Abstract
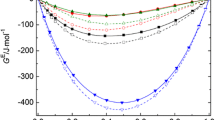
Second-order thermodynamic derivative properties, such as isobaric thermal molar expansions, isothermal and adiabatic molar compressibilities, and isochoric molar heat capacities of (ethanol, decan-1-ol, 2-methyl-2-butanol) + heptane mixtures at pressures up to 100 MPa and in the temperature range from 293.15 K to 318.15 K were derived from experimental speed-of-sound u(T, p), density ρ(T, p = 0.1 MPa), and isobaric heat-capacity C p (T, p = 0.1 MPa) data using appropriate thermodynamic relations. Excess values for the given properties were calculated according to the criterion of thermodynamic ideality of a mixture (Douhéret et al., Chem. Phys. Chem. 2, 148 (2001)), i.e., assuming that the chemical potential of component i in the ideal liquid mixture is equal to the chemical potential of component i in the mixture of perfect gases. The deviations from ideality for the mixtures under test have been explained in terms of the self-association of alcohols in solution which produces a strong departure from random mixing, the change in the non-specific interactions during mixing, and the packing effects.
Similar content being viewed by others
References
Davis L.A., Gordon R.B.: J. Chem. Phys. 46, 2650 (1967)
Dzida M.: J. Sol. Chem. 33, 527 (2004)
Sun T.F., Ten Seldam C.A., Kortbeek P.J., Trappeniers N.J., Biswas S.N.: Phys. Chem. Liq. 18, 107 (1988)
Dzida M., Żak A., Ernst S.: J. Chem. Thermodyn. 37, 405 (2005)
Dzida M.: J. Phys. Chem. B 113, 11649 (2009)
Dzida M., Waleczek L.: J. Chem. Thermodyn. 42, 312 (2010)
Dzida M., Marczak W.: J. Chem. Thermodyn. 37, 826 (2005)
Dzida M., Góralski P.: J. Chem. Thermodyn. 38, 962 (2006)
Dzida M., Góralski P.: J. Chem. Thermodyn. 41, 402 (2009)
Douhéret G., Davis M.I., Reis J.C.R., Blandamer M.J.: Chem. Phys. Chem. 2, 148 (2001)
Peleteiro J., González-Salgado D., Cerdeiriña C.A., Romaní L.: J. Chem. Thermodyn. 34, 485 (2002)
Cerdeiriña C.A., Troncoso J., González-Salgado D., García-Miaja G., Hernández-Segura G., Bessières D., Medeiros M., Romaní L., Costas M.: J. Phys. Chem. B 111, 1119 (2007)
Costas M., Patterson D.: Thermochim. Acta 120, 161 (1987)
Cerdeiriña C.A., Tovar C.A., Carballo E., Romaní L., Delgado M.C., Torres L.A., Costas M.: J. Phys. Chem. B 106, 185 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dzida, M., Kaczmarczyk, A. Second-Order Derivatives of the Gibbs Energy for Liquid Mixtures of Alcohol + Heptane at Pressures up to 100 MPa. Int J Thermophys 33, 583–626 (2012). https://doi.org/10.1007/s10765-011-1148-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-011-1148-y