT = 4.0 K;  , averaged final results for λ. The error bars represent the expanded (k = 2) uncertainties which are exemplarily shown for the results with ΔT = 3.0 K and the final results for λ. The expanded (k = 2) uncertainties for the experimental data are between (2.24 and 2.28)%
, averaged final results for λ. The error bars represent the expanded (k = 2) uncertainties which are exemplarily shown for the results with ΔT = 3.0 K and the final results for λ. The expanded (k = 2) uncertainties for the experimental data are between (2.24 and 2.28)%
The measurements reveal a parabolic temperature dependency, showing a decrease in λ from (268.15 to 278.15) K, a nearly constant behavior from (278.15 to 313.15) K, and a subsequent weak linear increase from (313.15 to 363.15) K. Nevertheless, the maximum change in λ within the studied T range is only 1.6% based on the final data. Interestingly, the most pronounced change in the thermal conductivity as a function of T appears in the subcooled liquid state below Tm of glycerol around 291 K. The change in the orientation of the molecules at the transition from the liquid to the subcooled liquid state implies an increase in λ. Since the present database of thermal conductivities at T below Tm is limited, a deeper discussion of the effect of subcooling on λ is out of the scope of the present work.
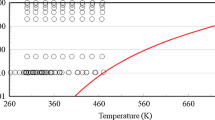
In upper part of Fig. 2, the final values for the thermal conductivity obtained from this work are compared with the experimental data in literature from three sources determined also by parallel-plate instruments [13,14,15]. For the three sources, information about the experimental uncertainties for λ and the water content of the studied samples is lacking. Overall, good agreement of all thermal conductivity datasets within ± 2.3% is found within the T range. The results from Kaye et al. [13] from (293.15 to 413.15) K with a stated “accuracy” of 1% exhibit an increasing trend in λ with increasing temperature, which is somewhat in contrast to our experimental results. The data published by Bates [14] between (283.14 and 353.12) K are in very good agreement with the present measurement results with an average absolute relative deviation (AARD) of 0.44% at matching temperature points. While Bates [14] reported a constant value for the thermal conductivity in the T range investigated, our results mirror this trend only in the range between (278.15 and 313.15) K, followed by a linear increase of λ at larger T. The results measured by Riedel [15] from (293.14 to 373.12) K agree with our experimental results with relative deviations between (− 0.19 and 1.23)%.
Measurement results for thermal conductivity of pure glycerol at atmospheric pressure as a function of temperature obtained in the present work and in literature (upper part) and their deviation from Eq. 1 (lower part):  , this work, averaged final results for λ (water content of 0.239 wt%);
, this work, averaged final results for λ (water content of 0.239 wt%);  , λcor according to Eq. 1;
, λcor according to Eq. 1;  , Kaye et al. [13];
, Kaye et al. [13];  , Bates [14];
, Bates [14];  , Riedel [15]. The error bars represent the expanded (k = 2) uncertainties which are exemplarily shown for the final results for λ. For the data reported by Kaye [13], the exemplary error bar corresponds to two times the stated “accuracy”
, Riedel [15]. The error bars represent the expanded (k = 2) uncertainties which are exemplarily shown for the final results for λ. For the data reported by Kaye [13], the exemplary error bar corresponds to two times the stated “accuracy”
Considering the sound agreement of the available experimental data with our measurement results within their expanded uncertainties, a correlation for the description of the thermal conductivity as a function of temperature is developed. It accounts for the four datasets shown in the upper part of Fig. 1, i.e., the three datasets from the literature [13,14,15] and the dataset from the present work given in Table 1 in the form of the averaged final results. The correlation for the thermal conductivity of glycerol at ambient pressure, λcor, is represented by a polynomial of second order with respect to temperature between (268 and 413) K according to
In the fitting procedure, the experimental data were considered with a statistical weight of 1/N, where N corresponds to the number of data points per dataset. In this way, a more balanced weighting of the datasets with scheme is selected, where datasets with a larger number of data points are weighted with a smaller weight. Table 2 summarizes the fit coefficients of the correlation in Eq. 1 and their uncertainties (k = 2). As can be seen in the lower part of Fig. 2, the maximum and minimal deviation of the four considered datasets for the thermal conductivities from λcor is (− 1.61 and 1.64)%, with an AARD value of 0.59%.
3.2 Influence of Water on Thermal Conductivity of Glycerol
The results for the thermal conductivity of the three additionally investigated glycerol-based samples with varying average water concentrations of (0.104, 1.02, and 2.01) wt% measured at atmospheric pressure from (283.14 to 358.14) K are listed in Table 3 and are shown in Fig. 3 as a function of T. Due to the used smaller sample thickness s = 1.30 mm at a given ΔT = 3.5 K, the expanded (k = 2) uncertainties for λ are slightly larger with 3.2% on average. Here, the average expanded uncertainty (k = 2) of the mean temperatures T is 13 mK. In Fig. 3, also averaged measurement results in Table 1 for a water content of 0.239 wt% are included.
Thermal conductivity of glycerol-based samples with varying average water contents at atmospheric pressure as a function of temperature obtained by GPPI for s = 1.30 mm and ΔT = 3.5 K:  , 0.104 wt%;
, 0.104 wt%;  , 1.016 wt%;
, 1.016 wt%;  , 2.014 wt%;
, 2.014 wt%;  , averaged final results for glycerol sample with water content of 0.239 wt%. Exemplary error bars are given for each set of data, which correspond to the measured expanded (k = 2) uncertainties of the thermal conductivity (Δλ/λ = ± 3.1%)
, averaged final results for glycerol sample with water content of 0.239 wt%. Exemplary error bars are given for each set of data, which correspond to the measured expanded (k = 2) uncertainties of the thermal conductivity (Δλ/λ = ± 3.1%)
The measurement results indicate a weak increase of λ of the glycerol-based samples with increasing water content for all studied T. This behavior is also in agreement with experimental results from Bates [14] for glycerol-water mixtures and is related to the about two times larger λ value of water compared to that of glycerol. In comparison with the results for the dried glycerol with a water concentration of 0.102 wt%, an average increase of 1.5% is observed in the thermal conductivity when the water content is increased to 2.014 wt%. Importantly, these changes in λ fall within the measurement uncertainties of the individual data. These findings suggest a very minor impact of water traces on λ of glycerol up to water contents of 2% in mass.
4 Conclusion
This work contributes to a reliable experimental database for the thermal conductivity of liquid glycerol at atmospheric pressure across a wide temperature range from (268.15 to 363.15) K including temperatures below 283 K in the subcooled liquid state. The measurements were performed in an absolute manner employing a steady-state guarded parallel-plate instrument. The experimental results for the thermal conductivity of glycerol with an average expanded (k = 2) uncertainty of 2.3% exhibit reasonable agreement with the few available experimental data in literature. Considering all available experimental datasets, a correlation for the thermal conductivity of pure glycerol at 0.1 MPa between (268 and 413) K was developed. Furthermore, a systematic study on the effect of the water concentration on the thermal conductivity of glycerol-based solutions up to 2 wt% led to an average increase of 1.5% compared to the values for the almost water-free samples. These minor variations within the measurement uncertainties highlight the negligible influence of water traces on the thermal conductivity of glycerol.
References
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), A. Mortensen, F. Aguilar, R. Crebelli, A. DiDomenico, B. Dusemund, M.J. Frutos, P. Galtier, D. Gott, U. Gundert-Remy, J.-C. Leblanc, O. Lindtner, P. Moldeus, P. Mosesso, D. Parent-Massin, A. Oskarsson, I. Stankovic, I. Waalkens-Berendsen, R.A. Woutersen, M. Wright, M. Younes, P. Boon, D. Chrysafidis, R. Gürtler, P. Tobback, A.M. Rincon, A. Tard, C. Lambré, Re-evaluation of glycerol (E 422) as a food additive. EFSA J. 15, e04720 (2017). https://doi.org/10.2903/j.efsa.2017.4720
J. Wuttke, J. Hernandez, G. Li, G. Coddens, H.Z. Cummins, F. Fujara, W. Petry, H. Sillescu, Neutron and light scattering study of supercooled glycerol. Phys. Rev. Lett. 72, 3052–3055 (1994). https://doi.org/10.1103/PhysRevLett.72.3052
K. Schröter, E. Donth, Viscosity and shear response at the dynamic glass transition of glycerol. J. Chem. Phys. 113, 9101–9108 (2000). https://doi.org/10.1063/1.1319616
A. Sanz, K. Niss, Liquid dynamics in partially crystalline glycerol. J. Chem. Phys. 146, 044502 (2017). https://doi.org/10.1063/1.4974831
B. Yang, M.M. Sarafraz, M. Arjomandi, Marangoni effect on the thermal performance of glycerol/water mixture in microchannel. Appl. Therm. Eng. 161, 114142 (2019). https://doi.org/10.1016/j.applthermaleng.2019.114142
P.-Q. Zhang, P.-C. Tan, Y.-M. Gao, X.-J. Zhang, Y. Xie, D.-N. Zheng, S.-B. Zhou, Q.-F. Li, The effect of glycerol as a cryoprotective agent in the cryopreservation of adipose tissue. Stem Cell Res. Ther. 13, 152 (2022). https://doi.org/10.1186/s13287-022-02817-z
A.A. Minea, L. Zupcu, A short overview on graphene-based nanofluids. Int. J. Thermophys. 43, 161 (2022). https://doi.org/10.1007/s10765-022-03093-y
K.G. Sundari, L.G. Asirvatham, S. Joseph John Marshal, S. Manova, M. Sahu, M. Jesse Aaron, Heat transfer studies using glycerin based nanocoolant for car radiator cooling applications. Mater. Today Proc. 47, 7045–7049 (2021). https://doi.org/10.1016/j.matpr.2021.06.104
F.E. Berger Bioucas, C. Köhn, A. Jean-Fulcrand, G. Garnweitner, T.M. Koller, A.P. Fröba, Effective thermal conductivity of nanofluids containing silicon dioxide or zirconium dioxide nanoparticles dispersed in a mixture of water and glycerol. Int. J. Thermophys. 43, 167 (2022). https://doi.org/10.1007/s10765-022-03084-z
J. Tang, S. Liu, W. Liu, Y. Wang, L. Li, Z. Li, J. Wang, Comparative study on tribological performance and mechanism of eco-friendly solvent-free covalent MXene nanofluids in glycerin and polyethylene glycol. Tribol. Int. 190, 109051 (2023). https://doi.org/10.1016/j.triboint.2023.109051
TP02 Manual v2211, Non-Steady-State Probe for Thermal Conductivity Measurement (2003).
V.R. Tarnawski, T. Momose, W.H. Leong, Thermal conductivity of standard sands II. Saturated conditions. Int. J. Thermophys. 32, 984–1005 (2011). https://doi.org/10.1007/s10765-011-0975-1
G.W.C. Kaye, W.F. Higgins, J.E. Petavel, The thermal conductivities of certain liquids. Proc. R. Soc. Lond. Ser. 117, 459–470 (1928). https://doi.org/10.1098/rspa.1928.0010
O.K. Bates, Binary mixtures of water and glycerol - thermal conductivity of liquids. Ind. Eng. Chem. 28, 494–498 (1936). https://doi.org/10.1021/ie50316a033
L. Riedel, Wärmeleitfähigkeitsmessungen an Mischungen verschiedener organischer Verbindungen mit Wasser. Chem. Ing. Tech. 23, 465–469 (1951). https://doi.org/10.1002/cite.330231902
F.E. Berger Bioucas, M.H. Rausch, J. Schmidt, A. Bück, T.M. Koller, A.P. Fröba, Effective thermal conductivity of nanofluids: measurement and prediction. Int. J. Thermophys. 41, 55 (2020). https://doi.org/10.1007/s10765-020-2621-2
F.E. Berger Bioucas, M.H. Rausch, T.M. Koller, A.P. Fröba, Guarded parallel-plate instrument for the determination of the thermal conductivity of gases, liquids, solids, and heterogeneous systems. Int. J. Heat Mass Transf. 212, 124283 (2023). https://doi.org/10.1016/j.ijheatmasstransfer.2023.124283
F.E. Berger Bioucas, T.M. Koller, A.P. Fröba, Effective thermal conductivity of microemulsions consisting of water micelles in n-decane. Int. J. Heat Mass Transf. 200, 123526 (2023). https://doi.org/10.1016/j.ijheatmasstransfer.2022.123526
A.P. Fröba, M.H. Rausch, K. Krzeminski, D. Assenbaum, P. Wasserscheid, A. Leipertz, Thermal conductivity of ionic liquids: measurement and prediction. Int. J. Thermophys. 31, 2059–2077 (2010). https://doi.org/10.1007/s10765-010-0889-3
M.H. Rausch, K. Krzeminski, A. Leipertz, A.P. Fröba, A new guarded parallel-plate instrument for the measurement of the thermal conductivity of fluids and solids. Int. J. Heat Mass Transf. 58, 610–618 (2013). https://doi.org/10.1016/j.ijheatmasstransfer.2012.11.069
Acknowledgements
The authors gratefully acknowledge funding of the Erlangen Graduate School in Advanced Optical Technologies (SAOT) by the Bavarian State Ministry for Science and Art.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was financially supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) via the project Grant FR 1709/20-1.
Author information
Authors and Affiliations
Contributions
Experimental investigations and evaluations were performed by Francisco E. Berger Bioucas. The first draft of the manuscript was written by Francisco E. Berger Bioucas. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare they have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10765_2024_3347_MOESM1_ESM.xlsx
Raw data related to the thermal conductivity measurements are available in two Microsoft Excel files. The file with the name “1_Raw_data_thermal_conductivity_pure_glycerol.xlsx” contains the raw data for pure glycerol, while the raw data for the three glycerol-water mixtures are given in the file “2_Raw_data_thermal_conductivity_glycerol_water_Mixtures.xlsx.” Supplementary file1 (XLSX 392 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bioucas, F.E.B., Koller, T.M. & Fröba, A.P. Thermal Conductivity of Glycerol at Atmospheric Pressure Between 268 K and 363 K by Using a Steady-State Parallel-Plate Instrument. Int J Thermophys 45, 52 (2024). https://doi.org/10.1007/s10765-024-03347-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-024-03347-x