Abstract
Climate change is associated with more frequent extreme weather conditions and an overall increase in temperature around the globe. Its impact on individual ecosystems is not yet well known. Long-term data documenting climate and the temporal abundance of food for primates are scarce. We used long-term phenological data to assess climate variation, fruit abundance and home range sizes of the endemic and Critically Endangered crested macaques (Macaca nigra) in Tangkoko forest, Sulawesi, Indonesia. Between January 2012 and July 2020, every month, we monitored 498 individual trees from 41 species and 23 families. We noted each tree’s phenophase and assessed variation in climate (daily temperature and rainfall) and fruit abundance. We also investigated whether individual trees of known key food sources for macaques (New Guinea walnut trees, Dracontomelon spp, two species, N = 10 individual trees; fig trees, Ficus spp, four species, N = 34, and spiked peppers, Piper aduncum, N = 4) showed regular and synchronised fruiting cycles. We used 2877 days of ranging data from four habituated groups to estimate home ranges between January 2012 and July 2020. We created models to evaluate the impact of ecological factors (temperature, rainfall, overall fruit abundance, fig abundance). We found that the temperature increased in Tangkoko forest, and the overall fruit abundance decreased across the study. Top key fruits showed different trends in fruiting. Figs seem to be present year-round, but we did not detect synchrony between individuals of the same species. The macaque home ranges were about 2 km2. Monthly temperature was the main predictor of home range size, especially in disturbed forest with previously burnt areas. This information will help to further monitor changes in the macaques’ habitat, and better understand ranging and foraging strategies of a Critically Endangered species and hence contribute to its conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change impacts the world in unprecedented ways: in recent decades, we have experienced severe extreme events and a global rise in temperatures (Cook et al., 2014; Coumou & Rahmstorf, 2012; Zachos et al., 2001). Irregular events such as the El Niño—Southern Oscillation (ENSO) add periods of extreme weather conditions such as droughts (Allen et al., 2010; Dai, 2011). With global warming, phenological changes in diverse ecosystems are visible (Cleland et al., 2007; Mendoza et al., 2017; Walther et al., 2002). Hence, understanding plant phenology is crucial to understand variation in food abundance and its impact on animal behaviour and survival. For instance, in Kibale forest, Uganda, great annual variation in fruiting was observed over a period of 15 years, mostly explained by several factors such as an increase in temperature, rainfall, and solar radiation (Chapman et al., 2005, 2018). Plant phenological events, such as flowering and fruiting, are rarely reported in tropical regions, however, probably due to the prevalence of short-term studies and the enormous effort that is needed to conduct such data collection (but see Bush et al., 2017; Chapman et al., 2005, 2018, 2021).
Wild non-human primates mainly inhabit subtropical and tropical forests, which are highly diverse and dynamic ecosystems (IUCN, 2022). To get enough daily energetic input, frugivorous primates are under constant pressure to find edible items (Milton, 1981, 1988). In rainforests, flowering and fruiting is often episodic, and temporal peaks in fruit abundance are typically recorded (van Schaik et al., 1993). Fruiting synchrony between individual trees seems indeed to provide an adaptive advantage at species level, by reducing predation (Ims, 1990; Kelly, 1994; van Schaik et al., 1993).
The timing of these events depends on the plant species and is influenced by climatic and biotic factors. For instance, variation in rainfall and irradiance appears to be two of the most significant climatic factors influencing the phenologies of flowering and fruiting in many tropical habitats (Chapman et al., 2018; Zimmerman et al., 2007).
Climatic factors, together with food abundance and group mass, are likely to affect primate home range sizes (Campos et al., 2014). While group mass was the best predictor of home range size in seven different groups of white-faced capuchins (Cebus capucinus) in north-western Costa Rica, maximum temperature and fruit availability also shaped their ranging behaviour (Campos et al., 2014). The hotter the weather, and the greater the fruit availability, the smaller the home range size. Interestingly, these factors also affected the home range composition: capuchins prefer to use evergreen and more mature forest under hotter conditions.
For primate field sites, phenological data are therefore crucial to better understand the ecology and the flexibility of local species, see e.g. data for the Kibale forest, Uganda, (Chapman et al., 2021; Potts et al., 2020), Lopé National Park, Gabon (Bush et al., 2020), Tana River forest, Kenya (Wieczkowski & Kinnaird, 2008), and Gunung Palung National Park, Indonesia (Marshall et al., 2014).
Because of its biogeography, Sulawesi has a unique ecosystem with great differences in climate and vegetation even compared to neighbouring islands. For instance, in a study comparing data from Sulawesi (collected between 1992 and 1995) and Sumatra (collected between 1997 and 2002), fruiting events were more seasonal on Sulawesi and more associated with rainfall than on Sumatra (Kinnaird & O’Brien, 2005). A recent report highlighted that El Niño events bring extreme drought and risk of fire in the forests in Sulawesi (Lestari et al., 2018). We do not know yet how drought events impact plant phenology. Compared to Sumatran forests, Sulawesian forests have larger trees and larger crops but smaller fruits (Kinnaird & O’Brien, 2005). Larger mammals, such as macaques, and birds rely mostly on figs; fig abundance impacted macaques’ resource defence and grouping behaviour (Kinnaird & O’Brien, 2005).
Crested macaques (Macaca nigra) are one of the 23 described macaque species (IUCN, 2022). They are endemic to North Sulawesi and are currently categorised as Critically Endangered on the IUCN red list (Lee et al., 2020). The population of crested macaques in Tangkoko Nature Reserve seems to be one of the only viable populations in its native range (Engelhardt et al., 2017; Johnson et al., 2020). Crested macaques belong to the 10% of primate species that are highly vulnerable to droughts (Zhang et al., 2019). Their social organisation and structure and social behaviour has been well described through the work of the Macaca Nigra project (see Duboscq et al., 2013; Micheletta et al., 2012; Neumann et al., 2011, and other contributions to this Special issue for an overview). Crested macaques are well adapted to living in a variety of ecosystems from old-growth lowland and mountain forest to disturbed forest by fires and agricultural areas (O’Brien & Kinnaird, 1997; Palacios et al., 2012; Rosenbaum et al., 1998). They are mainly frugivorous (O’Brien & Kinnaird, 1997) and thrive best in old growth forests: macaque abundance correlates positively with canopy tree density and tree mean basal area size (O’Brien & Kinnaird, 1997; Palacios et al., 2012; Rosenbaum et al., 1998). As a key species in their habitat, the presence of M. nigra is a good indicator of the current health of the ecosystem and M. nigra is considered an important umbrella species for biodiversity conservation (Hilser et al., 2013). Therefore, it is important to assess ecosystem health over the long term, in terms of interrelated ecological factors important to the macaques’ survival and well-being: food and climate.
An 18-month study in 1993–1994 found that crested macaques in Tangkoko spent more than 60% of their feeding time on fruits and ate 145 different species of fruits (> 36 families) (O’Brien & Kinnaird, 1997). The preferred fruit species belonged to the Anacardiaceae and Moraceae families (> 50% of fruit feeding records). The top fruits consumed were from Dracontomelon dao (New Guinea walnut, Anacardiaceae), Ficus spp. (Fig, Moriaceae) and Piper aduncum (spiked pepper, Piperaceae), an introduced species common in disturbed habitats (O’Brien & Kinnaird, 1997). Among fig trees, macaques favoured large canopy-size strangler trees. In the same study, the home range size varied between groups: over 18 months the smallest group home range recorded was around 1.6 km2 and the largest 4.1 km2 (O’Brien & Kinnaird, 1997).
Here we present findings from a longterm study in Tangkoko forest. We aimed to characterise temporal variation in the habitat of a Critically Endangered macaque species. Specifically, we (1) report long-term trends in the temperature and rainfall, (2) assess long-term flowering and fruiting states of the vegetation, (3) document long-term trends in the fruiting of key food sources for crested macaques, (4) identify phenological cycles and fruiting synchrony, if any, in previously reported key food sources for crested macaques, and (5) monitor the home ranges of crested macaques and test potential ecological predictors of the variation in their size.
Methods
Field Site
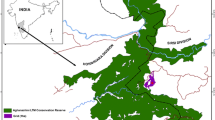
We collected data in Tangkoko Reserve (1°31′N 125°11′E; North Sulawesi, Indonesia). The habitat has been described in detail elsewhere (Kinnaird & O’Brien, 2005; O’Brien & Kinnaird, 1997; Rosenbaum et al., 1998). Briefly, Tangkoko Reserve is characterised by lowland tropical forest with great diversity and high endemism in flora. The study area is characterised mainly by old-growth forest but also by disturbed areas such as previously burnt patches and a recreation area. The study area is situated on the lower slope of Mount Tangkoko, along the shoreline of the Molucca Sea (Fig. 1).
Climate
We recorded minimum and maximum temperatures (in °C) and rainfall (in mm) daily between the beginning of June 2006 and the end of May 2020. We recorded rainfall in a rain gauge placed in the middle of the open courtyard of the field station. We emptied the gauge every morning. Every morning, at around 5 am, we read the lowest and highest marks indicated on a thermometer placed at eye level on a big tree in the forest behind the field station. We therefore recorded lowest temperature of the current day and highest temperature of the previous day, before resetting both marks to the current temperature.
Temperature data were missing for all of November 2015 (because of a broken thermometer) and April 2017 and data were missing for several individual days. Altogether, we computed mean monthly minimum and maximum temperatures for 79% (± 22%) of the daily temperature data in a month. The data quality (percentage of days with available data in a month) varied between years, ranging from 58% in 2015 to 92% in 2007. Similarly, it fluctuated across the months, ranging from 75% in April to 88% in August.
Rainfall data allowed us to estimate the amount of rain occurring in Tangkoko forest. Rainfall recordings were missing or insufficient for April 2013 and April 2017, and some data were missing for several individual days. Ninety-one percent of daily recordings were available over the 15-year data period. We computed yearly rainfall calculations and monthly variation, with a slight underestimation of the overall total amount of rainfall in the forest because of missing daily recordings.
Phenological Dataset
The phenological dataset includes data collected every month. We collected these data in 20 botanical plots (100 m × 100 m each) in the Tangkoko forest and geo-localised using GPS coordinates (Fig. 1). We placed the plots within a home range grid established previously by the Macaca Nigra Project researchers, based on ranging data from O’Brien and Kinnaird (1997) and our own experience of the macaque groups. We selected these 20 plots randomly from all the 100 m × 100 m grid squares across the range of all the macaque groups. Within a botanical plot we marked all trees with a diameter at breast height (dbh) > 10 cm with a blue cross and a round alloy number tag. We marked trees that macaques ate (phenology trees) with red rings above and below the blue cross.
We selected at least 15 trees of all tree species consumed by the macaques in such a way that the dbh distribution in the plots included some trees with small, some with medium, and some with large dbh relative to species values. We selected phenology trees of the same species from the botanical plots in the home ranges of two habituated crested macaque groups (PB and R1 groups). If there were not enough trees for a specific food tree species in the plots, we marked trees outside the plots that researchers passed when going from plot to plot. During the long-term study, we added at least 15 individuals of all new identified food-tree species that crested macaques fed on to the phenology sample. In addition to the food tree species, we included some other tree species using convenience sampling and selecting individual trees matching in their dbh, but not identified as feeding trees for macaques (nine species, Table I).
Every month, usually at the middle of the month, we determined the abundance of each phenophase for all trees in the phenology sample (this took two people 6 days). We recorded the presence of following plant parts — Leaves: leaf buds, young leaves, mature leaves); Flowers: flower buds, flowers; Fruits: unripe fruit, ripe fruit, old fruit. We further analysed the abundance of some specific food items (e.g., the ripe fruit of a particular fig species). We estimated the abundance of items by quantifying the items in the canopy. For this, we divided the tree crown into smaller equal-sized sections, counted items in one section, then multiplied the count by the number of sections to get the total estimate. We used a logarithmic scale (base 10) for estimation, whereby 0 = 0–9; 1 = 10–99; 2 = 100–999; 3 = 1000–9999; 4 = 10,000–99,999; 5 = 100,000–999,999; 6 = 1,000,000 + items present in the tree crown. To evaluate the overall flower and fruit abundance in Tangkoko forest, we collated the data for all individuals monitored each month (mean 529 ± 72 standard deviation, SD; Table S1). We calculated the proportion of individual bearing flowers (including all trees with flower buds and flowers) and fruits (including all trees bearing unripe to mature fruits) per month. To compare the data over the years, we selected 498 individual trees of 41 species (23 families) for which we had continuous data between January 2012 and July 2020 (Table I).
Phenological Analysis
To identify and characterise cycles of fruiting abundance in Tangkoko forest, we used Fourier analysis (Bush et al., 2017). This analytical approach seems to be robust and useful to describe long-term observational phenology data, even when the data are noisy. We used Fourier analysis with confidence intervals to quantify fruiting phenology of previously reported top fruit trees and evaluated cycles at an individual and species level for fig trees (Ficus spp, four species, N = 34 trees), New Guinea walnut trees (Dracontomelon spp, two species, N = 10 trees) and spiked peppers (Piper aduncum, N = 4 plants) for which we had continuous data between January 2012 and July 2020 (Tables S3, S4, and S5). We focused on the top fruit trees only as they were previously described as the main drivers shaping the ranging patterns of crested macaques (Kinnaird & O’Brien, 2005; O’Brien & Kinnaird, 1997). We included only individuals with at least one recording of a flowering or fruiting event during the study. The Fourier transform decomposes a time series into a series of sine and cosine waves of different frequencies, quantifying the power of each using a spectral estimate. These parameters can be visualised as a periodogram. We used the same method and code as in Bush et al. (2017) and applied the Daniel Kernel method to smooth the periodograms for each individual and extract length and power of the cycles. We also identified dominant cycles and their significance using confidence intervals. To compare the power of the dominant cycle across individuals, we normalised the spectrum so that the mean power across frequencies was equal to one (Bush et al., 2017). To estimate the level of synchrony at the species-level, we ran co-Fourier analysis for each individual with a significant dominant cycle equal to the modal cycle length for that species, including only species with more than five such individuals. Altogether, we quantified fruiting cycle confidence, length, power, timing, and synchrony for individuals of a single species.
We analysed data as time series between January 2012 to July 2020. The time series had three gaps, with a total of ten missing observations. These missing values were in January 2017, April 2017 to June 2017, and November 2017 to March 2018, with a maximum of five missing continuous values. Since the method requires regular time intervals between observations, we interpolated missing values using the linear estimator from R package ‘wql’ (Jassby et al., 2017). We used the function spectrum from the R base package ‘stats’ (R Core Team, 2021) for all Fourier analyses, similar to Bush et al. (2017).
Crested Macaque Home Ranges
We followed four wild groups of crested macaques (PB, PB1, R1, and R2) fully habituated by the Macaca Nigra project team (see www.macaca-nigra.org). R1 and R2 were first followed and studied by O’Brien & Kinnaird in1993 and 1994 (1997) and by the Macaca Nigra Project team since 2006 (Marty et al., 2016). PB has been followed continuously since 2008 (Marty et al., 2016). In 2013, PB split into two groups (PB1 and PB2). The four groups comprise 30–80 individuals each, with more females than males (Table S2). In Tangkoko, crested macaques usually spend more than half of the day on the ground, and they rest and move over long distances on the ground rather than in trees (O’Brien & Kinnaird, 1997). Researchers followed the animals from dawn to dusk, and recorded GIS data every minute using a Garmin GPSMAP 64 device (Garmin Ltd., US). The groups move through the home range as a unit, and the observer followed them closely. GPS data correspond to the observer’s position, which is an estimation of the group position. We imported the output of each day of data collection into QGIS software (version 3.22.7; QGIS.org, 2022). We cleaned each file and removed any outliers from each series of waypoints. We estimated monthly home range sizes using the minimum convex polygon (MCP) method and the minimum bounding geometry tool in QGIS.
We computed the overlap between home ranges of consecutive months and consecutive years to estimate the stability of the home ranges. To do this, we clipped the two areas in QGIS (version 3.22.7; QGIS.org, 2022) and calculated the percentage of the home range that was reused in the following month or year. We also calculated the overlap between the home ranges recorded in the first and the last year of the study to identify any long-term shift in space use. We made these calculations where we had at least 12 days of data collection. Home-range size estimates are correlated with the amount of data available, and preliminary inspection of the data for each group indicated that 12 days was an adequate minimum number to reach a plateau for estimation. We focused on the period between January 2012 and July 2020 to match the botanical dataset. The home range size analysis included 2577 days of collection (Table S3). The time frame of collection and number of data varied according to the group. For group PB, we had data for 213 days between January 2012 and December 2013. PB then split, and we used the data for sub-group PB1. For PB1, we had 561 days recorded between January 2014 and December 2016. For R1, we had 1147 days of data collected between January 2012 and January 2020. Lastly, for R2, we had 656 days of data collected between January 2012 and December 2016.
Statistical Analysis
We analysed trends in the time series in minimum and maximum temperatures, rainfall, and fruit abundance between January 2012 and July 2020. We used Sen’s Slope Estimator to examine the extent of the trend over the whole time series (Sen, 1968), and the Mann–Kendall (MK) test (Kendall, 1948; Mann, 1945) to analyse the significance of the trend in the seasonal and annual data series, with a 1% significance level. We used R packages ‘trend’ (Pohlert, 2020) and ‘Kendall’ (McLeod, 2022) to calculate the Sen’s slope and seasonal Mann–Kendall tests.
To identify factors impacting the home range sizes of the macaque groups, we computed linear mixed models (LMMs; R package ‘lme4’ v. 1.1–29, Bates et al., 2015). We built a-priori models to describe the relationship between monthly temperature, monthly rainfall, monthly overall fruit abundance (overall proportion of individual trees bearing fruits), monthly proportion of fig trees fruiting, and monthly home-range size. Because temperature, rainfall, and overall fruit abundance used very different scales that could affect the fit of the model, we standardised these measures. We added group identity as a random factor. To validate a model, we checked for the homogeneity of variance, and independence and normality of the model residuals. We selected the best model using Akaike Information criterion (AIC) calculated using the R package ‘MuMIn’ (v.1.46 Bartoń, 2022). We then inspected the 95% confidence intervals for the predictors of interest.
We performed all statistical analyses in R version 4.0.3 (R Core Team, 2021).
Ethical Note
The Indonesian State Ministry of Research and Technology (RISTEK), the Directorate General of Forest Protection and Nature Conservation (PHKA), and the Department for the Conservation of Natural Resources (BKSDA), North Sulawesi, provided permission to conduct research in Tangkoko Reserve. Data collection was non-invasive and adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research.
Conflict of Interest
The authors declare that they have no conflict of interest.
Results
Climate
Temperature — the temperatures in Tangkoko forest are quite stable throughout the year (Fig. 2). The mean minimum temperature was 24.29 °C (± 0.89 SD) ranging between 23.82 °C (± 1.10) in January and 24.66 °C (± 1.10) in August (Fig. 2A). The mean minimum values were recorded in 2015 (23.34 °C) and the maximum in 2019 (25.22 °C, Fig. 2C). The mean maximum temperature recorded in the forest was 28.51 °C (± 1.56 SD), ranging between 27.15 °C (± 1.87) in January and 30.04 °C (± 1.35) in September (see Fig. 2B). The mean minimum values were recorded in 2012 (27.48 °C) and maximum in 2016 (30.30 °C, see Fig. 2D).
Recorded minimum and maximum temperatures [°C] in Tangkoko Reserve, Sulawesi, 2006–2020. A and B: Mean temperatures recorded in 1 year (A: Minimum temperatures. B: Maximum temperatures). The data are represented using a violin plot for each month. Each violin depicts the distributions of the temperature data for 1 month over the years. The width of each curve corresponds with the frequency of data points. Densities are accompanied by an overlaid layer of individual data points representing the temperature recorded each year. C and D: The temperature records are represented as a heatmap (C: Minimum temperatures; D: Maximum temperatures): each tile corresponds to the temperature record for 1 year (in row) and 1 month (in column) during the study period. A grey data tile indicates a missing data point.
Rainfall — considering calendar years with complete data only, we recorded a mean of 1780 mm (± 404 mm SD) rainfall per year. Rainfall was highly seasonal: the minimum was recorded in August (51 ± 46 mm SD) and the maximum in January (252 ± 76 mm, see Fig. 3A). The yearly rainfall range varied greatly from a total of 957 mm in 2015 (with 100% daily data available for this year) to 2525 mm in 2010 (91% data available for the year). The data from 2015–2016 shows a severe drought and unusual minimal amounts of rain (Fig. 3B).
Recorded rainfall [mm] in Tangkoko Reserve, Sulawesi, Indonesia, 2006–2020. A: Distribution of rainfall across the year. The data are represented using a violin plot for each month. Each dark blue violin represents the distributions of the rainfall data for 1 month over the years. The width of each curve corresponds with the frequency of data points. Densities are accompanied by an overlaid layer of individual data points (green dots) representing the rainfall recorded each year. B: Total amount of rainfall for each month and each year of the study period represented as a heatmap. Each row represents a year, each column represents a month. The colour of the tile indicates the amount of rainfall in a month. A grey tile indicates a missing data point.
We detected no significant trend in rainfall over the study (Sen’s estimator, z = − 0.82, p = 0.41) and both annual and seasonal rainfall variations were stable (seasonal Mann–Kendall test, tau = − 0.10, p = 0.21). However, there was an overall significant positive trend in the minimum and maximum temperatures between January 2012 and July 2020 (minimum temperature: Sen’s estimator, z = 2.67, p = 0.008; 95% confidence interval (CI): 0.0025–0.014; maximum temperature: Sen’s estimator, z = 2.76, p = 0.006; 95% CI: 0.0068–0.028). This was confirmed by a significant positive trend in both annual and seasonal minimum and maximum temperature variations (seasonal Mann–Kendall test, minimum temperature: tau = 0.20, p = 0.016; maximum temperature: tau = 0.26, p = 0.001). The minimum and maximum temperatures in Tangkoko forest increased slightly over the 9-year period (minimum temperature: Sen's slope = 0.008; maximum temperature: Sen’s slope = 0.018). This corresponds to an increase of 0.11 °C per year in minimum temperature and 0.19 °C per year in maximum temperature.
Phenological Patterns in Flowering and Fruiting Trees and Plants
We recorded flowers and fruits throughout the year in Tangkoko forest. Of all species, 7% (± 14% SD) of the trees and plants bore flowers during our monthly monitoring, and 11% (± 16% SD) of the monitored trees and plants bore fruits (N = 529 ± 72 individuals, Fig. 4). At community level, there was no obvious seasonal flowering or fruiting pattern. Species showed great inter- and intraspecific variation in their fruiting cycles (Fig. 4, Table S1). There was an overall significant decrease in the fruiting proportion between January 2012 and July 2020 (Sen’s estimator, z = − 5.65, p < 0.001). This was supported by a significant decrease in both annual and seasonal fruiting proportion variations (seasonal Mann–Kendall test, z = − 0.493, p < 0.001, Fig. 4). The overall proportion of trees and plants bearing fruits in Tangkoko forest decreased over the 9-year period (Sen’s slope = − 0.0005), with a decline of about 1% in the proportion of fruiting trees per year).
Overall proportion of flowering and fruiting trees and plants across the year (A and B) and along the study period (C and D) in Tangkoko Reserve, Sulawesi, Indonesia. The sample includes a mean of 529 (± 72 SD) individual plants or trees observed monthly between June 2012 and June 2020. A and B represent the proportion data using box plots. The length of the box is equal to the interquartile distance, which is the difference between the third and first quartiles of the data for the proportion of trees. The black line inside the box represents the median. The whiskers (the lines extending from the top and bottom parts of the box) go to 1.5 times the interquartile distance from the centre of the data. Outliers are shown as plain circles.
Figs (Ficus spp)
We detected significant regular fruiting cycles for two of nine individual trees of Ficus altissima (Fig. 5A and B; detailed results in Table S8). Both individuals had sub-annual fruiting cycles (median cycle: 4.50 months, range: 2.25–6.75 months; Tables S7 and S8). The other Ficus altissima trees did not have a regular fruiting cycle during our study and we could not detect any synchrony of fruiting events between individuals (Fig. 5B, Table S8).
Fruiting proportion in four species of Ficus trees over a year and fruiting score for individual trees between January 2012 and July 2020, in Tangkoko Reserve, Sulawesi, Indonesia. A, C, E and G show the proportion of individual trees bearing fruits for each month. Data are represented using boxplots. The length of the box is equal to the interquartile distance, which is the difference between the third and first quartiles of the data for the proportion of fruiting trees. The black line inside the box represents the median. The whiskers (the lines extending from the top and bottom parts of the box) go to 1.5 times the interquartile distance from the centre of the data. Outliers are shown as plain circles. B, D and F show the time series of fruiting scores for each individual fruiting tree. The fruiting score followed a logistic scale and ranged from 0 to 4 whereby a score of 1 reflects an estimated 10–99 fruits, a score of 2 100–999 fruits, a score of 3, 1000–9999 fruits and a score of 4, 10,000–99,999 fruits. Only trees with at least one fruiting event during the study period were included. Number of individual trees for Ficus altissima is N = 9, Ficus microcarpa is N = 10, Ficus variegata is N = 5 and Ficus virens is N = 10. Individual trees are ranked according to their dbh, the time series for the largest individual is at the top (Table S4).
We detected significant regular fruiting cycles for three of ten individuals of Ficus macrocarpa (Fig. 5C and D; detailed results in Table S9). All individuals had sub-annual fruiting cycles (median cycle: 6 months, range: 4.5–6.35 months; Tables S7 and S9). We could not detect any synchrony of fruiting events between individuals (Fig. 5D, Table S9).
We detected significant regular fruiting cycles for two of five Ficus variegata (Fig. 5E and F; detailed results in Table S10). Both individuals had sub-annual fruiting cycles (median cycle: 7.46 months, range: 7.20–7.71 months; Tables S7 and S10). We could not detect any synchrony of fruiting events between individuals (Fig. 5F, Table S10).
We detected significant regular fruiting cycles for three of ten Ficus virens (Fig. 5G and H; detailed results in Table S11). Two individuals had sub-annual fruiting cycles and one had a supra-annual cycle (median cycle: 4.95 months; range: 2.20–13.5 months; Tables S7 and S11). We could not detect any synchrony of fruiting events between individuals (Fig. 5H, Table S11).
Altogether, after applying a confidence test, we found that only ten out of the 34 Ficus individuals showed significant dominant cycles; nine out of ten were sub-annual cycles.
We found no significant trend in the fruiting proportion over the whole 2012 to 2020 period for F. altissima (Table II). Both annual and seasonal fruiting variations were stable for F. altissima and F. microcarpa, too (Table II). However, we found an increasing trend in F. variegata fruiting events and a decreasing trend in F. microcarpa and F. virens fruiting events. Annual and seasonal fruiting variations showed an increasing trend for F. variegata and a decreasing trend for F. virens.
New Guinea Walnuts (Dracontomelon dao and D. mangiferum)
We could not detect any significant cycles for D. dao individuals (N = 5, Fig. 6A and B; detailed results in Tables S7 and S12), nor for D. mangiferum individuals (N = 6, Fig. 6C and D; detailed results in Tables S7 and S13). Hence, we could not detect any synchrony in their fruiting events. We found no significant trend in fruiting over the whole 2012 to 2020 period (Sen’s estimator, z = − 0.26, p = 0.79, Fig. 6) and both annual and seasonal fruiting variations were stable (seasonal Mann–Kendall test, tau = − 0.115, p = 0.21).
Fruiting states of the Dracontomelon trees over a year and between January 2012 and July 2020, in Tangkoko Reserve, Sulawesi, Indonesia. Data is presented for each of the two species: Dracontomelon dao and Dracontomelon mangiferum. A and C represent the proportion of individual trees bearing fruits for each month. Data are represented using boxplots. The length of the box is equal to the interquartile distance, which is the difference between the third and first quartiles of the data for the proportion of fruiting trees. The black line inside the box represents the median. The whiskers (the lines extending from the top and bottom parts of the box) go to 1.5 times the interquartile distance from the centre of the data. Outliers are shown as plain circles. B and D represent the time series of fruiting scores for each individual fruiting tree. Fruiting score followed a logistic scale and ranged from 0 to 4 whereby a score of 1 reflects an estimated 10–99 fruits, a score of 2, 100–999 fruits, a score of 3, 1000–9999 fruits and a score of 4, 10,000–99,999 fruits. Only trees with at least one fruiting event during the study period were included. Number of individual trees for Dracontomelon dao is N = 5, and Dracontomelon mangiferum is N = 6. Individual trees have been ranked according to their dbh, the time series for the largest individual is at the top (see supplementary material, Table S4). Data was collated monthly between January 2012 and July 2020.
Spiked Pepper (Piper aduncum)
Spiked peppers had fruits year-round (Fig. 7). In the whole phenological dataset, 42% of spiked peppers carried fruits in any one month, making them the species that fruited most. None of the individual spiked pepper plants did show any cyclicity (N = 4, Fig. 7A and B; detailed results in Tables S7 and S14). We detected a significant trend in the fruiting proportion overall the whole 2012 to 2020 period (Sen’s estimator, z = − 5.79, p < 0.001). Annual and seasonal fruiting variations also show a decreasing trend (seasonal Mann–Kendall test, tau = − 0.44, p < 0.001).
Fruiting states of the spiked peppers plants over a year and between January 2012 and July 2020 in Tangkoko Reserve, Sulawesi, Indonesia. A represents the proportion of individual plants bearing fruits for each month. Data is represented using a boxplot. The length of the box is equal to the interquartile distance, which is the difference between the third and first quartiles of the data for the proportion of fruiting plants. The black line inside the box represents the median. The whiskers (the lines extending from the top and bottom parts of the box) go to 1.5 times the interquartile distance from the centre of the data. Outliers are shown as plain circles. B represents the time series of fruiting scores for each individual fruiting plant. Fruiting score followed a logistic scale and ranged from 0 to 4, whereby a score of 1 reflects an estimated 10–99 fruits, a score of 2, 100–999 fruits, a score of 3, 1000–9999 fruits and a score of 4, 10,000–99,999 fruits. Only plants with at least one fruiting event during the study period were included. Number of individual trees is N = 4. Individual plants have been ranked according to their dbh, the time series for the largest individual is at the top (see supplementary material, Table S5). Data was collated monthly between January 2012 and July 2020.
Estimated Home Range and its Drivers
Crested macaques in Tangkoko ranged over a mean of 2.32 km2 in a month (± 1.12; range 1.31 km2 for PB to 3.01 km2 for R1; Fig. 8; Table S15). The best-fit and most parsimonious model was Home Range ~ Temperature + (1 | Group). We found a main effect of the monthly temperature only on the home range size (estimate = 0.25, SE = 0.06, t = 4.34; CI = 0.14–0.37; p < 0.0001). The hotter it was in the forest, the larger the home ranges (Fig. 8).
Standardised monthly temperature and monthly home range size of four groups of crested macaques in Tangkoko Reserve, Sulawesi, Indonesia, between January 2012 and December 2016. We included monthly measurements of home ranges if at least 12 daily data points were available. Each dot represents a monthly home range size for one group. We added smoothing lines using the geomsmooth() function and used the Loess method of fitting; the grey areas represent 95% confidence interval around the regression lines.
We found that the two groups (PB and PB1) which ranged almost exclusively in the old-growth forest have smaller home ranges than the two groups (R1 and R2) that use disturbed habitat (Fig. 9A). Home ranges overlapped by 86.8% between consecutive months (± 9.83, range: 77.5 for PB group in 2012 and 2013 to 88.8 for R1 from 2012 to 2020; Table III). The overlap in home ranges between consecutive years was 88.0% (± 11.1, range: 77.7 for PB group to 92.9 for R1) and between the first and last year during our study was 85.2% (± 7.8, range: 80.1 for PB1 group to 94.2 for R2, Fig. 9B).
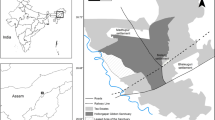
Distribution of the home ranges of crested macaques in Tangkoko Reserve, Sulawesi. We used a Google Earth satellite image (February 2023) as the background layer. A. Home ranges of groups PB1, R1, and R2 in 2016. B. Overlap between home ranges in consecutive years for the largest group (R1). We estimated home range using the minimum convex polygon method. We show years for which we had data for every month: 2012 (N = 164 days), 2013 (N = 62 days), 2014 (N = 288 days), 2015 (N = 304 days), and 2016 (N = 261 days).
Discussion
We found that, over 9 consecutive years, about 10% of monitored individual trees bore flowers or fruits during our monthly recording. Between 2012 and 2020, temperatures increased by up to 0.2 °C per year in the forest, and the overall fruit abundance decreased by 1% per year. Crested macaques in Tangkoko have roamed in the same area over several years. Home-range sizes varied with group identity and overlapped greatly between consecutive months, with no large shift between years. We also found that the monthly temperature in the forest was the best predictor of home range size: the hotter the temperature, the larger the home ranges.
These findings give important information, and add to published climatic and botanical data in Tangkoko from the early 1990s (O’Brien & Kinnaird, 1997). In the mid-1990s, crested macaques had similar home range sizes to those reported here (range 1.6 km2 to 4.1 km2 in O’Brien & Kinnaird, 1997). The home range sizes greatly varied according to the group and had great overlap, which is still true 25 years later. The groups still are in the same forest area, suggesting maintenance of a healthy local population. Groups PB, PB1, and R2 had quite similar composition to one another during the study, with R1 the largest group. Group size might influence home range size (e.g., Campos et al., 2014). However, as described previously (O’Brien & Kinnaird, 1997), we found that the two groups which range mainly in primary forest have smaller home ranges than the two groups that use disturbed habitat. This pattern might explain for the impact of temperature on home range sizes. Looking at the data, the monthly temperature had more of an effect on the larger home ranges, covering both old-growth and disturbed forests, than on the smaller ranges in the old-growth forest only. It is unclear which attribute has the biggest influence on this difference in ranging behaviour. However, crested macaques depend on large-sized canopy trees for food and sleeping (O’Brien & Kinnaird, 1997). This dependence might explain why they prefer old-growth forest. Disturbed areas have a smaller density of big trees (Palacios et al., 2012), which might offer fewer resources and less cover against the heat, and macaques would need to travel larger distances than in primary forest.
Our findings are in line with other studies investigating the influence of climatic factors on home-range size and space use in primates. For instance, similar patterns in ranging were found in capuchins (Cebus capucinus) living in a mixed deciduous forest (Campos et al., 2014). Capuchins preferred to use old-growth and primary forest under hotter conditions. Similarly, temperature, but not rainfall, negatively impacted the distance travelled by red-tailed monkeys (Cercopithecus ascanius) in both evergreen forest and mixed savanna–woodland habitat (McLester et al., 2019). Food abundance also played a role in shaping ranging patterns, with closed habitats with more food being preferred to more open habitats, which was reflected in smaller home ranges. Further investigation on time budget and movements at a small spatial scale would be helpful to test the exact drivers of home-range size (such as tree canopy for thermoregulation) in Tangkoko forest.
We found that ten of 34 fig trees have significant fruiting cycles and nine out of ten fruit sub-annually (3- to 6-month cycles). Guinea walnuts seemed to have no regular fruiting cycles. Spiked peppers fruited frequently and year-round, and did not show any cyclicity. None of the trees within a species produced fruits synchronously. Trends in the fruiting proportions varied greatly between species, even within the same genus (e.g. Ficus spp). Our study supports previous assumptions and shorter-term data that fruits, and especially figs, are available year-round in Tangkoko forest (Kinnaird & O’Brien, 2005; O’Brien & Kinnaird, 1997).
We found that trees fruited less at the end of our study in 2020 than in 2012. Our climate data are in line with the recording of a severe drought and unusual minimal amounts of rain reported in the area due to a major El Niño event (Lestari et al., 2018). The decreasing fruiting trend may therefore be due to this ENSO event or the result of the global climate change. We also found that most of the fig trees fruited irregularly with no clear timing pattern and were not predictable over time. This is in line with previous studies of the phenology of fig trees in the area (Kinnaird & O’Brien, 2005) and in other tropical forests (Milton, 1991). Fig fruiting phenologies are very complex and need further investigation. For instance, some fig tree species are dioecious, with distinct female and male trees which produce flowers and fruits at a different timing (Kuaraksa et al., 2012). Drought can also have severe impacts on fig fruiting (Harrison, 2001). In our study, the absence of detected pattern in fruiting may be explained by abiotic factors, such as rainfall (Chapman et al., 2018; Zimmerman et al., 2007). Alternatively, the absence of pattern could be due to the temporal resolution of the monitoring of fruiting events, which might not allow us to detect more ephemeral fruiting states. Monitoring the forest requires a tremendous amount of effort. However, new technologies might help to get more data with a better spatial and temporal resolution. For instance, ForestScanner (Tatsumi et al., 2022) enables the free use of Light Detection And Ranging (LiDAR) technology with a mobile phone to do a plant inventory. The application integrates measurement of stem diameters and a map of the vegetation using spatial coordinates, hence reconstructing a precise 3D visualisation of the scanned habitat using cost-efficient technology.
Crested macaques in Tangkoko keep stable home ranges over several years. We used a minimum convex polygon method to estimate home ranges. These are likely to overestimate the area, and may not reflect the complexity of ranging behaviour. Kernel analysis has been proposed as a more accurate method to calculate home range, as it considers the density based on the clustering of the GPS data points (Seaman & Powell, 1996). However, kernel analysis also tends to overestimate the home ranges and is of limited use in interpretation if we do not have the spatial coordinates of the key food trees, as in this study.
Further investigations using this dataset could look at daily movements to learn more about foraging strategies and underlying possible cognitive skills. For instance, it would be useful to inspect timing of visits and potential monitoring of fig trees depending on the weather conditions. Studies of whether primates use specific temporal patterns to visit key resources are rare (but see Ban et al., 2014; Jang et al., 2021; Janmaat et al., 2012). So far, it seems that some primate species time their visit to trees according to their fruiting status (Jang et al., 2021; Janmaat et al., 2012), or to whether they have recently visited and depleted them (Ban et al., 2014). As proposed recently (Janmaat et al., 2021), the analysis of temporal patterns should focus on the frequency of visits and its regularity as well as the predictability of visits between patches or their connectedness. This will help us disentangle potential memory of spatial locations only, or whether these specific areas are visited at specific times (i.e., planning). Evidence from species living in different habitats showing different predictability in their food sources will be of utmost importance to assess subtle changes in behavioural strategies (Janmaat et al., 2021).
Large phenology datasets from the tropics are crucial sources of insight into long-term and current regional and global changes, and enable us to understand and predict phenological events as well as their impact on primate foraging and movement. This paper sets the scene for further investigation of spatial use, animal movement, and ultimately their link to social dynamics, such as intergroup competition. It provides further details on socio-ecological challenges that a species faces and, among other considerations, encourages future investigations to better understand primate cognition in the wild and its relevance to primate conservation.
Data Availability
The data are available in the supplementary material.
References
Allen, C. D., Macalady, A. K., Chenchouni, H., Bachelet, D., McDowell, N., Vennetier, M., Kitzberger, T., Rigling, A., Breshears, D. D., Hogg, E. H. (Ted), Gonzalez, P., Fensham, R., Zhang, Z., Castro, J., Demidova, N., Lim, J.-H., Allard, G., Running, S. W., Semerci, A., & Cobb, N. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management, 259(4), 660–684.https://doi.org/10.1016/j.foreco.2009.09.001
Ban, S. D., Boesch, C., & Janmaat, K. R. L. (2014). Taï chimpanzees anticipate revisiting high-valued fruit trees from further distances. Animal Cognition, 17(6), 1353–1364. https://doi.org/10.1007/s10071-014-0771-y
Bartoń, K. (2022). MuMIn: Multi-Model Inference (1.46.0). https://CRAN.R-project.org/package=MuMIn
Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015). Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01
Bush, E. R., Abernethy, K. A., Jeffery, K., Tutin, C., White, L., Dimoto, E., Dikangadissi, J., Jump, A. S., & Bunnefeld, N. (2017). Fourier analysis to detect phenological cycles using long-term tropical field data and simulations. Methods in Ecology and Evolution, 8(5), 530–540. https://doi.org/10.1111/2041-210X.12704
Bush, E. R., Whytock, R. C., Bahaa-el-din, L., Bourgeois, S., Bunnefeld, N., Cardoso, A. W., Dikangadissi, J. T., Dimbonda, P., Dimoto, E., EdzangNdong, J., Jeffery, K. J., Lehmann, D., Makaga, L., Momboua, B., Momont, L. R. W., Tutin, C. E. G., White, L. J. T., Whittaker, A., & Abernethy, K. (2020). Long-term collapse in fruit availability threatens Central African forest megafauna. Science, 370(6521), 1219–1222. https://doi.org/10.1126/science.abc7791
Campos, F. A., Bergstrom, M. L., Childers, A., Hogan, J. D., Jack, K. M., Melin, A. D., Mosdossy, K. N., Myers, M. S., Parr, N. A., Sargeant, E., Schoof, V. A. M., & Fedigan, L. M. (2014). Drivers of home range characteristics across spatiotemporal scales in a Neotropical primate, Cebus capucinus. Animal Behaviour, 91, 93–109. https://doi.org/10.1016/j.anbehav.2014.03.007
Chapman, C. A., Chapman, L. J., Struhsaker, T. T., Zanne, A. E., Clark, C. J., & Poulsen, J. R. (2005). A long-term evaluation of fruiting phenology: Importance of climate change. Journal of Tropical Ecology, 21(1), 31–45. https://doi.org/10.1017/S0266467404001993
Chapman, C. A., Valenta, K., Bonnell, T. R., Brown, K. A., & Chapman, L. J. (2018). Solar radiation and ENSO predict fruiting phenology patterns in a 15-year record from Kibale National Park, Uganda. Biotropica, 50(3), 384–395. https://doi.org/10.1111/btp.12559
Chapman, C. A., Galán-Acedo, C., Gogarten, J. F., Hou, R., Lawes, M. J., Omeja, P. A., Sarkar, D., Sugiyama, A., & Kalbitzer, U. (2021). A 40-year evaluation of drivers of African rainforest change. Forest Ecosystems, 8(1), 66. https://doi.org/10.1186/s40663-021-00343-7
Cleland, E., Chuine, I., Menzel, A., Mooney, H., & Schwartz, M. (2007). Shifting plant phenology in response to global change. Trends in Ecology & Evolution, 22(7), 357–365. https://doi.org/10.1016/j.tree.2007.04.003
Cook, B. I., Smerdon, J. E., Seager, R., & Coats, S. (2014). Global warming and 21st century drying. Climate Dynamics, 43(9–10), 2607–2627. https://doi.org/10.1007/s00382-014-2075-y
Coumou, D., & Rahmstorf, S. (2012). A decade of weather extremes. Nature Climate Change, 2(7), 491–496. https://doi.org/10.1038/nclimate1452
Dai, A. (2011). Drought under global warming: A review. Wires Climate Change, 2(1), 45–65. https://doi.org/10.1002/wcc.81
Duboscq, J., Micheletta, J., Agil, M., Hodges, K., Thierry, B., & Engelhardt, A. (2013). Social tolerance in wild female crested macaques (Macaca nigra) in Tangkoko-Batuangus Nature Reserve, Sulawesi, Indonesia. American Journal of Primatology, 75, 361–375. https://doi.org/10.1002/ajp.22114
Engelhardt, A., Muniz, L., Perwitasari-Farajallah, D., & Widdig, A. (2017). Highly polymorphic microsatellite markers for the assessment of male reproductive skew and genetic variation in Critically Endangered crested macaques (Macaca nigra). International Journal of Primatology, 38(4), 672–691. https://doi.org/10.1007/s10764-017-9973-x
Harrison, R. D. (2001). Drought and the consequences of El Niño in Borneo: A case study of figs. Population Ecology, 43(1), 63–75. https://doi.org/10.1007/PL00012017
Hilser, H., Siwi, Y., Agung, I., Masson, G., Bowkett, A., Plowman, A. B., Taronga, V. M., & Tasirin, J. S. (2013). A non-native population of the Critically Endangered Sulawesi crested black macaque persists on the island of Bacan. Oryx, 47(4), 479–480. https://doi.org/10.1017/S0030605313000872
Ims, R. A. (1990). The ecology and evolution of reproductive synchrony. Trends in Ecology & Evolution, 5(5), 135–140. https://doi.org/10.1016/0169-5347(90)90218-3
IUCN. (2022). The IUCN Red List of Threatened Species. Version 2022–2. https://www.iucnredlist.org. Accessed on 03 June 2023.
Jang, H., Oktaviani, R., Kim, S., Mardiastuti, A., & Choe, J. C. (2021). Do Javan gibbons (Hylobates moloch) use fruiting synchrony as a foraging strategy? American Journal of Primatology, 83(10), e23319. https://doi.org/10.1002/ajp.23319
Janmaat, K. R. L., Chapman, C. A., Meijer, R., & Zuberbühler, K. (2012). The use of fruiting synchrony by foraging mangabey monkeys: A ‘simple tool’ to find fruit. Animal Cognition, 15(1), 83–96. https://doi.org/10.1007/s10071-011-0435-0
Janmaat, K. R. L., de Guinea, M., Collet, J., Byrne, R. W., Robira, B., van Loon, E., Jang, H., Biro, D., Ramos-Fernández, G., Ross, C., Presotto, A., Allritz, M., Alavi, S., & Van Belle, S. (2021). Using natural travel paths to infer and compare primate cognition in the wild. IScience, 24(4):102343. https://doi.org/10.1016/j.isci.2021.102343
Jassby, A., Cloern, J., & Stachelek, J. (2017). wql: Exploring Water Quality Monitoring Data (0.4.9). https://CRAN.Rproject.org/package=wql
Johnson, C. L., Hilser, H., Linkie, M., Rahasia, R., Rovero, F., Pusparini, W., Hunowu, I., Patandung, A., Andayani, N., Tasirin, J., Nistyantara, L. A., & Bowkett, A. E. (2020). Using occupancy-based camera-trap surveys to assess the Critically Endangered primate Macaca nigra across its range in North Sulawesi, Indonesia. Oryx, 54(6), 784–793. https://doi.org/10.1017/S0030605319000851
Kelly, D. (1994). The evolutionary ecology of mast seeding. Trends in Ecology & Evolution, 9(12), 465–470. https://doi.org/10.1016/0169-5347(94)90310-7
Kendall, M. G. (1948). Rank correlation methods. Griffin.
Kinnaird, M. F., & O’Brien, T. G. (2005). Fast Foods of the Forest: The influence of figs on primates and hornbills across Wallace’s line. In J. L. Dew & J. P. Boubli (Eds.), Tropical Fruits and Frugivores (pp. 155–184). Springer-Verlag. https://doi.org/10.1007/1-4020-3833-X_9
Kuaraksa, C., Elliott, S., & Hossaert-Mckey, M. (2012). The phenology of dioecious Ficus spp. Tree species and its importance for forest restoration projects. Forest Ecology and Management, 265, 82–93. https://doi.org/10.1016/j.foreco.2011.10.022
Lee, R., Riley, E., Sangermano, F., Cannon, C. & Shekelle, M. (2020). Macaca nigra. The IUCN red list of threatened species 2020: e.T12556A17950422. https://doi.org/10.2305/IUCN.UK.2020-3.RLTS.T12556A17950422.en
Lestari, D. O., Sutriyono, E., Sabaruddin, & Iskandar, I. (2018). Severe drought event in Indonesia following 2015/16 El Niño/positive Indian dipole events. Journal of Physics: Conference Series, 1011, 012040. https://doi.org/10.1088/1742-6596/1011/1/012040
Mann, H. B. (1945). Nonparametric tests against trend. Econometrica, 13(3), 245. https://doi.org/10.2307/1907187
Many Primates: Altschul, D. M., Beran, M. J., Bohn, M., Call, J., DeTroy, S., Duguid, S. J., Egelkamp, C. L., Fichtel, C., Fischer, J., Flessert, M., Hanus, D., Haun, D. B. M., Haux, L. M., Hernandez-Aguilar, R. A., Herrmann, E., Hopper, L. M., Joly, M., Kano, F., … Watzek, J. (2019). Establishing an infrastructure for collaboration in primate cognition research. PLoS ONE, 14(10), e0223675. https://doi.org/10.1371/journal.pone.0223675
Marshall, A., Beaudrot, L., & Wittmer, H. (2014). Responses of primates and other frugivorous vertebrates to plant resource variability over space and time at Gunung Palung National Park. International Journal of Primatology, 35(6), 1178–1201. https://doi.org/10.1007/s10764-014-9774-4
McLeod, A. I. (2022). Kendall: Kendall Rank Correlation and Mann-Kendall Trend Test (2.2.1). https://CRAN.Rproject.org/package=Kendall
McLester, E., Brown, M., Stewart, F. A., & Piel, A. K. (2019). Food abundance and weather influence habitat-specific ranging patterns in forest- and savanna mosaic-dwelling red-tailed monkeys ( Cercopithecus ascanius ). American Journal of Physical Anthropology, 170(2), 217–231. https://doi.org/10.1002/ajpa.23920
Mendoza, I., Peres, C. A., & Morellato, L. P. C. (2017). Continental-scale patterns and climatic drivers of fruiting phenology: A quantitative Neotropical review. Global and Planetary Change, 148, 227–241. https://doi.org/10.1016/j.gloplacha.2016.12.001
Micheletta, J., Waller, B. M., Panggur, M. R., Neumann, C., Duboscq, J., Agil, M., & Engelhardt, A. (2012). Social bonds affect anti-predator behaviour in a tolerant species of macaque, Macaca nigra. Proceedings of the Royal Society B-Biological Sciences, 279(1744), 4042–4050.
Milton, K. (1981). Distribution patterns of tropical plant foods as an evolutionary stimulus to primate mental development. American Anthropologist, 83, 534–548.
Milton, K. (1988). Foraging behaviour and the evolution of primate intelligence. In R. W. Byrne & A. Whiten (eds.), Machiavellian intelligence: Social expertise and the evolution of intellect in monkeys, apes, and humans (pp. 285–305). Clarendon Press/Oxford University Press
Milton, K. (1991). Leaf change and fruit production in six neotropical Moraceae species. Journal of Ecology, 79(1), 1–26. https://doi.org/10.2307/2260781
Neumann, C., Duboscq, J., Dubuc, C., Ginting, A., Irwan, A. M., Agil, M., Widdig, A., & Engelhardt, A. (2011). Assessing dominance hierarchies: Validation and advantages of progressive evaluation with Elo-rating. Animal Behaviour, 82(4), 911–921. https://doi.org/10.1016/j.anbehav.2011.07.016
O’Brien, T. G., & Kinnaird, M. F. (1997). Behavior, diet, and movements of the Sulawesi crested black macaque (Macaca nigra). International Journal of Primatology, 18(3), 321–351. https://doi.org/10.1023/A:1026330332061
Palacios, J. F. G., Engelhardt, A., Agil, M., Hodges, K., Bogia, R., & Waltert, M. (2012). Status of, and conservation recommendations for, the Critically Endangered crested black macaque Macaca nigra in Tangkoko, Indonesia. Oryx, 46(2), 290–297. https://doi.org/10.1017/S0030605311000160
Pohlert, T. (2020). trend: Non-Parametric Trend Tests and Change-Point Detection (1.1.4). https://CRAN.Rproject.org/package=trend
Potts, K. B., Watts, D. P., Langergraber, K. E., & Mitani, J. C. (2020). Long-term trends in fruit production in a tropical forest at Ngogo, Kibale National Park. Uganda. Biotropica, 52(3), 521–532. https://doi.org/10.1111/btp.12764
QGIS.org. (2022). QGIS Geographic Information System (3.22) [Computer software]. QGIS Association. http://www.qgis.org
R Core Team. (2021). R: A language and environment for statistical computing. [R Foundation for Statistical Computing,]. https://www.R-project.org/.
Rosenbaum, B., O’Brien, T. G., Kinnaird, M., & Supriatna, J. (1998). Population densities of Sulawesi crested black macaques (Macaca nigra) on Bacan and Sulawesi, Indonesia: Effects of habitat disturbance and hunting. American Journal of Primatology, 44(2), 89–106. https://doi.org/10.1002/(SICI)1098-2345(1998)44:2%3c89::AID-AJP1%3e3.0.CO;2-S
Seaman, D. E., & Powell, R. A. (1996). An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology, 77(7), 2075–2085. https://doi.org/10.2307/2265701
Sen, P. K. (1968). Estimates of the regression coefficient based on Kendall’s Tau. Journal of the American Statistical Association, 63(324), 1379–1389. https://doi.org/10.1080/01621459.1968.10480934
Tatsumi, S., Yamaguchi, K., & Furuya, N. (2022). ForestScanner: A mobile application for measuring and mapping trees with LiDAR-equipped iPhone and iPad. Methods in Ecology and Evolution. https://doi.org/10.1111/2041-210X.13900
van Schaik, C. P., Terborgh, J. W., & Wright, S. J. (1993). the phenology of tropical forests: Adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics, 24(1), 353–377. https://doi.org/10.1146/annurev.es.24.110193.002033
Walther, G.-R., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J. C., Fromentin, J.-M., Hoegh-Guldberg, O., & Bairlein, F. (2002). Ecological responses to recent climate change. Nature, 416(6879), 389. https://doi.org/10.1038/416389a
Wieczkowski, J., & Kinnaird, M. (2008). Shifting forest composition and primate diets: A 13-year comparison of the Tana River mangabey and its habitat. American Journal of Primatology, 70(4), 339–348. https://doi.org/10.1002/ajp.20495
Zachos, J., Pagani, M., Sloan, L., Thomas, E., & Billups, K. (2001). Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292(5517), 686–693. https://doi.org/10.1126/science.1059412
Zhang, L., Ameca, E. I., Cowlishaw, G., Pettorelli, N., Foden, W., & Mace, G. M. (2019). Global assessment of primate vulnerability to extreme climatic events. Nature Climate Change, 9(7), 554–561. https://doi.org/10.1038/s41558-019-0508-7
Zimmerman, J. K., Wright, S. J., Calderón, O., Pagan, M. A., & Paton, S. (2007). Flowering and fruiting phenologies of seasonal and aseasonal neotropical forests: The role of annual changes in irradiance. Journal of Tropical Ecology, 23(2), 231–251. https://doi.org/10.1017/S0266467406003890
Acknowledgements
We thank the Indonesian State Ministry of Research and Technology (RISTEK), the Directorate General of Forest Protection and Nature Conservation (PHKA) and the Department for the Conservation of Natural Resources (BKSDA), North Sulawesi, for permission to conduct research in the Tangkoko Nature Reserve. We thank all members of the Macaca Nigra Project for their support. We would like to thank the guest editor, the editor-in-chief, and two anonymous reviewers for their helpful comments on previous drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Inclusion and Diversity Statement
The author list includes contributors from the location where the research was conducted, who participated in study conception, study design, and data collection. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Additional information
Handling Editor: Julie Duboscq
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joly, M., Tamengge, M., Pfeiffer, JB. et al. Climate, Temporal Abundance of Key Food Sources and Home Ranges of Crested Macaques (Macaca nigra) in Sulawesi, Indonesia: A Longitudinal Phenological Study. Int J Primatol 44, 670–695 (2023). https://doi.org/10.1007/s10764-023-00377-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-023-00377-4