Abstract
Ecological niches are the environmental conditions under which an organism can maintain viable populations. A detailed understanding of an organisms’ ecological niche can provide information on its taxonomy and biogeography, and ecological niche modelling allows researchers to investigate how closely-related species are able to coexist. Ecological niche models also enable conservationists to determine species’ habitat requirements, map distributions, and assess threats. We used this approach to investigate the conservation biogeography of the dwarf lemurs (genus Cheirogaleus), a group of cryptic, nocturnal primates endemic to Madagascar. Using climatic and vegetation-related variables, we constructed ecological niche models for three species to investigate niche overlap among taxa. We also constructed maps of the availability of forest habitat, and we assessed anthropogenic risk and protection. Our ecological niche models and background tests indicated that each of the three analysed Cheirogaleus species occupies distinct environmental space. The area of suitable habitat (realized niche) varied interspecifically (28,889–41,934 km2). This also was mirrored by variation in the percentage of each species’ realized niche within protected areas (20,065–25,266 km2) and near anthropogenic features (5,744–16,999 km2). Our results support the 2020 taxonomy of the dwarf lemurs recognised by the IUCN Red List and provides information on their biogeography. Furthermore, our ecological niche models have highlighted that the habitat of some dwarf lemur species, such as C. crossleyi, are more threatened than other species, such as C. medius and C. major, and these species require urgent conservation attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological niche models (ENMs) are well-suited for studies of demography, taxonomy, and species conservation, because they can be utilised to assess how well-protected species are throughout their ranges and their susceptibility to anthropogenic pressures (Elith et al., 2006; Thorn et al., 2009; Urbina-Cardona & Loyola, 2008). ENMs can provide valuable information about the potential geographic distributions of species (Peterson & Soberón, 2012) and information on niche separation among sympatric species and between closely related taxa (Zimmerman, 2006) and integrated taxonomy (Padial & De La Riva, 2010). It is therefore useful for species delineation (Raxworthy et al., 2007; Rissler & Apodaca, 2007). Furthermore, ENMs can reveal information about the potential geographic ranges of understudied species (Guisan & Zimmermann, 2000) and thus facilitate their conservation and future-study (Ferrer-Sánchez & Rodríguez-Estrella, 2016). ENMs are particularly relevant tools to estimate the distributions of elusive animals that are hard to observe and study in the wild (Coudrat & Nekaris, 2013). Cryptic species are such an example of this, because their similar morphologies make them difficult to visually identify (Sattler et al., 2007). Unfortunately, the geographic distributions of many of these newly described species are unknown (Rissler & Apodaca, 2007). It also is difficult to determine the presence and absence of cryptic species at specific localities (Black, 2020), because they often are small, elusive, and inconspicuous (Gibson et al., 2007; Sutherland, 2000). This dearth of knowledge can be detrimental to their conservation, as many cryptic species are already threatened with extinction (Black, 2020; Morais et al., 2013).
The dwarf lemurs (genus Cheirogaleus) are a group of small-bodied, nocturnal, and cryptic primates endemic to Madagascar (Mittermeier et al., 2008). Like many other nocturnal lemurs (Groves, 2001; Yoder et al., 2000), the dwarf lemur genus has undergone taxonomic expansion in recent years, despite opposition from Groeneveld et al., (2009, 2011) who provided genetic and morphological evidence against this taxonomic splitting. Nine species of dwarf lemur are now formally described and are recognized by the IUCN (Frasier et al., 2016; IUCN, 2020a; Lei et al., 2015; McLain et al., 2017). Although dwarf lemurs are arboreal and require forest habitat for their survival (Lehman et al., 2016), some species, such as C. medius and C. major, are capable of surviving in degraded and anthropogenically disturbed areas (Hending, 2021a). Unlike nocturnal lemurs of the Microcebus, Lepilemur, and Phaner genera, whose species-specific distributions often are restricted by biogeographical and hydrological features (Craul et al., 2007; Hending et al., 2020; Olivieri et al., 2007; Wilmé et al., 2006), many dwarf lemur species are more geographically widespread (IUCN, 2020b; Lei et al., 2014), making their ranges difficult to predict. Studies of Cheirogaleus biogeography are made more challenging by the hibernation that they undergo for up to 7 months annually (Dausmann & Blanco, 2016), making them undetectable for large periods of the year. Despite this, many new dwarf lemur populations have been discovered recently (Gardner & Jasper, 2015; Hending et al., 2017), but morphological similarities among species make these new populations difficult to identify (Groeneveld et al., 2009).
Sympatry among multiple dwarf lemur species at numerous localities, such as C. crossleyi with C. sibreei and C. major with C. thomasi, further confuses our understanding of their geographic distributions (Blanco et al., 2009; Groeneveld et al., 2010) and raises questions of their habitat requirements and niche overlap (Hapke et al., 2005; Herrera et al., 2016; Lahann, 2007). Whereas the full ranges of the dwarf lemurs remain little known, it is already apparent that their forest habitat is being cleared at an alarmingly high rate, and much of what remains is now fragmented (Vieilledent et al., 2018). Eight of the nine dwarf lemur species are now listed as threatened on the IUCN Red List, whereas C. grovesi is listed as Data Deficient (IUCN, 2020a). An informed understanding of Cheirogaleus ecological niches, the area of suitable habitat that is available to them, and their potential geographic distributions is needed to conserve and manage their remaining populations and alleviate the threats that they face (Schwitzer et al., 2013).
We investigated the biogeography of Madagascar’s dwarf lemurs with ENMs. The specific objectives of this study were:
-
1.
To compare ecological niche-overlap among the dwarf lemurs. As dwarf lemur sympatry sometimes occurs (Blanco et al., 2009; Herrera et al., 2016; Lahann, 2007), we hypothesized that little niche overlap would occur between species to maintain reproductive isolation.
-
2.
To assess the total area of suitable forest habitat (realized niche—the area in which each species can survive) of each species. Some dwarf lemurs are regarded as adaptable, generalist primates (Schäffler & Kappeler, 2014), and we therefore hypothesized that their ENMs would encompass a large area of suitable habitat. We also predicted that the realized niche area (suitable habitat) would be lower in area than the total occupiable area (fundamental niche) identified in the ENMs due to widescale deforestation and forest fragmentation of Madagascar (Harper et al., 2007; Vieilledent et al., 2018).
-
3.
To assess how much of each species’ realized niche is currently protected, and to determine how this habitat is affected by humans. We hypothesized that a large percentage of the realized niches would be located within protected areas due to the substantial efforts of conservationists to protect Madagascar’s remaining biota (Goodman et al., 2018). We also predicted that much of the realized niches would be at significant risk of anthropogenic disturbance, and that habitat suitability would correlate negatively with disturbance intensity.
Methods
Ethics
We certify that all work undertaken in this manuscript complied with the protocols of our institutions and the UK Home Office, the legal requirements of the UK, and the UK Animals (Scientific Procedures) Act 1986 Amendment Regulations (SI 2012/3039).
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on request.
Competing Interests
All authors state that they have no conflict of interest.
Occurrence Data
We collected known Cheirogaleus species’ occurrence data from both published and unpublished sources. To find occurrence data within the published literature, we searched the full volume-catalogue of the primatology journals, American Journal of Primatology, International Journal of Primatology, Primates, Folia Primatologica, Primate Conservation, and Lemur News, as well as the Madagascar-specific journals, Malagasy Nature and Madagascar Conservation and Development. This method ensured that we did not miss any studies from local or regional journals (e.g., Malagasy Nature). In addition to these primate-specific journals, we used Google Scholar to search for published articles in nonprimate-specific journals, dissertations, and edited book volumes. We used the keywords “population,” “distribution,” “presence,” “dwarf lemur, and “Cheirogaleus” in our literature search, in addition to the common and scientific name keywords for all nine Cheirogaleus species. Additionally, we supplemented these published datasets with occurrence records from unpublished sources (e.g., unpublished reports, conference posters, personal observations) listed in the most recent IUCN Red List assessments (Blanco et al., 2020a, b, c, d, e, f; Ganzhorn et al., 2020; Sgarlata et al., 2020a, b).
We included all occurrence points as separate data points in our database. For each point, we listed the geographic coordinate of the location (to as many decimal points listed in the source), the corresponding Cheirogaleus species, and their conservation status. Many records in our database were from studies conducted before recent species descriptions. We thus updated the species names to reflect the latest taxonomy using the geographic location of the respective point and species-specific range information available in the most recent Red List assessments (IUCN, 2020a, b) (Fig. 1); this range information is informed by genetic studies that have confirmed the identity of Cheirogaleus populations (Frasier et al., 2016; Lei et al., 2015; McLain et al., 2017). Although Groeneveld et al., (2009, 2011) argue for taxonomic deflation of the dwarf lemurs, we use the Cheirogaleus taxonomy as per the IUCN Red List due to the conservation biogeography theme of this paper (IUCN, 2020a). However, the IUCN assessments do not include the full range of some Cheirogaleus species (e.g., C. medius), so we used the wider published literature to identify species occurrences that fell outside of the IUCN polygons (Fig. 1). We excluded any points where we could not reliably confirm the identity of the species using this method (i.e., Cheirogaleus sp.), and we checked the validity of the point coordinates in the literary sources using ArcGIS. Our database included occurrence points from studies that occurred 1964–2020; due to ongoing deforestation in Madagascar (Vieilledent et al., 2018), we checked to ensure that all occurrence points were still forested using current high-resolution (< 1 m/pixel), cloud-free, satellite imagery in Google Earth Pro (version 7.3.3, Google LLC, Mountain View, CA).
Geographic ranges of the dwarf lemurs (genus Cheirogaleus), as described by the IUCN Red List (IUCN, 2020b), and our occurrence point dataset (triangles). The IUCN ranges do not include all localities at which dwarf lemurs are known to occur, as indicated by the occurrence points that we extracted from literary sources.
Environmental Variables
We considered 21 environmental variables for the construction of our ENMs (Table I). The specific variables were chosen based on their relevance to the ecology and natural history of the study-species, and because they have been frequently used to model distributions of primates in previous studies (Chetan et al., 2014; Thorn et al., 2009). Of our 21 chosen environmental variables, 19 were bioclimatic variables related to precipitation and temperature (Hijmans et al., 2005; Booth et al., 2014), and two were vegetation-cover related variables: Normalised Difference Vegetation Index (NDVI, a proxy of plant productivity) and Leaf Area Index (LAI, a proxy of tree cover density). We downloaded global geoTiff raster layers of all BIOCLIM variables from the WorldClim database (Hijmans et al., 2005, 1-km2 resolution). For the NDVI and LAI variables, we downloaded monthly geoTiff layers (250- × 250-m resolution) from January 2000 until January 2020, and then stacked these layers in R Studio (R Core Team, 2017) using the packages “raster” (Hijmans, 2017), “sp” (Bivand et al., 2013), and “rgdal” (Bivand et al., 2019) to create mean NDVI and LAI layers. Next, we resampled all layers to a resolution of 1 km2. In order to draw pseudo-absences from a species-appropriate area when constructing our ENMs, we then clipped the Madagascar-wide raster layers for each species to an area to which each species can realistically disperse (Barve et al., 2011) (Appendix 1). These areas were chosen based on topographic features (including the central highlands that separate Madagascar’s western regions from the eastern regions), major rivers (Goodman et al., 2018), and forest cover (Vieilledent et al., 2018). We chose the extent of these species-specific input layers based on the occurrence points available for each species, the extent of occurrence of each species, and known geographic dispersal barriers (Blanco et al., 2020a, b, c, d, e, f; Ganzhorn et al., 2020; Sgarlata et al., 2020a, b).
It is recommended to remove correlated variables before running ENMs (Merow et al., 2013). To do this, we generated 2,000 random geographic points for Madagascar, and we extracted the corresponding values for each environmental layer. We then analysed the pairwise correlations between each variable for these 2,000 points by using Pearson correlation tests (Appendix 2, as in Hending, 2021b). When pairs of variables had correlation coefficients ≥ 0.70, we retained one variable from the pairwise analysis for creating our ENMs (Cobos et al., 2019). This approach reduced the total number of environmental variables from 21 to nine (Table I). The retained variables are likely drivers of dwarf lemur ecology and physiology, and proxies for the different habitats of Madagascar (Kamilar & Muldoon, 2010; Kamilar et al., 2016). The retained climatic variables represent temperature seasonality (Bio2 and Bio3), water availability (Bio12), and forest presence and quality (NDVI, and LAI), along with proxies of potential instances of extreme heat (Bio5), frost (Bio6), and drought (Bio17) (Blair et al., 2013), which are relevant for lemur infant mortality (Wright, 1999) and seasonal hibernation in dwarf lemurs (Dausmann & Blanco, 2016).
Ecological Niche Modelling and Model Validation
We used the maximum entropy algorithm in Maxent version 3.4.1 (Phillips et al., 2006) to construct ENMs of potential geographic distribution and habitat suitability for the dwarf lemurs. We opted to use the Maxent software because it is now a widely used method to identify species’ ecological niches (Elith et al., 2006; Merow et al., 2013), and it only requires presence points (it does not need absence points to run a model). This is advantageous for this study, as dwarf lemurs are small, nocturnal, cryptic, and they undergo long periods of seasonal hibernation (Dausmann & Blanco, 2016), making it difficult to confirm their absence from a location, similarly to mouse lemurs (Kamilar et al., 2016).
Because we could only find a high number (N > 30) of occurrence points for three of the Cheirogaleus species, we did not run ENMs for the other six species. Whilst models for these species may be useful for exploratory analysis (Wisz et al., 2008), they may not be reliable enough to draw any robust conclusions from (Riley et al., 2019). Before creating the ENMs, we used the “ENMeval” package (Muscarella et al., 2014) in R Studio to create species-specific bias files for inclusion in the Maxent algorithm. These bias files enabled us to control for spatial sampling bias in the occurrence points, by projecting the raster stack of environmental variables and occurrence points with two-dimensional Kernel density estimation (Kramer-Schadt et al., 2013); this was not possible to do via occurrence point thinning due to the small number of occurrence points for the thinly distributed species (Boria et al., 2014). We also used the “ENMeval” package to evaluate the optimal Maxent model features and regularization multiplier parameters (model selection) to use for each species (Table II), using 10,000, generated, geographic, background points and a tenfold cross-validation technique. We selected the model features and parameters to use based on the Akaike Information Criterion (AIC).
For all Maxent models, we set the maximum number of iterations to 500, the convergence threshold to 0.001, and the number of background points to 10,000 (as in Nazeri et al., 2012), and we fine-tuned the species-specific models with different features and multiplier parameters (Table II). We used a fourfold cross-validation procedure to run our models (as in Blair et al., 2013; Kamilar et al., 2016; Pearson et al., 2007, 2011). We used the area under response curve (AUC) method to evaluate the performance of our ENMs (Merow et al., 2013). In summary, higher AUC values equate to a better-performing model, where an AUC value equal to 1 indicates perfect model performance and an AUC value < 0.5 indicates that the model does not have the ability to perform reliably (Phillips, 2006). However, we also used binomial tests of omission to validate the performance of the ENMs, because AUC does not penalize for overfitting (Pearson et al., 2007).
Niche Overlap
We calculated the pair-wise niche overlap between dwarf lemur species in environmental space based on our ENMs by using Schoener’s D (Warren et al., 2008) and Hellinger’s I (Schoener, 1968) indices in the “ENMtools” package (Warren & Dinnage, 2021). This method calculates the difference in standardized suitability score for each cell used in the ENMs between species and assigns a score between 0 (no niche overlap) and 1 (complete niche overlap/identical niches); pair-wise scores > 0.8 often indicate significant niche overlap between species (Warren et al., 2008). We then conducted background tests of whether the pair-species niche overlaps were equivalent to each other. Background tests are suitable to compare niche overlap among allopatric and sister species (Warren et al., 2010), and these tests pool all occurrence data for each species-pair and then randomly assigns localities to two new samples of equal size to the two original species samples. We specified for this procedure to produce 99 additional replicates of random species-pairs with values of niche overlap. We then compared our original species-pair niche overlap values to the null distribution of the pseudo-replicate niche overlap values. If our original pair-species overlap values were within the bottom 5% of the null distribution, then we determined that the ENMs of the two species were not equivalent and significantly more diverged than expected considering the habitat available to each species. This is comparable to a one-sided test with an alpha level of 0.05.
Suitable Habitat
Maxent ENMs contain a habitat suitability output ranging from 0 (not suitable habitat) to 1 (completely suitable habitat). To interpret and visualize the remaining suitable niche areas of each species, we set a binary threshold for each model to distinguish presence from absence (Liu et al., 2005; Pearson et al., 2007). Assigning arbitrary thresholds for each species would lack any ecological basis (Liu et al., 2005), so we calculated minimum training presence thresholds (MTP) based on our ENMs, The MTP threshold integrates the prevalence of model-building data; it is conservative and robust regarding a species’ likely distribution (i.e., it avoids overfitting), and it has the lowest false-positive and false-negative error rates of all MaxEnt-generated thresholds (Liu et al., 2005). We used these binary ENMs to calculate the total area suitable for each species in ArcMap.
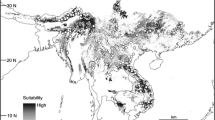
The habitat suitability of the ENMs were predicted by using a range of climatic and vegetation cover-related environmental variables. ENMs more-heavily influenced by nonvegetation cover-related variables (e.g., precipitation, temperature) can often result in the inclusion of areas outside of their natural habitat types (Hartel et al., 2010). Because all lemurs depend on forest habitat for their survival (Schwitzer et al., 2013), we downloaded the most recent forest cover raster data for Madagascar (resolution 30 m2, Vieilledent et al., 2018; Fig. 2A), and we clipped the binary ENMs to exclude areas outside of forest cover. We then measured the total area of the clipped ENMs to calculate the realized niche area for each Cheirogaleus species.
Remaining forest cover (A); distribution of protected areas (B); Human Footprint (HFP) map (C)) of anthropogenic activity throughout Madagascar. Cooler, blue colours indicate higher levels of anthropogenic activity in C. Figures were created using raster layers of forest cover (Vieilledent et al., 2018), protected areas (UNEP-WCMC, 2020), and HFP (Venter et al., 2016), with a scale of 1:6,500,000 for Madagascar.
Conservation and Anthropogenic Risk Assessment
We downloaded a raster layer of Madagascar’s protected area network (UNEP-WCMC, 2020) to assess whether the realized niche areas of each dwarf lemur species are protected. This layer is composed of all protected areas in Madagascar, including 29 National Parks, 24 Special and Strict Nature Reserves, and 26 Classified Protected Areas (Fig. 2B). We overlaid this protected area layer with the realized niche of each species in ArcGIS, and we calculated the total area that was within Madagascar’s protected area network.
We also assessed the risk of anthropogenic threats on each realized niche area by using the Human Footprint (HFP) raster (Venter et al., 2016), a commonly used proxy of anthropogenic disturbance (Campera et al., 2020; Di Marco et al., 2018). The HFP layer accounts for areas of anthropogenic activity, such as roads, settlements, agricultural areas, and buildings, and maps them based on cumulative intensity on a scale of 0 (no anthropogenic activity) to 50 (intense anthropogenic activity) at a resolution of 1 km2 (Fig. 2C). In ArcGIS, we filtered out all features within the HFP layer with a < 10 score. We then converted the continuous raster to a binary layer containing all features with a score of 10–50; this new layer still contained all roads, settlements, agricultural areas, and pastures. To assess the risk of anthropogenic threats to Cheirogaleus within their realized niches, we applied 5-km and 10-km buffers to all HFP features. We then calculated the area of each species’ realized niche that fell within these buffers to determine the areas at high risk (< 5 km), medium risk (5–10 km), and low risk (> 10 km) from anthropogenic disturbance features. Cheirogaleus spp. are hunted throughout Madagascar (Herrera et al., 2018), and therefore we selected these distances to classify anthropogenic risk based on the distance that people travel from settlements and roads to hunt and gather wood and other resources from the forest (Peres & Lake, 2002; Thorn et al., 2009).
Finally, we extracted the HFP and habitat suitability scores from 100 random 1-km2 pixels of the unfiltered HFP and ENM raster layers clipped to the realized niche area of each species. We chose 100 random pixels rather than using every pixel, because the sample size would have varied significantly among species. We then performed linear regression analyses on the HFP and habitat suitability scores to investigate whether anthropogenic disturbance affects dwarf lemur habitat suitability. Shapiro–Wilk tests confirmed that the HFP and habitat score datasets met the assumptions of normal distribution, homoscedasticity, and linearity for linear regression. We then bootstrapped each analysis to 10,000 replicates with bias-corrected and accelerated confidence intervals (BCAs) in the R package “boot” (Canty & Ripley, 2020). An alpha level of 0.05 was used for all analyses described in this article.
Results
ENM Performance and Niche Overlap
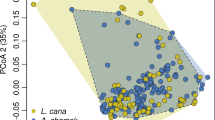
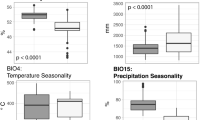
Our Maxent ENMs (Appendix 3) performed well for the three Cheirogaleus species, with mean AUCs for each species ranging from 0.801 to 0.917 (Table II). All three ENMs also had statistically significant results for all model fold omission tests (Table II). The ENM percentage contributions of the nine environmental variables varied greatly between species (Table III). The species-pair Schoener’s D overlap indices ranged from 0.127 (between C. crossleyi and C. medius) to 0.653 (between C. crossleyi and C. major), and Hellinger’s I overlap indices ranged from 0.323 (between C. crossleyi and C. medius) to 0.798 (between C. crossleyi and C. major). These results indicate significant environmental niche separation and among pair-species (all P < 0.01; Table IV).
Cheirogaleus Realized Niches
With the MTP threshold applied, the fundamental niche area of the three analysed Cheirogaleus species ranged from 92,127 km2 for C. crossleyi to 336,494 km2 for C. medius (Table V). The realized niches (forest area) of the three species were lower in comparison, ranging from 28,889 km2 for C. crossleyi to 41,934 km2 for C. medius (Fig. 3; Table V).
Conservation and Anthropogenic Threats
Of the realized niche areas of the analysed dwarf lemurs, the percentage that was within Madagascar’s protected area network varied between species, ranging from 47.8% (20,065 km2) for C. medius to 66.0% (25,266 km2) for C. major (Table V; Fig. 4). The percentage of the realized niche area that was less than 5 km from an HFP feature, and therefore at high risk of anthropogenic disturbance, also varied between species, ranging from 19.9% (5,744 km2) for C. crossleyi to 40.5% (16,999 km2) for C. medius (Table V). This also was the case for the percentage of the realized niche area in a medium-risk area (35.2% (10,157 km2) for C. crossleyi to 36.6% (15,334 km2) for C. medius) and percentage of the realized niche in a low-risk area (22.9% (9,601 km2) for C. medius to 44.9% (12,988 km2) for C. crossleyi) (Fig. 4).
The habitat suitability scores for two analysed species (C. major and C. medius) had no significant relationship with anthropogenic disturbance, as indicated by confidence intervals overlapping with zero (Table VI). C. crossleyi had a significantly negative habitat score relationship with HFP (Table VI).
Discussion
Ecological Niche Models
Our ENMs define the ecological niches of three dwarf lemur species. The AUC values and significance levels of the binomial tests of omission suggest that the ENMs of these species are useful for predicting their distributions (Table II). The performance of these ENMs mirror the success of other studies that have used climatic and vegetation cover-related variables to model the niches of cryptic species (Hending, 2021b; Kamilar et al., 2016; Rissler & Apodaca, 2007; Sattler et al., 2007; Schüßler et al., 2021, 2023; Thorn et al., 2009).
Cheirogaleus Niche Determinants and Species Niche Overlap
Our results suggest that the geographic distributions of some dwarf lemur species are more-strongly influenced by climatic variables. For example, the distribution of C. crossleyi appears most-strongly related to temperature, indicating that this species may be more sensitive to high temperatures than other Cheirogaleus or that the upper thermal limits of its preferred feeding/sleeping trees may be low (Sentinella et al., 2020). Precipitation appears the primary driver of C. major distribution; this species is a lowland rainforest specialist (Blanco et al., 2020d) and is thus sensitive to drought and unable to survive in dry habitats. The occurrence of C. medius appears to be influenced by vegetation presence and productivity (Table III). This indicates that this species may be sensitive to habitat degradation (but see Hending et al., in press). Dwarf lemurs spend large periods of the austral winter in seasonal hibernation, a trait evolved to enable their survival during periods of water crises and food shortages associated with the dry season (Dausmann & Blanco, 2016). Heterothermy might therefore buffer dwarf lemurs from spatial variations in temperature and rainfall and might allow some species to persist in a wider range of seasonal environments and be more widespread. Whereas the influence of climate on the ENMs can be explained by dwarf lemur heterothermy, the influence of NDVI and LAI is primarily explained by habitat requirements, because, such as many other primates, dwarf lemurs require forest habitat for their survival (Schwitzer et al., 2013). Species with higher NDVI and LAI ENM percentage contributions are more sensitive to vegetation density and have niche preferences of dense-vegetation forest (eastern rainforest: C. major), rather than more open-vegetation forest (western/southern dry forests: C. medius).
Significant niche divergence among species pairs, as per our original prediction (Table IV), suggests that each dwarf lemur occupies a distinct ecological niche (Chetan et al., 2014). The taxonomy of the dwarf lemurs has undergone significant upheaval in recent years (Lei et al., 2014, 2015), and while visual discrimination among members of this cryptic primate group may be difficult (Groeneveld et al., 2009), our niche-overlap results support the existing genetic and morphological data that classifies these species as distinct (Frasier et al., 2016; Herrera et al., 2016; McLain et al., 2017). Dwarf lemur sympatry does however occur in some areas (Blanco et al., 2009; Lahann, 2007). Local-scale resource partitioning needs to be further studied so that we understand how dwarf lemur coexistence can occur.
Realized Niches
Unfortunately, many areas of the fundamental niches identified in species ENMs often do not contain the habitat types that the organism would require to survive (realized niche) due to habitat loss and disturbance (Escobar et al., 2015). This is the case for our study, as is demonstrated when the suitable habitat areas are clipped to account for the availability of forest (Vieilledent et al., 2018). Although dwarf lemur populations are likely to exist outside of natural forest (Hending et al., 2018; Webber et al., 2020), the conservative habitat area estimates of the forest-clipped maps (realized niches) are the most appropriate means to gauge their current status. This is because some dwarf lemur species now only occur over small areas, particularly C. shethi, C. lavasoensis and C. thomasi (Fig. 1), although we did not construct ENMs for these taxa due to low occurrence point sample sizes. Coupled with the high rate of deforestation in Madagascar (Vieilledent et al., 2018), this information highlights the necessity for urgent conservation of the remaining dwarf lemur populations and the habitats within their ranges.
Protected Areas and Anthropogenic Disturbance
The results of our study show that 58.9% of the realized niche areas of the analysed species were within Madagascar’s protected area network (Fig. 4A). Such results provide information on which species are well-protected (or underprotected) within their geographic range, and they are therefore important to assign conservation priorities so that key habitat areas are preserved (Sharma et al., 2018; Thorn et al., 2009). Our results also suggest that dwarf lemurs, and their forest habitats, are well-protected within their ranges. As deforestation, habitat fragmentation, and climate change continue to threaten Madagascar’s forests (Hending et al., 2022; Vieilledent et al., 2018), this is encouraging for their survival. However, the percentage of the realized niche area that is protected varies between species, and more than 50% of the realized niche of C. medius is under no protection at all. Due to its wide distribution, this is unlikely to be detrimental for C. medius conservation. However, species with smaller distributions (e.g., C. lavasoensis and C. thomasi) would be much more threatened and likely to depend on protected areas for survival. Species-specific conservation measures therefore need to be implemented to protect their remaining populations, including ecological research and conservation action plans. For example, less than half (47.8%) of C. medius’ realized niche area is protected, and so conservation efforts for this species could focus on identifying additional areas for consideration for protection. Furthermore, improved enforcement within protected areas and community conservation initiatives to counteract local community reliance on cutting down forest are needed. If such actions are successfully implemented, extensions of Madagascar’s protected area network may be a viable strategy.
Although some of the results are encouraging for dwarf lemur conservation, this study also highlights that 63.9% of realized niche areas are within 10 km of an anthropogenic feature. Considering that people venture several kilometres from roads and villages to hunt and harvest from neighbouring forests (Peres & Lake, 2002), this of great conservation concern and it suggests that dwarf lemurs may be at great risk. The effect of anthropogenic disturbance on habitat suitability is not a significant factor for some species (Table VI) and may in fact be a positive influence (Hending, 2021a), which is reflected by observations of dwarf lemurs within anthropogenic habitats (Hending et al., 2018; Webber et al., 2020). However, habitat suitability of C. crossleyi appears to be negatively impacted by human activity. These results suggest that this species may be more-specialized than others that can tolerate and adapt to anthropogenic disturbance (Devictor et al., 2008). It has been hypothesised that disturbance gradients have greater effect on ecologically specialized, narrow-niched species than broader-niched generalists (Botts et al., 2013; Devictor et al., 2008). Our results demonstrate that the dwarf lemurs reflect this to some degree.
Study Limitations
While this investigation has provided an overview of dwarf lemur conservation biogeography, there are some limitations that originate from the occurrence point dataset and the environmental layers. Foremostly, the number of occurrence points of six species (C. andysabini, C. grovesi, C. lavasoensis, C. shethi, C. sibreei, and C. thomasi) were too low to construct reliable ENMs. Increased sampling efforts of the occurrence of more widespread species may alleviate this issue, which would allow the construction of reliable ENMs for these species. This would be highly useful to inform their conservation. However, this would not work for species with small, restricted geographic ranges, such as C. thomasi. Also, the resolution of our environmental layers was limited to 1 km2, which was the finest resolution available (resolution of all input-layers must be identical). Higher-resolution layers often result in improved Maxent model performance (Ross et al., 2015), but until such layers become available, this aspect cannot be improved. Higher-resolution layers would enable studies such as this to find out more information about species with restricted ranges (i.e., more pixels would be available). Finally, the percentage values of dwarf lemur realized niches that occur within protected areas may be influenced by the preference of researchers to work within protected areas. However, we were able to account for this sampling bias by including bias raster layers in our Maxent models.
Implications and Conclusion
The analyses of our ENMs provide evidence to suggest that dwarf lemur distribution is affected by both climatic and vegetation cover-related factors. Our results support the theory of divergent ecological niches among closely related species (Kamilar et al., 2016; Rissler & Apodaca, 2007). The dwarf lemurs, and indeed many other Cheirogaleid lemurs, often are described as generalist, adaptable species in the literature (Kamilar & Muldoon, 2010; Schäffler & Kappeler, 2014). Although our results provide evidence of this for C. medius, our ENMs suggest that others, such as C. crossleyi, are much more specialized and as a consequence are at much greater risk from anthropogenic disturbance. These findings underpin the importance of ecological niches for the informed understanding of the taxonomy and biogeography of cryptic organism groups, such as nocturnal strepsirrhine primates, and highlight their importance for the assessment of threats and assignment of conservation priorities.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on request.
References
Barve, N., Barve, V., Jiménez-Valverde, A., Lira-Noriega, A., Maher, S. P., Peterson, A. T., Soberón, J., & Villalobos, F. (2011). The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecological Modelling, 222(11), 1810–1819.
Bivand, R., Keitt, T., & Rowlingson, B. (2019). rgdal: Bindings for the 'Geospatial' Data Abstraction Library. R package version 1.4–8. https://CRAN.R-project.org/package=rgdal. [Accessed 10 September 2020].
Bivand, R. S., Pebesma, E., & Gomez-Rubio, V. (2013). Applied Spatial Data Analysis with R (2nd ed.). Springer.
Black, S. A. (2020). Assessing presence, decline, and extinction for the conservation of difficult-to-observe species. In F. M. Angeleici & L. Rossi (Eds.), Problematic Wildlife II (pp. 359–392). Springer.
Blair, M. E., Sterling, E. J., Dusch, M., Raxworthy, C. J., & Pearson, R. (2013). Ecological divergence and speciation between lemur (Eulemur) sister species in Madagascar. Journal of Evolutionary Biology, 26(8), 1790–1801.
Blanco, M. B., Godfrey, L. R., Rakotondratsima, M., Rahalinarivo, V., Samonds, K. E., Raharison, J. L., & Irwin, M. T. (2009). Discovery of sympatric dwarf lemur species in the high-altitude rain forest of Tsinjoarivo, Eastern Madagascar: Implications for biogeography and conservation. Folia Primatologica, 80(1), 1–17.
Blanco, M., Dolch, R., Donati, G., Ganzhorn, J., Greene, L. K., et al. (2020a). Cheirogaleus grovesi. The IUCN red list of threatened species. e.T163021927A163021999. Accessed 15 June 2020.
Blanco, M., Dolch, R., Donati, G., Ganzhorn, J., Greene, L. K., et al. (2020b). Cheirogaleus lavasoensis. The IUCN red list of threatened species. e.T163022131A163022293. Accessed 15 June 2020.
Blanco, M., Dolch, R., Ganzhorn, J., Greene, L. K., Le Pors, B., et al. (2020c). Cheirogaleus medius. The IUCN red list of threatened species 2020e. e.T163023599A115588562. Accessed 15 June 2020.
Blanco, M., Borgerson, C., Dolch, R., Donati, G., Ganzhorn, J., et al. (2020d). Cheirogaleus major. The IUCN red list of threatened species. e.T54778911A115588708. Accessed 15 June 2020.
Blanco, M., Dolch, R., Ganzhorn, J., Greene, L. K., Le Pors, B., et al. (2020e). Cheirogaleus sibreei. The IUCN red list of threatened species 2020f: e.T41576A115579719. Accessed 15 June 2020.
Blanco, M., Borgerson, C., Dolch, R., Donati, G., Ganzhorn, J., et al. (2020f). Cheirogaleus crossleyi. The IUCN red list of threatened species 2020a: e.T163021377A115581154. Accessed 15 June 2020.
Booth, T. H., Nix, H. A., Busby, J. R., & Hutchinson, M. F. (2014). BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MAXENT studies. Diversity and Distributions, 20(1), 1–9.
Boria, R. A., Olson, L. E., Goodman, S. M., & Anderson, R. P. (2014). Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecological Modelling, 275, 73–77.
Bosso, L., Russo, D., Di Febbraro, M., Cristinzio, G., & Zoina, A. (2016). Potential distribution of Xylella fastidiosa in Italy: A maximum entropy model. Phytopathologia Mediterranea, 55(1), 62–72.
Botts, E. A., Erasmus, B. F., & Alexander, G. J. (2013). Small range size and narrow niche breadth predict range contractions in South African frogs. Global Ecology and Biogeography, 22(5), 567–576.
Campera, M., Santini, L., Balestri, M., Nekaris, K. A. I., & Donati, G. (2020). Elevation gradients of lemur abundance emphasise the importance of Madagascar’s lowland rainforest for the conservation of endemic taxa. Mammal Review, 50(1), 25–37.
Canty, A., & Ripley, B. D. (2020). boot: Bootstrap R (S-Plus) Functions. R package version 1.3–25. [Accessed 1 November 2020].
Cao, Y., DeWalt, R. E., Robinson, J. L., Tweddale, T., Hinz, L., & Pessino, M. (2013). Using Maxent to model the historic distributions of stonefly species in Illinois streams: The effects of regularization and threshold selections. Ecological Modelling, 259, 30–39.
Chetan, N., Praveen, K. K., & Vasudeva, G. K. (2014). Delineating ecological boundaries of Hanuman langur species complex in peninsular India using MaxEnt modeling approach. PLoS ONE, 9(2), e87804.
Cobos, M. E., Peterson, A. T., Osorio-Olvera, L., & Jiménez-García, D. (2019). An exhaustive analysis of heuristic methods for variable selection in ecological niche modeling and species distribution modeling. Ecological Informatics, 53, 100983.
Coudrat, C. N., & Nekaris, K. (2013). Modelling niche differentiation of co-existing, elusive and morphologically similar species: A case study of four macaque species in Nakai-Nam Theun National Protected Area, Laos. Animals, 3(1), 45–62.
Craul, M., Zimmermann, E., Rasoloharijaona, S., Randrianambinina, B., & Radespiel, U. (2007). Unexpected species diversity of Malagasy primates (Lepilemur spp.) in the same biogeographical zone: a morphological and molecular approach with the description of two new species. BMC Evolutionary Biology, 7, 83.
Dausmann, K. H., & Blanco, M. B. (2016). Possible causes and consequences of different hibernation patterns in Cheirogaleus species: Mitovy fatsy sahala. In S. Lehman, U. Radespiel, & E. Zimmermann (Eds.), Dwarf and Mouse Lemurs of Madagascar: Biology, Behavior and Conservation Biogeography of the Cheirogaleidae (pp. 335–349). University Press.
Devictor, V., Julliard, R., & Jiguet, F. (2008). Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos, 117(4), 507–514.
Di Marco, M., Venter, O., Possingham, H. P., & Watson, J. E. (2018). Changes in human footprint drive changes in species extinction risk. Nature Communications, 9(1), 1–9.
Elith, J. H., Graham, C. P., Anderson, R., Dudík, M., Ferrier, S., Guisan, A. J., Hijmans, R., Huettmann, F. R., Leathwick, J., Lehmann, A., Li, J., Lohmann, L. G., Loiselle, B. A., Manion, G., Moritz, C., Nakamura, M., Nakazawa, Y., Overton, J. M. C. M., Peterson, A. T., … Zimmermann, N. K. (2006). Novel methods improve prediction of species distributions from occurrence data. Ecography, 29(2), 129–151.
Escobar, L. E., Awan, M. N., & Qiao, H. (2015). Anthropogenic disturbance and habitat loss for the red-listed Asiatic black bear (Ursus thibetanus): Using ecological niche modelling and nighttime light satellite imagery. Biological Conservation, 191, 400–407.
Ferrer-Sánchez, Y., & Rodríguez-Estrella, R. (2016). How rare species conservation management can be strengthened with the use of ecological niche modelling: The case for endangered endemic Gundlach’s Hawk and Cuban Black-Hawk. Global Ecology and Conservation, 5, 88–99.
Frasier, C. L., Lei, R., McLain, A. T., Taylor, J. M., Bailey, C. A., Ginter, A. L., Nash, S. D., Randriamampionona, R., Groves, C. P., Mittermeier, R. A., & Louis, E. E. (2016). A new species of dwarf lemur (Cheirogaleidae: Cheirogaleus medius group) from the Ankarana and Andrafiamena-Andavakoera Massifs, Madagascar. Primate Conservation, 30, 59–72.
Ganzhorn, J., Donati, G., Eppley, T. M., Lahann, P., Rakotondranary, S. J., et al. (2020). Cheirogaleus thomasi. The IUCN red list of threatened species. e.T163022885A163312222. Accessed 15 June 2020.
Gardner, C. J., & Jasper, L. D. (2015). Discovery of an island population of dwarf lemurs (Cheirogaleidae: Cheirogaleus) on Nosy Hara, far northern Madagascar. Primates, 56(4), 307–310.
Gibson, L., Barrett, B., & Burbidge, A. (2007). Dealing with uncertain absences in habitat modelling: A case study of a rare ground-dwelling parrot. Diversity and Distributions, 13, 704–713.
Goodman, S. M., Raherilalao, M. J., & Wohlhauser, S. (2018). The Terrestrial Protected Areas of Madagascar: Their History, Description, and Biota. University Press.
Groeneveld, L. F., Blanco, M. B., Raharison, J. L., Rahalinarivo, V., Rasoloarison, R. M., Kappeler, P. M., Godfrey, L. R., & Irwin, M. T. (2010). MtDNA and nDNA corroborate existence of sympatric dwarf lemur species at Tsinjoarivo, eastern Madagascar. Molecular Phylogenetics and Evolution, 55(3), 833–845.
Groeneveld, L. F., Rasoloarison, R. M., & Kappeler, P. M. (2011). Morphometrics confirm taxonomic deflation in dwarf lemurs (Primates: Cheirogaleidae), as suggested by genetics. Zoological Journal of the Linnean Society, 161(1), 229–244.
Groeneveld, L. F., Weisrock, D. W., Rasoloarison, R. M., Yoder, A. D., & Kappeler, P. M. (2009). Species delimitation in lemurs: Multiple genetic loci reveal low levels of species diversity in the genus Cheirogaleus. BMC Evolutionary Biology, 9(1), 30.
Groves, C. P. (2001). Primate Taxonomy. Smithsonian Institution Press.
Guisan, A., & Zimmermann, N. E. (2000). Predictive habitat distribution models in ecology. Ecological Modelling, 135, 147–186.
Hapke, A., Fietz, J., Nash, S. D., Rakotondravony, D., Rakotosamimanana, B., Ramanamanjato, J. B., Randria, G. F., & Zischler, H. (2005). Biogeography of dwarf lemurs: Genetic evidence for unexpected patterns in southeastern Madagascar. International Journal of Primatology, 26(4), 873–901.
Harper, G. J., Steininger, M. K., Tucker, C. J., Juhn, D., & Hawkins, F. (2007). Fifty years of deforestation and forest fragmentation in Madagascar. Environmental Conservation, 34(4), 325–333.
Hartel, T., Nemes, S., Oellerer, K., Cogalniceanu, D., Moga, C., & Arntzen, J. W. (2010). Using connectivity metrics and niche modelling to explore the occurrence of the northern crested newt Triturus cristatus (Amphibia, Caudata) in a traditionally managed landscape. Environmental Conservation, 37(2), 195–200.
Hending, D. (2021a). Environmental drivers of Cheirogaleidae population density: Remarkable resilience of Madagascar’s smallest lemurs to habitat degradation. Ecology and Evolution, 11, 5874–5891.
Hending, D. (2021b). Niche-separation and conservation biogeography of Madagascar’s fork-marked lemurs (Cheirogaleidae: Phaner): Evidence of a new cryptic species? Global Ecology and Conservation, 29, e01738.
Hending, D., Andrianiaina, A., Rakotomalala, Z., & Cotton, S. (2017). Range extension and behavioural observations of the recently described Sheth’s dwarf lemur (Cheirogaleus shethi). Folia Primatologica, 88(5), 401–408.
Hending, D., Andrianiaina, A., Rakotomalala, Z., & Cotton, S. (2018). The use of vanilla plantations by lemurs: Encouraging findings for both lemur conservation and sustainable agroforestry in the Sava region, northeast Madagascar. International Journal of Primatology, 39(1), 141–153.
Hending, D., Holderied, M., McCabe, G., & Cotton, S. (2022). Effects of future climate change on the forests of Madagascar. Ecosphere, 13(4), e4017.
Hending, D., Randrianarison, H., Andriamavosoloarisoa, NNM., Ranohatra-Hending, C., Solofondranohatra, JS., Tongasoa, HR., Ranarison, HT., Gehrke, V., Andrianirina, N., Holderied, M., McCabe, G. and Cotton, S. (2023) Seasonal differences in the encounter rate of the fat-tailed dwarf lemur (Cheirogaleus medius) in the transitional forests of North West Madagascar: implications for reliable population density assessment. International Journal of Primatology.
Hending, D., Sgarlata, G. M., LePors, B., Rasolondraibe, E., Jan, F., Rakotonanahary, A. N., Ralantoharijaona, T. N., Debulois, S., Andrianiaina, A., Cotton, S., Rasoloharijaona, S., Zaonarivelo, J. R., Andriaholinirina, N. V., Chikhi, L., & Salmona, J. (2020). Distribution and conservation status of the Endangered Montagne d’Ambre fork-marked lemur (Phaner electromontis). Journal of Mammalogy, 101(4), 1049–1060.
Herrera, J. P., Borgerson, C., Tongasoa, L., Andriamahazoarivosoa, P., Rasolofoniaina, B. R., Rakotondrafarasata, E. R., Randrianasolo, J. R. R., Johnson, S. E., Wright, P. C., & Golden, C. D. (2018). Estimating the population size of lemurs based on their mutualistic food trees. Journal of Biogeography, 45(11), 2546–2563.
Herrera, J. P., Lydia, T., & Wright, P. C. (2016). Contact zones and species sympatry in dwarf lemurs (genus Cheirogaleus): The roles of ecological adaptation and sexual selection. In S. Lehman, U. Radespiel, & E. Zimmermann (Eds.), The Dwarf and Mouse Lemurs of Madagascar: Biology, Behaviour and Conservation Biogeography of the Cheirogaleidae (pp. 113–132). University Press.
Hijmans, R. J. (2017). Raster: Geographic Data Analysis and Modeling. R package version 2.6–7. https://CRAN.R-project.org/package=raster [Accessed 10 September 2020].
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology., 25, 1965–1978.
IUCN (2020a). IUCN Red List of Threatened Species. Available from https://www.iucnredlist.org/ [Accessed 10 July 2020b].
IUCN (2020b). Cheirogaleus andysabini, Cheirogaleus crossleyi, Cheirogaleus grovesi, Cheirogaleus lavasoensis, Cheirogaleus major, Cheirogaleus medius, Cheirogaleus shethi, Cheirogaleus sibreei, Cheirogaleus thomasi. The IUCN Red List of Threatened Species. Version 2020a-1. http://www.iucnredlist.org. [Downloaded 28 September 2020a].
Kamilar, J. M., Blanco, M. B., & Muldoon, K. M. (2016). Ecological niche modelling of mouse lemurs (Microcebus spp.) and its implications for their species diversity and biogeography. In Lehman, SM., Radespiel, U., & Zimmermann, E. (Eds. The Dwarf and Mouse Lemurs of Madagascar: Biology, Behavior, and Conservation Biogeography of the Cheirogaleidae. pp. 451–463. University Press.
Kamilar, J. M., & Muldoon, K. M. (2010). The climatic niche diversity of Malagasy primates: A phylogenetic perspective. PLoS ONE, 5(6), e11073.
Kramer-Schadt, S., Niedballa, J., Pilgrim, J. D., Schröder, B., Lindenborn, J., Reinfelder, V., Stillfried, M., Heckmann, I., Scharf, A. K., Augeri, D. M., Cheyne, S. M., Hearn, A. J., Ross, J., Macdonald, D. W., Mathai, J., Eaton, J., Marshall, A. J., Semiadi, G., Rustam, R., … Wilting, A. (2013). The importance of correcting for sampling bias in MaxEnt species distribution models. Diversity and Distributions, 19(11), 1366–1379.
Lahann, P. (2007). Feeding ecology and seed dispersal of sympatric cheirogaleid lemurs (Microcebus murinus, Cheirogaleus medius, Cheirogaleus major) in the littoral rainforest of south-east Madagascar. Journal of Zoology, 271(1), 88–98.
Lehman, S., Radepsiel, U., & Zimmermann, E. (2016). Dwarf and Mouse Lemurs of Madagascar: Biology. University Press.
Lei, R., Frasier, C. L., McLain, A. T., Taylor, J. M., Bailey, C. A., Engberg, S. E., Ginter, A. L., Randriamampionona, R., Groves, C. P., Mittermeier, R. A., & Louis, E. E. (2014). Revision of Madagascar’s dwarf lemurs (Cheirogaleidae: Cheirogaleus): Designation of species, candidate species status and geographic boundaries based on molecular and morphological data. Primate Conservation, 28, 9–35.
Lei, R., McLain, A. T., Frasier, C. L., Taylor, J. M., Bailey, C. A., Engberg, S. E., Ginter, A. L., Nash, S. D., Randriamampionona, R., Groves, C. P., Mittermeier, R. A., & Louis, E. E. (2015). A new species in the genus Cheirogaleus (Cheirogaleidae). Primate Conservation, 29, 43–54.
Liu, C., Berry, P. M., Dawson, T. P., & Pearson, R. G. (2005). Selecting thresholds of occurrence in the prediction of species distributions. Ecography, 28(3), 385–393.
McLain, A. T., Lei, R., Frasier, C. L., Taylor, J. M., Bailey, C. A., Robertson, B. A. D., Nash, S. D., Randriamanana, J. C., Mittermeier, R. A., & Louis, E. E. (2017). A new Cheirogaleus (Cheirogaleidae: Cheirogaleus crossleyi group) species from southeastern Madagascar. Primate Conservation, 31, 27–36.
Merow, C., Smith, M. J., & Silander, J. A. (2013). A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography, 36(10), 1058–1069.
Mittermeier, R. A., Ganzhorn, J. U., Konstant, W. R., Glander, K., Tattersall, I., Groves, C. P., Rylands, A. B., Hapke, A., Ratsimbazafy, J., Mayor, M. I., Louis, E. E., Rumpler, Y., Schwitzer, C., & Rasoloarison, R. (2008). Lemur diversity in Madagascar. International Journal of Primatology, 29(6), 1607–1656.
Morais, A. R., Siqueira, M. N., Lemes, P., Maciel, N. M., De Marco, P., & Brito, D. (2013). Unravelling the conservation status of Data Deficient species. Biological Conservation, 166, 98–102.
Muscarella, R., Galante, P. J., Soley-Guardia, M., Boria, R. A., Kass, J. M., Uriarte, M., & Anderson, R. P. (2014). ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods in Ecology and Evolution, 5(11), 1198–1205.
Nazeri, M., Jusoff, K., Madani, N., Mahmud, A. R., Bahman, A. R., & Kumar, L. (2012). Predictive modelling and mapping of Malayan Sun Bear (Helarctos malayanus) distribution using maximum entropy. PLoS ONE, 7, e8104.
Olivieri, G., Zimmermann, E., Randrianambinina, B., Rasoloharijaona, S., Rakotondravony, D., Guschanski, K., & Radespiel, U. (2007). The ever-increasing diversity in mouse lemurs: Three new species in north and northwestern Madagascar. Molecular Phylogenetics and Evolution, 43, 309–327.
Padial, J. M., & De La Riva, I. (2010). A response to recent proposals for integrative taxonomy. Biological Journal of the Linnean Society., 101(3), 747–756.
Pearson, R. G., Raxworthy, C. J., Nakamura, M., & Peterson, A. T. (2007). Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. Journal of Biogeography, 34(1), 102–117.
Peres, C. A., & Lake, I. R. (2002). Extent of nontimber resource extraction in tropical forests: Accessibility to game vertebrates by hunters in the Amazon Basin. Conservation Biology, 17, 521–535.
Peterson, A. T., & Soberón, J. (2012). Species distribution modeling and ecological niche modeling: Getting the concepts right. Natureza & Conservação, 10(2), 102–107.
Peterson, A. T., Soberón, J., Pearson, R. G., Anderson, R. P., Martínez-Meyer, E., Nakamura, M., & Araújo, M. B. (2011). Ecological Niches and Geographic Distributions. University Press.
Phillips, S. J. (2006). A Brief Tutorial on Maxent. AT&T Research, Florham Park, New Jersey [Accessed 22 September 2020].
Phillips, S. J., Dudík, M., & Schapire, R. E. (2006). Maxent software for modelling species niches and distributions (Version 3.4.1). Available from url: biodiversityinformatics.amnh.org/open_source/maxent/ [Accessed 22nd September 2020].
R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
Raxworthy, C. J., Ingram, C. M., Rabibisoa, N., & Pearson, R. G. (2007). Applications of ecological niche modelling for species delimitation: A review and empirical evaluation using day geckos (Phelsuma) from Madagascar. Systematic Biology, 56(6), 907–923.
Riley, R. D., Snell, K. I., Ensor, J., Burke, D. L., Harrell, F. E., Moons, K. G., & Collins, G. S. (2019). Minimum sample size for developing a multivariable prediction model: PART II-binary and time-to-event outcomes. Statistics in Medicine, 38(7), 1276–1296.
Rissler, L. J., & Apodaca, J. J. (2007). Adding more ecology into species delimitation: Ecological niche models and phylogeography help define cryptic species in the black salamander (Aneides flavipunctatus). Systematic Biology, 56(6), 924–942.
Ross, L. K., Ross, R. E., Stewart, H. A., & Howell, K. L. (2015). The influence of data resolution on predicted distribution and estimates of extent of current protection of three ‘listed’ deep-sea habitats. PLoS ONE, 10(10), e0140061.
Sattler, T., Bontadina, F., Hirzel, A. H., & Arlettaz, R. (2007). Ecological niche modelling of two cryptic bat species calls for a reassessment of their conservation status. Journal of Applied Ecology, 44(6), 1188–1199.
Schäffler, L., & Kappeler, P. M. (2014). Distribution and abundance of three cheirogaleid species in Menabe Central, Western Madagascar. Lemur News, 18, 38–43.
Schoener, T. W. (1968). Sizes of feeding territories among birds. Ecology, 49(1), 123–141.
Schüßler, D., Rabemananjara, N. R., Radriarimanga, T., Rafamantanantsoa, S. M., Randimbiharinirina, R. D., Radespiel, U. & Hending, D. (2023). Habitat and ecological niche characteristics of the elusive Hairy‐eared Dwarf Lemur (Allocebus trichotis) with updated occurrence and geographic range data. American Journal of Primatology. e23473.
Schüßler, D., Rabemananjara, N. R., & Ratsimbazafy, J. (2021). The potential distribution of the giant mouse lemurs (Mirza coquereli, Mirza zaza) with implications for their conservation. Lemur News, 23, 44–48.
Schwitzer, C., Mittermeier, R. A., Davies, N., Johnson, S., Ratsimbazafy, J., Razafindramanana, J., Louis, E. E., & Rajaobelina, S. (2013). Lemurs of Madagascar: A strategy for their conservation 2013–2016. IUCN SSC Primate Specialist Group, Bristol Conservation and Science Foundation, and Conservation International.
Sentinella, A. T., Warton, D. I., Sherwin, W. B., Offord, C. A., & Moles, A. T. (2020). Tropical plants do not have narrower temperature tolerances, but are more at risk from warming because they are close to their upper thermal limits. Global Ecology and Biogeography, 29(8), 1387–1398.
Sgarlata, G. M., Le Pors, B., Blanco, M., Salmona, J., Chikhi, L., et al. (2020a). Cheirogaleus shethi. The IUCN red list of threatened species 2020a: e.T163020756A163020759. Accessed 15 June 2020.
Sgarlata, G. M., Le Pors, B., Frasier, C. L., Chikhi, L., Salmona, J., et al. (2020b). Cheirogaleus andysabini. The IUCN red list of threatened species 2020b: e.T163021607A163021799. Accessed 15 June 2020.
Sharma, S., Arunachalam, K., Bhavsar, D., & Kala, R. (2018). Modelling habitat suitability of Perilla frutescens with Maxent in Uttarakhand—A conservation approach. Journal of Applied Research on Medicinal and Aromatic Plants, 10, 99–105.
Sutherland, W. J. (2000). The Conservation Handbook: Research, Management and Policy. Blackwell Science.
Thorn, J. S., Nijman, V., Smith, D., & Nekaris, K. A. I. (2009). Ecological niche modelling as a technique for assessing threats and setting conservation priorities for Asian slow lorises (Primates: Nycticebus). Diversity and Distributions, 15(2), 289–298.
UNEP-WCMC. (2020). Protected Area Profile for Madagascar from the World Database of Protected Areas, September 2020. Available from: www.protectedplanet.net [Accessed 25th September 2020].
Urbina-Cardona, J. N., & Loyola, R. D. (2008). Applying niche-based models to predict endangered-hylid potential distributions: Are neotropical protected areas effective enough? Tropical Conservation Science, 1(4), 417–445.
Venter, O., Sanderson, E. W., Magrach, A., Allan, J. R., Beher, J., Jones, K. R., Possingham, H. P., Laurance, W. F., Wood, P., Fekete, B. M., Levy, M. A., & Watson, J. E. M. (2016). Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nature Communications, 7(1), 1–11.
Vieilledent, G., Grinand, C., Rakotomalala, F. A., Ranaivosoa, R., Rakotoarijaona, J. R., Allnutt, T. F., & Achard, F. (2018). Combining global tree cover loss data with historical national forest cover maps to look at six decades of deforestation and forest fragmentation in Madagascar. Biological Conservation, 222, 189–197.
Warren, D., & Dinnage, R. (2021). ENMTools: Analysis of niche evolution using niche and distribution models. R package version 1.0.4. https://CRAN.Rproject.org/package=ENMTools.
Warren, D. L., Glor, R. E., & Turelli, M. (2008). Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution: International Journal of Organic Evolution, 62(11), 2868–2883.
Warren, D. L., Glor, R. E., & Turelli, M. (2010). ENMTools: A toolbox for comparative studies of environmental niche models. Ecography, 33(3), 607–611.
Webber, A. D., Solofondranohatra, J. S., Razafindramoana, S., Fernández, D., Parker, C. A., Steer, M., Abrahams, M., & Allainguillaume, J. (2020). Lemurs in cacao: Presence and abundance within the shade plantations of Northern Madagascar. Folia Primatologica, 91(2), 96–107.
Wilmé, L., Goodman, S. M., & Ganzhorn, J. U. (2006). Biogeographic evolution of Madagascar’s microendemic biota. Science, 312(5776), 1063–1065.
Wisz, M. S., Hijmans, R. J., Li, J., Peterson, A. T., Graham, C. H., Guisan, A. and NCEAS Predicting Species Distributions Working Group (2008). Effects of sample size on the performance of species distribution models. Diversity and Distributions, 14(5), 763-773.
Wright, P. C. (1999). Lemur traits and Madagascar ecology: Coping with an island environment. American Journal of Physical Anthropology, 110(S29), 31–72.
Yoder, A. D., Rasoloarison, R. M., Goodman, S. M., Irwin, J. A., Atsalis, S., Ravosa, M. J., & Ganzhorn, J. U. (2000). Remarkable species diversity in Malagasy mouse lemurs (Primates, Microcebus). Proceedings of the National Academy of Sciences, 97(21), 11325–11330.
Zimmerman, N. E. (2006). Novel methods improve prediction of species’ distributions from occurrence data. Ecography, 29, 129–151.
Acknowledgements
The authors thank all of the researchers, students and field guides whose fieldwork efforts to gather distribution data on the dwarf lemurs made this study possible. They also thank Professor Martin Genner and Dr. Ulrike Bauer for insightful discussion and five anonymous reviewers for helpful advice.
Author information
Authors and Affiliations
Contributions
All authors conceptualized and designed the study. DH conducted all data collection and analyses. All authors contributed to the writing of the manuscript.
Corresponding author
Additional information
Handling Editor: Dr. Onja Razafindratsima
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hending, D., McCabe, G., Cotton, S. et al. Conservation Biogeography of the Dwarf Lemurs (Cheirogaleus) of Madagascar, Investigated via Ecological Niche Modelling. Int J Primatol 44, 960–983 (2023). https://doi.org/10.1007/s10764-023-00363-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-023-00363-w