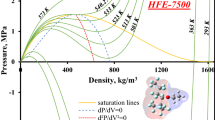
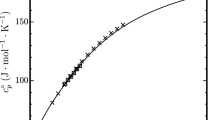
Abstract
Three SAFT equations of state: sPC-SAFT, Soft-SAFT and SAFT-BACK were fitted to five Hydrofluoroethers (HFEs): four perfluoroalkyl methyl ethers and one C6-fluoroketone. The fits were verified with experimental data consisting of vapor pressure, liquid and vapor density and the speed of sound. Two sets of PC-SAFT parameters for HFE fluids from the literature were also tested and compared with our results. The selected fluids are good candidates to replace perfluorocarbon heat transfer fluids, and the obtained fits are intended to support the challenging transition of cooling systems to more environment-friendly fluids. The SAFT equations of state coupled with group contribution methods enable mixtures of a multitude of compounds to be modeled and their equilibrium, solubility, absorption and other effects to be predicted. This can be helpful not only when studying the properties of useful mixtures but also when evaluating material compatibility and the effects of fluid contamination, which cause a lot of concern for cooling systems that require very high reliability.








Similar content being viewed by others
Abbreviations
- AAD:
-
Absolute average deviation
- VLE:
-
Vapor liquid equilibrium
- PVT:
-
Pressure volume temperature
- SoS:
-
Speed of sound
- HFC:
-
Hydrofluoroolefins
- HFE:
-
Hydrofluoroether
- HFO:
-
Hydrofluoroolefins
- A :
-
Helmholtz energy, J
- a :
-
Constant Eq. 10
- B :
-
First virial coefficient, m3·mol‒1
- b :
-
Constant Eq. 10
- c :
-
Constant Eq. 10
- c p :
-
Isobaric heat capacity, J·mol‒1·K‒1
- c v :
-
Isochoric heat capacity, J·mol‒1·K‒1
- k b :
-
Boltzmann constant, J⋅K−1
- M :
-
Molecular weight, g⋅mol−1
- m :
-
Average chain length
- OF :
-
Objective function
- p :
-
Pressure, Pa
- R :
-
Universal gas constant, J⋅K−1⋅mol−1
- T :
-
Temperature, K
- u :
-
Segment dispersion parameter, J
- w :
-
Speed of sound, m⋅s−1
- α :
-
Geometry hard convex body (Saft-BACK parameter)
- γ :
-
Heat capacity ratio, 1
- ε :
-
Potential minimum, J
- ν :
-
Segment volume parameter, cm3⋅mol−1
- ρ :
-
Density, kg⋅m−3
- σ :
-
Distance of zero potential, Å
- a:
-
Acoustic
- c:
-
Critical
- r:
-
Reduced
- 0:
-
Ideal gas
- calc:
-
Calculated
- chain:
-
Chain formation contribution
- dis:
-
Dispersion contribution
- exp:
-
Experimental
- hc:
-
Hard chain contribution
- hcb:
-
Hard convex body contribution
- pert:
-
Perturbed
- ref:
-
Reference
- res:
-
Residual
References
Online document P. Gorbounov, M. Battistin, E. Thomas, Comparison of liquid coolants suitable for single-phase detector cooling (CERN, 2016) https://twiki.cern.ch/twiki/pub/LHCb/C6K/Coolants_review.pdf. Accessed 16 Mar 2022
Online document P. Gorbounov, 3M Novec fluids as alternative to perfluorocarbons for detector cooling at CERN (CERN, 2015) https://indico.cern.ch/event/444246/attachments/1151300/1652860/FI_presentation_8.09.2015.pdf. Accessed 16 Mar 2022
A. Massafferri, J. Instrum. (2020). https://doi.org/10.1088/1748-0221/15/08/C08006
Online document P. Senger, CBM Progress Report 2019 (GSI Darmstadt Darmstadt, 2020) https://doi.org/10.15120/GSI-2020-00904. Accessed 16 Mar 2022
B. Verlaat, P. Petagna, L. Zwalinski et al., Proceedings of the 25th IIR International Congress of Refrigeration (2019) https://doi.org/10.18462/iir.icr.2019.1690
M. Battistin, S. Berry, A. Bitadze et al., Int. J. of Chem. React. Eng. 13, 4 (2015). https://doi.org/10.1515/ijcre-2015-0022
Online document J. Godlewski, ATLAS mono-phase cooling systems (CERN, 2008) https://indico.cern.ch/event/41288/contributions/1871983/attachments/842452/1171868/ATLAS_mono-phase_systems.pdf. Accessed 16 Mar 2022
E.W. Lemmon, I.H. Bell, M.L. Huber, M.O. McLinden, REFPROP—Reference Fluid Thermodynamic and Transport Properties, NIST Standard Reference Database 23, version 10.0 (2018)
M.A. Marcelino Neto, J.R. Barbosa Jr., J. Supercrit. Fluid. 50, 1 (2009). https://doi.org/10.1016/j.supflu.2009.04.006
Z. Hu, J. Yang, Y. Li, Fluid Phase Equilib. 205, 1 (2003). https://doi.org/10.1016/S0378-3812(02)00307-2
N. Chouireb, E.A. Crespo, L.M.C. Pereira, O. Tafat-Igoudjilene, L.F. Vega, J.A.P. Coutinho, P.J. Carvalho, J. Chem. Eng. Data 63, 7 (2018). https://doi.org/10.1021/acs.jced.7b00945
N. Solms, M.L. Michelsen, G.M. Kontogeorgis, Ind. Eng. Chem. Res. 44, 9 (2005). https://doi.org/10.1021/ie049089y
V. Vinš, A. Aminian, D. Celný, M. Součková, J. Klomfar, M. Čenský, O. Prokopová, Int. J. Refrig. (2021). https://doi.org/10.1016/j.ijrefrig.2021.06.029
Online document 3M™ Novec™ 649 Engineered Fluid Product Information (3M, 2021) https://multimedia.3m.com/mws/media/569865O/3m-novec-engineered-fluid-649.pdf. Accessed 16 Mar 2022
M.O. McLinden, R.A. Perkins, E.W. Lemmon, T.J. Fortin, J. Chem. Eng. Data (2015). https://doi.org/10.1021/acs.jced.5b00623
Online document 3M™ Novec™ 7000 Engineered Fluid Technical Data (3M, 2021) https://multimedia.3m.com/mws/media/121372O/3m-novec-7000-engineered-fluid-tds.pdf. Accessed 16 Mar 2022
Online document 3M™ Novec™ 7100 Engineered Fluid Product Information (3M, 2021) https://multimedia.3m.com/mws/media/199818O/3m-novec-7100-engineered-fluid.pdf. Accessed 16 Mar 2022
W.T. Tsai, J. Hazard. Mater. (2005). https://doi.org/10.1016/j.jhazmat.2004.12.018
N. Solms, I. Kouskoumvekaki, M. Michelsen, G. Kontogeorgis, Fluid Phase Equilib. (2006). https://doi.org/10.1016/J.FLUID.2006.01.001
J. Gross, G. Sadowski, Ind. Eng. Chem. Res. 40, 4 (2001). https://doi.org/10.1021/ie0003887
J. Vijande, M.M. Piñeiro, D. Bèssieres, H. Saint-Guironsb, J.L. Legido, Phys. Chem. Chem. Phys. (2004). https://doi.org/10.1039/B312223A
K. Řehak, M. Klajmon, M. Strejc, P. Moravek, J. Chem. Eng. Data 62, 11 (2017). https://doi.org/10.1021/acs.jced.7b00599
F. Llovell, L.F. Vega, J. Phys. Chem. B 110, 23 (2006). https://doi.org/10.1021/jp0608022
A.M.A. Dias, J.C. Pàmies, J.A.P. Coutinho, I.M. Marrucho, L.F. Vega, J. Phys. Chem. B 108, 4 (2004). https://doi.org/10.1021/jp036225o
Z. Zhang, J. Yang, Y. Li, Fluid Phase Equilib. 172, 2 (2000). https://doi.org/10.1016/S0378-3812(00)00386-1
T. Boublík, J. Mol. Liq. 134, 1–3 (2007). https://doi.org/10.1016/j.molliq.2006.12.008
Z. Zhang, Z. Hu, J. Yang, Y. Li, Tsinghua Sci. Technol. 7, 1 (2002)
M. Salimi, A. Bahramian, Petrol. Sci. Technol. 32, 4 (2014). https://doi.org/10.1080/10916466.2011.580301
M. Doubek, V. Vacek, Chem. Eng. Data 61, 12 (2016). https://doi.org/10.1021/acs.jced.6b00536
R.A. Perkins, M.O. McLinden, J. Chem. Thermodyn. 91, 43–61 (2015). https://doi.org/10.1016/j.jct.2015.07.005
Online document M.L. Huber, E.W. Lemmon, Properties of Fire Suppressant Systems “PROFISSY” (NIST, 2021) https://doi.org/10.6028/NIST.TN.2132. Accessed 11 Feb 2022
M.H. Rausch, L. Kretschmer, S. Will, A. Leipert, A.P. Fröba, J. Chem. Eng. Data 60, 12 (2015). https://doi.org/10.1021/acs.jced.5b00691
K. Tanaka, JSRAE 32, 3 (2015). https://doi.org/10.11322/tjsrae.15-15_OA
H. Ohta, Y. Morimoto, J.V. Widiatmo, K. Watanabe, J. Chem. Eng. Data (2001). https://doi.org/10.1021/je0002538
T. Lafitte, F. Plantier, M.M. Pineiro, J. Daridon, D. Bessieres, Ind. Eng. Chem. Res. (2007). https://doi.org/10.1021/ie0700462
H. Qi, D. Fang, X. Meng, J. Wu, J. Chem. Thermodyn. (2014). https://doi.org/10.1016/j.jct.2014.05.017
M.M. Piñeiro, F. Plantier, D. Bessières, J.L. Legido, J.L. Daridon, Fluid Phase Equilib. (2004). https://doi.org/10.1016/j.fluid.2004.06.013
B. An, Y. Duan, F. Yang, Z. Yang, J. Chem. Eng. Data (2015). https://doi.org/10.1021/acs.jced.5b00513
Y. Kayukawa, M. Hasumoto, T. Hondo, Y. Kano, K. Watanabe, J. Chem. Eng. Data (2003). https://doi.org/10.1021/je025657
J.V. Widiatmo, T. Tsuge, K. Watanabe, J. Chem. Eng. Data (2001). https://doi.org/10.1021/je0101247
S.B. Kiselev, J.F. Ely, Ind. Eng. Chem. Res. (1999). https://doi.org/10.1021/ie990387i
Acknowledgements
The work presented here received support from the European Regional Development Fund—Project “Center for Advanced Applied Science” (Grant No CZ.02.1.01/0.0/0.0/16_019/0000778) and also from the Project for the Large Research Structures of the Ministry of Education of the Czech Republic (Grant No. LM201804).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doubek, M., Vacek, V. SAFT Equations of State for Low GWP Hydrofluoroethers Heat Transfer Fluids. Int J Thermophys 43, 138 (2022). https://doi.org/10.1007/s10765-022-03063-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-022-03063-4