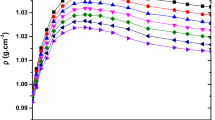
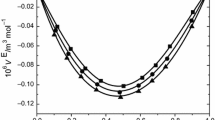
The sound speeds and densities of the 1-propanol + diethyl ether + 1-octanol ternary mixture and constituent binary mixtures, 1-propanol + diethyl ether, 1-propanol + 1-octanol, and diethyl ether + 1-octanol, have been measured at 298.15 K as a function of composition. Isentropic compressibilities, molar isentropic compressibilities, excess molar isentropic compressibilities, excess molar volumes, and excess sound speeds have been calculated from the experimental density and sound speed data. Excess molar volumes, excess molar isentropic compressibilities, and excess sound speeds of the binary mixtures were fitted to the Redlich–Kister equation. By using the free length theory (FLT), Schaaff’s collision factor theory (CFT), Nomoto’s relation (NR), Van Deal’s ideal mixing relation (IMR), and Junjie’s relation (JR), sound-speed values of the investigated mixtures were calculated. These values were compared with the experimental sound-speed results.
Similar content being viewed by others
References
Canosa J., Rodriguez A., Tojo J. (1999). Fluid Phase Equilib. 156:57
Rodriguez A., Canosa J., Tojo J. (1999). J. Chem. Thermodyn. 31:1009
Letcher T.M., Govender P.U. (1997). Fluid Phase Equilib. 140:207
Arce A., Rodil E., Soto A. (1997). J. Chem. Eng. Data 42:721
Savaroglu G., Aral E. (2006). Pramana-J. Phys. 66:435
Baker F. (1912). J. Chem. Soc. Trans. 101:1409
Jacobson B. (1952). J. Chem. Phys. 6:927
Jacobson B. (1952). Acta Chem. Scand. 6:1485
W. Schaaffs, Molekularakustik (Springer-Verlag, Berlin, Göttingen, Heidelberg, Germany, 1963).
Nomoto O. (1958). J. Phys. Soc. 13:1528
W. Van Deal and E. Vageel, Proc. First Int. Conf. Calorim. Thermodyn., Warsaw (1969), p. 556.
Junjie Z. (1984). J. Chem. Univ. Sci. Tech. 14:298
Cerdeirina C.A., Tovar C.A., Troncoso J., Carballo E., Romani L. (1999). Fluid Phase Equilib. 157:93
J. A. Riddich, W. B. Bunger, and T. K. Sokano, Organic Solvents. Physical Properties and Methods of Purification (Techniques of Chemistry), 4th Ed., Vol. 2 (Wiley/Interscience, New York, 1986).
Savaroglu G., Aral E. (2005). Int. J. Thermophys. 26:1525
Iglesias T.P., Legido J.L., Romani L., Paz Andrade M.I. (1993). Phys. Chem. Liq. 25:135
Gonzalez C., Iglesias M., Lanz J., Marino G., Orge B., Resa J.M. (2001). J. Food Eng. 50:29
Kiyohara O., Benson G.C. (1981). J. Solution Chem. 10:281
de Cominges B.E., Pineiro M.M., Iglesias T.P., Legido J.L., Paz Andrade M.I. (1998). J. Chem. Thermodyn. 30:1147
Pineiro A., Amigo A., Bravo R., Brocos P. (2000). Fluid Phase Equilib. 173:211
R. H. Perry and D. W. Green, Perry’s Chemical Engineers Handbook, 7th Ed. (McGraw-Hill, New York, 1997).
Pineiro A., Brocos P., Amigo A., Pintos M. (2002). J. Solution Chem. 31:369
CDATA, Database of Thermodynamic and Transport Properties for Chemistry and Engineering, Version 1.020 (Department of Physical Chemistry, Institute of Chemical Technology, Prague, Czech Republic, 1999).
Calvo E., Brocos P., Pineiro A., Pintos M., Amigo A., Bravo R., Roux- Desgranges A.H. (1999). J. Chem. Eng. Data 44:948
Douheret G., Pal A., Davis M.I. (1990). J. Chem. Thermodyn. 22:99
Douheret G., Salgado C., Davis M.I., Loya J. (1992). Thermochim. Acta 207:313
Douheret G., Davis M.I., Reis J.C.R., Blandamer M.J. (2001). Phys. Chem. Chem. Phys. 2:148
Redlich O., Kister A.T. (1948). Ind. Eng. Chem. 40:345
Cibulka I. (1982). Coll. Czech. Chem. Commun. 47:144
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Savaroglu, G., Tasagal, D. & Aral, E. Excess Molar Isentropic Compressibilities, Excess Molar Volumes, and Excess Sound Speeds of the 1-Propanol + Diethyl Ether + 1-Octanol Ternary Mixture and Constituent Binary Mixtures at 298.15 K. Int J Thermophys 28, 245–258 (2007). https://doi.org/10.1007/s10765-007-0166-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-007-0166-2