Abstract
In this work, we present normal spectral emissivity data of solid and liquid molybdenum at a wavelength of 684.5 nm. The presented results are novel measurements on molybdenum, a material, which was already measured 15 years ago by our group. The present results indicate a lower emissivity in the liquid phase. The novel measurements were done within the European Metrology Programme for Innovation and Research (EMPIR) project 17IND11 Hi-TRACE. The optimized measuring system is an ohmic pulse-heating apparatus combined with microsecond Division of Amplitude polarimetry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
When investigating thermophysical properties, i.e., temperature-dependent properties, of liquid metals and alloys at high temperatures, measurements need to be performed contactless and containerless. Especially contactless temperature measurement poses manifold complications. Above a temperature of 1234.93 K, the melting point of silver, the international temperature of 1990 (ITS-90) [1], is defined by spectral pyrometry. Pyrometry is based on the fact that every object with a temperature higher than absolute zero, 0 K, emits thermal radiation, which can be detected by, e.g., a photo diode. However, the ratio between emitted thermal radiation of an object and the emitted thermal radiation of a so-called black body (a perfect thermal emitter) under the same conditions needs to be known in order to obtain correct temperatures. This ratio is called the emissivity of the material. Especially, the normal spectral emissivity, meaning the temperature-dependent emissivity, perpendicular to the surface of the object analyzed at the wavelength of the pyrometer used for measurement, needs to be known.
At the thermo- and metalphysics group of the institute of experimental physics of Graz University of Technology, a fast ohmic pulse-heating system is used to measure thermophysical properties of liquid metals and alloys, see, e.g. [2]. The setup was extended by a microsecond Division of Amplitude Photopolarimeter (\(\upmu \)s-DOAP) in 2001 [3]. The \(\upmu \)s-DOAP allows the measurement of normal spectral emissivity at a wavelength of 684.5 nm. After a longer period of inactivity, the \(\upmu \)s-DOAP system was re-established as part of Graz University of Technology’s contribution to the European Metrology Programme for Innovation and Research (EMPIR) project 17IND11 “Hi-TRACE.” After ensuring the functionality of the apparatus, the normal spectral emissivity of solid and liquid molybdenum at 684.5 nm was measured and compared to previously published data in 2004, Cagran et al. [4], 2005, Cagran et al. [5] and 2013, Pottlacher et al. [6].
A discrepancy between the previously published data and the newly obtained data was detected. After re-evaluating the original data, obtained by Cagran et al., we conclude that the previously reported data in the liquid phase are too high. Therefore, newly measured and evaluated values are published in this work.
2 Experimental Methods
The methods used to obtain normal spectral emissivity of solid and liquid molybdenum are a combination of an ohmic pulse-heating apparatus with a \(\upmu \)s-DOAP. Both measuring systems have been thoroughly described in previous publications, e.g. [7, 8]. Only a short description of the measuring system is given in this work.
2.1 Ohmic Pulse-Heating Apparatus (OPA)
The ohmic pulse-heating apparatus (OPA) works as follows: A large current pulse (some 1000 A) is distributed through a wire-shaped sample, with a diameter of 0.5 nm. Due to its electrical resistivity, the sample heats up, melts, and once it reaches the material’s boiling point, it explodes. Thus, the technique was also called exploding wire technique. The whole process of heating up through the solid phase, melting, and passing through the liquid phase only takes around 30 \(\upmu \)s to 50 \(\upmu \)s. Besides the prevention of chemical reactions with any surroundings, this short experimental time also ensures that the liquid wire can be observed, without collapsing due to gravitational forces. The temperature measurement is performed by pyrometry. An especially designed pyrometer for very fast time responses, operating at a wavelength of 649.7 nm, is used to measure the radiance temperature of the sample. The radiance temperature can later be used, to calculate the true temperature if the normal spectral emissivity of the sample is known, measured, or estimated. Additional information about the measuring system can be found, e.g., in [9].
2.2 Microsecond Division of Amplitude Photopolarimeter (\(\upmu \)s-DOAP)
Polarimetry is a powerful tool to measure normal spectral emissivity (see, e.g. [10]). Usually polarimetry instruments depend on rotating or other moveable parts. For fast experimental time scales, like OPA measurements, a polarimeter with moveable parts would suffer from insufficient resolution. Such instruments might even not be capable to obtain one data point during one OPA experiment. In 1982, Azzam [11] designed an instrument without moveable parts. The working principle is as follows: An incident laser beam with a wavelength of 684.5 nm passes the so-called polarization state generator (PSG), which as the name suggests generates a linearly polarized beam. This laser beam is then reflected by the wire-shaped sample inside the experimental chamber of the OPA, which changes the state of polarization. At an angle of incidence of \(70^\circ \), the reflected laser beam hits the polarization state detector (PSD). A coated beam splitter then splits up the laser beam and the two resulting beams each pass a Glan–Thompson prism. Finally, there are four laser beams, which are detected by separate photo diodes. By calibrating the measuring system beforehand, these four detector signals are used to calculate the so-called Stokes vector. From the Stokes vector, the normal spectral emissivity of the sample at the wavelength of the incident laser beam can be calculated. More information on the measuring system and the data analysis can be found in previous publications [3, 4, 8, 12, 13].
3 Results
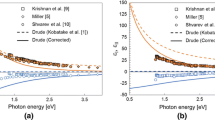
Normal spectral emissivity data for solid and liquid molybdenum are presented in the following. Figure 1 shows normal spectral emissivity data as a function of radiance temperature \(T_\mathrm {r}\) for both the solid and the liquid phase. Keep in mind, that normal spectral emissivity in the solid phase is highly dependent on the surface condition. The samples were prepared by polishing them with abrasive paper (grade 1200) and subsequently cleaned by acetone. This procedure was performed consistently over all experiments to ensure comparability.
The data were evaluated for a radiance temperature of 2520 K (at the pyrometer wavelength), the melting point of molybdenum [6]. The linear regression lines were calculated from 1650 K to 2490 K for the solid phase and from 2550 K to 3190 K for the liquid phase. The measurement range of the pyrometer limits the temperature ranges. In the solid phase, the linear regression line of the normal spectral emissivity is given by
However, in the solid phase, two clusters of data can be seen. Additional to the fit of all data in the solid phase, both clusters were also evaluated separately. There are three datasets in the upper cluster, denoted with top, which can be approximated by a linear regression line of
In the lower cluster, denoted with bottom, four datasets can be found. This suggests an approximately equal distribution between the two bulks. The following linear regression line approximates the lower bulk:
In the liquid phase, a linear regression line approximates the normal spectral emissivity by
By extrapolating the linear fit of the liquid phase towards the melting point, the normal spectral emissivity at the melting temperature can be estimated by
Normal spectral emissivity at 684.5 nm \(\epsilon _{{684.5}~{\text{nm}}}\) as a function of radiance temperature \(T_\mathrm {r}\) for 649.7 nm, seven independent measurements with linear regression lines and statistical uncertainty analysis, errorbars represent single standard deviations in the solid phase and k = 2 standard deviations in the liquid phase. gray lines: measurement data, solid red lines: linear regression lines for solid and liquid phase, dotted red lines: linear regression lines for the two clusters in the solid phase, blue line: literature data from 2004, Cagran et al. [4].
4 Discussion
The resulting normal spectral emissivity in the liquid phase is, close to the melting temperature, outside of the calculated k = 2 uncertainty interval and overall lower by about 20 % than the values presented in the articles 2004, Cagran et al. [4], 2005, Cagran et al. [5], and 2013, Pottlacher et al. [6]. After revisiting of the original data from 2004, Cagran et al. [4], one dataset was omitted because of an incorrect temperature allocation and one dataset was taken out because of an atypical evolution of the emissivity in the liquid phase. By removing these two datasets, the resulting emissivity decreases significantly.
Within this work, two different clusters of data were measured, with a significant difference in emissivity in the solid phase. The surface treatment was performed consistently over all experiments with a certain number of strokes in certain directions with abrasive paper and acetone. Although the experiments were performed over several days, no correlation of the data with any external influences was found. Measurements with results in both clusters were performed on each day and the room temperature as well as the nitrogen atmosphere was comparable during all experiments.
5 Uncertainty
Uncertainty estimation of the emissivity measurements with the \(\upmu \)s-DOAP is challenging. While at first glance the obtained data seem to have a rather high uncertainty of about 30 \(\%\), comparison with other institutes in the past showed a good agreement of the measured data. The uncertainty is increased by single data points, which do not follow the characteristic, linear temperature evolution.
In 1970, Jaeger [14] suggested that these peaks are caused by magnetohydrodynamic oscillations on the surface. Before the experiments, the measurement devices are adjusted to the position of the wire. If the wire would start oscillating, the measurement devices are not adjusted properly any more because the reflection of the laser after the wire might not be correctly directed in the detector. Therefore, the oscillations are influencing the measurement of the emissivity by changing the geometry. If only the normal spectral emissivity is considered, the uncertainty can be estimated significantly lower. Therefore, only the linear regression lines for every single experiment are used for the uncertainty estimation of the normal spectral emissivity. The uncertainty is calculated by looking at the deviation between these averages. To quantify a reasonable uncertainty, we suggest the following routine:
The uncertainty estimation was performed separately for the solid and the liquid phase. A linear regression line was calculated for every single measurement, for both the solid and the liquid phase. Then, the average of the linear regression lines together with the standard deviation was calculated. In Fig. 1, the average of the standard deviation, together with the average as a function of the radiance temperature, is shown.
This method weights single peaks in the data only very lightly, because of the averaging process. On the other hand, it allows a good understanding of the deviations between the different experiments. For the solid phase, an average single standard deviation of Δε684.5 nm,s(Tr) = 0.068 was calculated if all data points are included. If only the upper bulk is considered, the single standard deviation for the solid phase is Δε684.5 nm,s,top(Tr) = 0.024. If only the lower bulk is considered, the single standard deviation for the solid phase is Δε684.5 nm,s,bottom(Tr) = 0.016. For the liquid phase, an average single standard deviation of Δε684.5 nm,l(Tr) = 0.027 is reached with this evaluation method. This equates to about 10 % of the value for the liquid phase. After considering several different types of B uncertainties, we concluded that the combined uncertainty is mainly governed by the statistical uncertainty.
References
W. Blanke, Die Internationale Temperaturskala von 1990: ITS-90 (Physikalisch-Technische Bundensanstalt) (1989)
P. Pichler, B.J. Simonds, J.W. Sowards, G. Pottlacher, J. Mater. Sci. 55, 4081 (2019). https://doi.org/10.1007/s10853-019-04261-6
A. Seifter, F. Sachsenhofer, S. Krishnan, G. Pottlacher, Int. J. Thermophys. 22, 1537 (2001)
C. Cagran, B. Wilthan, G. Pottlacher, Int. J. Thermophys. 25, 1551 (2004)
C. Cagran, G. Pottlacher, M. Rink, W. Bauer, Int. J. Thermophys. 26, 1001 (2005). https://doi.org/10.1007/s10765-005-6680-1
G. Pottlacher, K. Boboridis, C. Cagran, T. Huepf, A. Seifter, B. Wilthan, Temperature: its measurement and control in science and industry, volume 8. In: Proceedings of the Ninth International Temperature Symposium, AIP Publishing, vol. 1552, pp. 704–709 (2013)
E. Kaschnitz, G. Pottlacher, H. Jaeger, Int. J. Thermophys. 13, 699 (1992). https://doi.org/10.1007/BF00501950
A. Seifter, F. Sachsenhofer, G. Pottlacher, Int. J. Thermophys. 23, 1267 (2002)
M. Leitner, T. Leitner, A. Schmon, K. Aziz, G. Pottlacher, Metall. Mater. Trans. A 48, 3036 (2017). https://doi.org/10.1007/s11661-017-4053-6
H.G. Thompson, E.A. Irene (eds.), Handbook of Ellipsometry. William Andrew Inc., New York
R. Azzam, Opt. Acta Int. J. Opt. 29, 685 (1982). https://doi.org/10.1080/713820903
A. Seifter, Bestimmung des normalen spektralen Emissionskoeffizienten von flüssigen pulsgeheizten Metallen mittels eines schnellen Photopolarimeters. Ph.D. Thesis (2001)
C. Cagran, Untersuchung des Emissionsverhaltens flüssiger Metalle mittels Photopolarimetrie und Mehrwellenlängenpyrometrie. Ph.D. Thesis (2004)
H. Jaeger. Die physikalischen Vorgänge bei elektrischen Drahtexplosionen. Habilitationsschrift (1970)
Acknowledgements
Hi-TRACE Project has received funding from the EMPIR initiative co-financed by the Participating States and from the European Union’s Horizon 2020 research and innovation programme.
Funding
Open access funding provided by Graz University of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eber, A., Pichler, P. & Pottlacher, G. Re-investigation of the Normal Spectral Emissivity at 684.5 nm of Solid and Liquid Molybdenum. Int J Thermophys 42, 17 (2021). https://doi.org/10.1007/s10765-020-02769-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-020-02769-7