Abstract
The progressive fragmentation of forest habitat is causing an increase in edge areas that may differ structurally and in quality from forest interiors. We investigated the impact of edge effects on habitat structure, behaviour, and ecology of the small, nocturnal, and highly arboreal Sahamalaza sportive lemur, Lepilemur sahamalaza. To understand edge effects, we established edge-to-interior gradients using temperature, humidity, and light intensity measurements along transects. From 773 h of behavioural observations on 14 individual sportive lemurs between 2015 and 2016, we compared home range sizes, activity budgets, and habitat use of animals inhabiting the edge area and those in the core forest. We found that microclimatic edge effects penetrated the forest up to 165 m, but that there was no significant edge effect on vegetation; forest vegetation was structurally variable throughout. Individual sportive lemurs living in the edge area used more trees with a diameter at breast height of less than 5 cm but showed no other behavioural differences to individuals inhabiting the core forest. The study shows that this species may not be impacted by edge effects, at least in situations in which vegetation structure is not affected, despite microclimatic differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forests are frequently cleared for agricultural use, breaking up large, continuous habitat into small and increasingly isolated fragments (Bolt et al., 2019; Fahrig, 2003; Gardner et al., 2009; Lenz et al., 2014; Marsh, 2003; Turner, 1996). Ongoing fragmentation is leading to an increase in forest edge areas that differ from forest interiors in abiotic (i.e., temperature, humidity, sun exposure) and biotic (i.e., vegetation composition) variables (Chen et al., 1992, 1995; Davies-Colley & Payne, 2000; Gehlhausen et al., 2000; Lenz et al., 2014; Malcolm, 1994; Newmark, 2001). This in turn affects species composition and relative abundances of animal communities in fragments, with some species avoiding edge areas while population densities of others increase in response to variation in required resources. (Brodie et al., 2015; Murcia, 1995; Ries et al., 2004).
Although edge areas are often seen as lower-quality habitat, they are becoming an increasingly large part of forest-dependent species’ ranges (Andriatsitohaina et al., 2020; Arroyo-Rodríguez & Mandujano, 2006; Lehman et al., 2006a). In particular, species that hibernate (e.g. greater dwarf lemur, Cheirogaleus major: Lehman et al., 2006b) or are highly frugivorous (e.g., Coquerel’s sifaka, Propethicus coquereli: Kun-Rodrigues et al., 2014; McGoogan, 2011; brown lemur, Eulemur fulvus: Lehman, 2007; red-faced spider monkey, Ateles paniscus: Lenz et al., 2014) have shown negative responses, due to higher temperatures, more exposed vegetation, and fewer feeding resources at forest edges. In contrast, insectivorous (e.g., golden-brown mouse lemur, Microcebus ravelobensis: Burke & Lehman, 2014; pygmy tarsier, Tarsius pumilus: Grow et al., 2013; gray mouse lemur, Microcebus murinus: Lehman et al., 2006a) and folivorous species (e.g., Guyanan red howler, Alouatta macconnelli, red-backed bearded saki, Chiropotes chiropotes, golden-handed tamarin, Saguinus midas, and tufted capuchin, Sapajus apella apella: Lenz et al., 2014; white-faced capuchins, Cebus capucinus: Bolt et al., 2020; weasel sportive lemur, Lepilemur mustelinus: Lehman, 2007; golden-crowned sifaka, Propithecus tattersalli: Quéméré et al., 2010) are often drawn towards forest edges due to an increase in insect abundance and leaf quality in these areas. Because most primates now live within 1 km of a forest edge, it is crucial to understand the impacts that edge areas have on primate ecology and behaviour (Estrada et al., 2017).
While some primate species tolerate forest edges (Schreier et al., 2021), others alter their activity patterns, feeding behaviour, and group cohesion when in forest edges compared to the forest interior (Coquerel’s sifaka, Propethicus coquereli: McGoogan, 2011; Verreaux's sifakas, Propithecus verreauxi: Dinter et al., 2021). Primates may also face nutritional stress because of low food availability in the forest edge. This is particularly true of frugivores (Dinter et al., 2021), but may also hold true for species with other dietary strategies. For example, mantled howler monkey (Alouatta palliata) in Costa Rica feed more on stems and less on leaves, and feed from a higher number of tree families in the forest edge compared to the interior (Bolt et al., 2021). In contrast, groups of moor macaque (Macaca maura) that inhabit forest areas bisected by a major road, increase their daily travel distances, and preferentially feed in edges of forest near roads (Riley et al., 2021). Behavioural changes as a response to altered habitat structure and quality may have long-term effects on fitness of populations (Bolt et al., 2019; Dinter et al., 2021; Ukizintambara, 2010; Woodroffe & Ginsberg, 1998). As more primate species become threatened with extinction, it is increasingly important to understand the effects of habitat alterations and primates’ responses to them in order to effectively mitigate any negative impact forest edges may have on primates.
With the ongoing fragmentation of forests in Madagascar (Harper et al., 2007), understanding how forest edges affect lemur populations is of utmost importance (Irwin, 2006). As of 2007, up to 80% of Madagascar’s remaining forest was within 1 km of an edge and little connectivity persisted between fragments (Harper et al., 2007), and further fragmentation has continued to occur. Edge-related microclimatic changes penetrate forest up to 1 km across forest types and affect plant and animal responses up to 300 m from forest edges (Laurance et al., 1997; Marsh, 2003). The spatial extent of edge influence (i.e., the distance from the edge of the forest into the interior that shows difference in microclimate and habitat: Chen et al., 1992; Harper & Macdonald, 2011; Harper et al., 2005) makes it likely that very little of Madagascar’s forests currently remain unaffected. The encroaching human population may put additional pressure on the lemurs to avoid edge areas, despite potentially favourable resource distributions (McLennan et al., 2017).
Sportive lemurs of the genus Lepilemur are small (600–1200 g), nocturnal and highly arboreal primates that range throughout Madagascar (Mittermeier et al., 2010). All sportive lemur species are classified as threatened by the IUCN, and local extinctions and measurable genetic consequences of habitat fragmentation have already occurred (red-tailed sportive lemur, Lepilemur ruficaudatus: Bachmann et al., 2000; Milne Edwards sportive lemur, Lepilemur edwardsi: Craul et al., 2009). Although this genus can persist even in small fragments, population sizes correlate with forest fragment sizes, with fewer individuals in smaller fragments (red-tailed sportive lemur, Lepilemur ruficaudatus: Bachmann et al., 2000; Madame Fleurette's sportive lemur, Lepilemur fleuretae: Campera, 2018). Sportive lemurs are considered generalist folivores (Ganzhorn et al., 2004; Hladik & Charles-Dominique, 1974; Mittermeier et al., 2010), and forest edges provide folivores with valuable resources, as increased solar radiation increases the protein-to-fibre ratio in leaves and enhances leaf production (Ganzhorn, 1995). However, sportive lemurs travel mainly via clinging and leaping, for which bigger trees—which are not commonly found at forest edges—are necessary (Blanchard et al., 2015; Crompton et al., 2010). Although previous studies indicate that some sportive lemur species seem more affected by the overall size of forest fragments and level of degradation than edge effects per se (Lehman, 2007; Lehman et al., 2006a, c; Seiler et al., 2014), no information is available on whether they adapt their behaviour to changes in microclimates or vegetation structures in forest edges. If sportive lemurs persist in edge areas, research into their behaviour and ecology is required to determine whether individuals living in edge areas behave differently than those inhabiting core forest, especially in small forest fragments.
We studied a population of Sahamalaza sportive lemurs, Lepilemur sahamalaza (formerly L. sahamalazensis: Andriaholinirina et al., 2017) inhabiting a forest small (~ 50 ha) fragment of Ankarafa Forest on the Sahamalaza Peninsula in north-western Madagascar. The fragment consists predominantly of regenerated (but degraded) forest (Hending, 2021; Seiler et al., 2013b; Volampeno et al., 2013). Human activities such as logging, fires, and hunting, while forbidden, still occur occasionally throughout the forest (Ruperti, 2007; Seiler et al., 2013b; Volampeno et al., 2013). The goal of this study was to investigate whether forest edges are suitable habitats for sportive lemurs in Ankarafa or if living at the edge, with its potential variations in resources or differences in microclimate, incurs potential fitness costs, is reflected in changes in activity budget and home range size.
We hypothesized that if edge effects play a role in Ankarafa forest, there would be a distinct edge-to-interior gradient in microclimate. If this was the case, we predicted that the forest fragment would show higher temperatures and lower humidity towards the forest edges, as recorded in other forests (i.e., Denyer et al., 2006; Gehlhausen et al., 2000). We also hypothesized that if there were distinct changes along a microclimatic gradient, that these would be reflected in canopy cover and vegetation composition from edge to interior. Here we predicted that vegetation changes would be reflected in the presences of more large trees and a more closed canopy in the forest interior, compared to the edge. In addition, we hypothesized that if the microhabitat of forest edges was different to that of the forest interior in Ankarafa Forest, and if this negatively impacted Sahamalaza sportive lemurs, individuals inhabiting edge areas would show differences in activity budgets and home range sizes to those in the forest interior.
Methods
Study Site and Seasons
We collected data in Ankarafa Forest, the most western forest in the Sahamalaza–Iles Radama National Park, north-western Madagascar, located between 13°52'S and 14°27'S and 45°38'E and 47°46'E. Ankarafa Forest is highly fragmented with many smaller blocks that are connected by narrow corridors. The vegetation is characterized by a mix of dry deciduous and Sambirano rainforest vegetation structures (Volampeno et al., 2013) with canopies of up to 25 m high (Dumetz, 1999; Gouvenain & Silander, 2003). Ankarafa Forest fragments are surrounded by an abrupt edge bordered by savannah grassland with very little wooded vegetation. Its accessible, open structure allows for anthropogenic disturbances, such as logging or fires, as well as hunting activities (Seiler et al., 2013b). The climate in north-west Madagascar shows two distinct seasons, with a hotter, rainy period between November and March and a colder, dry period between April and October. During the study period (October 2015 until August 2016, although no data collection was carried out in March 2016) we measured daily temperature and rainfall with digital thermometers (TFA Dostmann, Wertheim, Germany, measuring range: − 20 to + 50 °C, accuracy: ± 0.5 °C) and simple rain gauges (self-made). Maximum temperatures during the day reached 39 °C in the wet season and 21 °C in the dry season, while minimum temperatures at night were 28 °C and 13 °C in the wet and the dry season respectively (Mandl et al., 2018).
Study Subjects
We captured 14 individual Sahamalaza sportive lemurs, eight females and six males, and fitted them with radio collars (cable-tie VHF radio collars, 3.5 g, Biotrack, Wareham, UK; individual radio collars allowed for individual identification based on the unique radio signal of each collar). We captured individuals between September and October 2015 during the day at their sleeping trees and injected them with anaesthetic (0.1 mg/kg Zoletil 100) by hand or using a Telinject blowpipe. Immediately after capture, we weighed and measured each individual and equipped it subcutaneously with a microchip (8 mm × 1.4 mm ISO FDXB, Micro-ID, West Sussex, UK) for future identification in case of re-capture. Trained veterinarians performed all handling procedures, and we observed captured individuals for at least 6 h before we released them at the site of capture at the onset of their normal nocturnal activity period. At the end of the study, we re-captured all individuals using the same methods and removed the radio collars.
Depth of Edge Effects — Microclimate
To determine how far edge effects penetrated the forest fragment we set up five parallel transects (Fig. 1, crevasses within the forest limited accessibility resulting in only east/west transects). The transects were perpendicular to the edges, 400 m in length, covering approximately half the width of the fragment (Fig. 1). They were spaced at least 50 m apart. We selected non-random starting points of transects for their accessibility into the forest fragment. However, clearing vegetation to make the forest fragment more accessible was deemed counterproductive to the conservation aspect of this study. We marked transect points with flagging tape (non-adhesive coloured vinyl, C.H. Hanson, Naperville, IL, USA) at 10-m intervals, and removed all markers at the end of the data collection period.
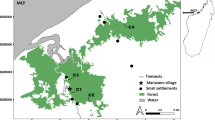
Location of study site within the Sahamalaza – Iles Radama National Park, in north-western Madagascar. The studied forest fragment was approximately 50 ha in size (black outline) and the habitat to the 14 studied Lepilemur sahamalaza. Dotted black lines indicate the location of five transects used to establish microclimatic and vegetational gradients from edge to core of the forest between October 2015 and August 2016.
We conducted transect walks at 10:00 h, walking slowly from the forest edge inwards and taking abiotic measurements every 10 m. We measured light intensity using a portable handheld digital Luxmeter (range: 0–200,000 Lux, YH-THINKING, Peking, China), and temperature and humidity with a portable weather station (TCM, Tschibo Gmbh., Hamburg, Germany), which we suspended from a branch at eye-level for measurements. At every marked point, we waited 2–5 min before taking temperature and humidity readings and took a picture of the canopy with a digital camera (Panasonic LUMIX DMC-ZS30, Tokyo, Japan), which we directed straight up towards the canopy at a consistent height of 1.8 m. We walked each transect sixteen times, eight times/season (wet: October–January, dry: April–July). We walked one transect per day.
Depth of Edge Effects — Assessment of Vegetation Structure
To investigate structural changes in the vegetation from the edge to the core, we set up nine vegetation plots on four out of the five transects at the 0-, 10-, 20-, 40-, 60-, 100-, 150-, 200- and 250-m points. Plots were 20 m × 5 m in size, with the long side running parallel to the edge of the fragment. Because of a severely degraded patch within the core area of the forest between the 300- and 350-m points on transect 1, we placed vegetation plots only up to the 250-m point. These distances are comparable with studies detecting edge-related changes in both boreal and tropical forests (Harper et al., 2005). We took the following measurements: total number and diameter at breast height (DBH, measured at 1.3 m above the ground) of all trees above 2.5 m in height and total number of all bamboo stems. We identified all trees to genus or species level where possible. We excluded all plants that could not be identified to at least genus level (n = 18) from the following analyses of diversity. For all data, in cases where we could not identify the species from the vernacular name, we treated all trees with the same vernacular name as the same species. Where we could only identify the tree to genus level, and the vernacular names differed from species in the same genus, we treated these as separate species. We measured mid-canopy heights using a laser distance meter (GLM 30, BOSCH Professional, Frankfurt, Germany). We chose mid-canopy height as a measurement instead of tree height due to the often-impenetrable nature of the canopy making it difficult to measure total tree height. We then estimated tree height using mid-canopy measurements. We collected vegetation plot data once per plot, only in the dry season between April and June 2016.
Behaviour, Home Range Size and Habitat Use
We conducted behavioural observations of the radio collared individuals throughout the entire study period at night between 18:00 h and midnight, with two teams of three observers following two individuals simultaneously. Both teams recorded behaviour continuously (Table I). We recorded a GPS point at every tree that had been visited by the focal individuals with a handheld GPS unit (GPSMAP 60CSx, Garmin Ltd., Schaffhausen, Switzerland). In addition to continuous recording, we marked the plant the focal individual was resting on with flagging tape every 15 min, as a standardized measure to enable comparison between individuals regardless of their activity levels, and recorded the height that the individual was at. We collected these scan sampling data for vegetation used every 15 min because it would not have been feasible to collect characteristics of every single tree the individuals visited, as well as to compare heights in which the individuals moved during observations. We then identified and measured the marked plants the following day, noting species (or genus), DBH, and mid-canopy height and taking a picture of the canopy.
Analysis
Depth of Edge Effects — Microclimate
We calculated canopy cover using a custom-written Matlab script (v2016a, MathWorks, Natick, Massachusetts, USA), transforming the photos into black and white at a luminance threshold of 70% (function im2bw; such that the sky — high luminance; blue and white pixels — was white, and vegetation — low luminance pixels — was black). The script then counted the percentage of black pixels for each picture and took the value as proxy for canopy cover. For photographs with a high percentage of sky, we set the threshold to 30% to avoid misclassification of dark blue pixels as vegetation. We compared converted images to original photographs manually for consistent classification of vegetation pixels. We also compared temperature, humidity and light intensity measurements between seasons using paired t-tests in R (ver. 3.3.1, R Core Team). Because comparisons of abiotic factors showed significant differences between seasons (paired t-test, temperature: t(204) = − 37.4, p < 0.01; humidity: t(204) = − 28.7, p < 0.01; light: t(204) = − 4.8, p < 0.01) we analysed depth of edge effects separately for each season. Canopy cover did not show seasonal differences (paired t-test, t(614) = − 1.1, p = 0.24) but we analysed canopy breakpoints separately for each season for consistency. We defined the depth of edge effects as an abrupt change, or breakpoint, in the variables recorded from the forest edge to the interior of the forest, as measured along the transects. This change describes a threshold up to which the surrounding non-forest matrix influence penetrates the forest fragment (McGoogan, 2011; Newmark, 2001; Nickerson et al., 1989). We used the breakpoints in the variables measured along transects as a guide to extrapolate the depth of edge effects for the entire forest fragment (~ 50 ha), following the methods of similar studies (Denyer et al., 2006; Gehlhausen et al., 2000; Harper et al., 2005). We used piecewise linear regression, in which the slope of the variables is determined, to estimate these breakpoints with the package segmented (Muggeo, 2008) in R (Newmark, 2001; Nickerson et al., 1989). Afterwards, we compared the values before and after the calculated breakpoints for temperature and humidity using paired t-tests and estimated the depth of edge effects (distance to breakpoints). To calculate the proportion of the studied forest fragment that was probably affected by microclimatic edge effects, we used the breakpoints as fixed distances into which the forest climate was impacted in each season. We then computed the affected area size by drawing a buffer from the edge to the interior breakpoints with QGIS (QGIS.org, 2021) and determining the size of the edge buffer as well as that of the ‘core’ area (forest after the breakpoints).
Depth of Edge Effects — Assessment of Vegetation Structure
We calculated the total number of trees, total number of species, total number of bamboo stems, mean height, mean DBH, and tree density for each vegetation plot, classifying trees either as small trees (measured DBH smaller than 5 cm) or larger trees (measured DBH bigger than 5 cm) following common ecological practise regarding trees with a diameter of < 5 cm as saplings (King et al., 1997). As a measurement of diversity, we computed the Shannon–Wiener index (H) and indicator of evenness (H’) for each plot (Spellerberg & Fedor, 2003).
We used a modification of the Critical Values Approach, introduced by Harper & Macdonald, (2002), to determine the depth of edge effects for the vegetation data. This methodology, labelled Randomization Test of Edge Influence (RTEI), is an Excel macro program that computes mean values of variables collected at varying distances from a forest edge, comparing them to reference values (i.e., variables collected in the forest interior), and calculates the magnitude of edge influence (MEI) for each distance (Harper et al., 2005). The concept for the RTEI method is based in testing the MEI for each distance from the edge and the reference values via randomization tests, using the formula MEI = (e-r)/(e+r), where e = mean value of the targeted variable at a given distance from the edge and r = mean value of the reference data set, for each distance from the edge (Harper et al., 2005). MEI values vary between -1 and +1; a 0 implies that the mean value of a given variable at the edge is identical to the mean value for that variable in the interior (Harper & Macdonald, 2011). The test further evaluates the significance level of the calculated MEI 274 – the P-value is equal to the determined percentile of the calculated MEI (Harper et al., 2005). To estimate the depth of edge effects, we calculated the set of distances over which the MEI is significant (Harper et al., 2005). We performed the test with 5000 permutations for each of the following response variables (for each plot) using MS Excel (ver. Office 365, Microsoft, Redmond, USA): the total number of trees, total number of bamboo stems, total number of species, H, H’, density of larger trees/hectare, density of small trees/hectare, mean DBH of larger trees, mean DBH of small trees, mean height of larger trees and mean height of small trees. We used the data collected at the three innermost vegetation plots (150, 200, and 250 m) as reference values, as we deemed to be within the interior forest and not likely to be affected by edge effects.
Behaviour, Home Range Size and Habitat Use
To compute individual home ranges, we compiled GPS points taken during behavioural observations for each individual into 99% kernel density ranges in R (package: adehabitatHR (Calenge, 2006) using least squares cross-validation smoothing (LSCV) to adapt to the small size of the home ranges and autocorrelated GPS point collection (Steury et al., 2010). We then determined which study individuals inhabited the core area by overlaying the home ranges onto the established map. If more than 85% of all GPS locations fell within the zone after the microclimatic breakpoints, we classified the respective individual as a ‘core individual’. We then compared home range sizes of ‘core’ and ‘edge’ individuals using Student’s t-tests (de Winter, 2013).
We analysed recorded behaviours to establish year-round activity budgets for each individual, using only the time during which we could clearly see the lemurs and determine their behaviour. We calculated the percentage of time engaged in each behaviour over the course of each season and investigated the effect of season and of location (and the interaction between both) on the activity budgets through a linear mixed-effects model (LMM) in R, using the package lme4 (Bates et al., 2015). For the LMM we set the dependent variables as the times spent engaged in different behaviours (log-transformed to achieve normal distribution), the fixed effects (explanatory variables) to be season (wet vs dry) and location (edge vs core). We set random effects, which are the variables to be controlled for in the analysis, as individual identity, sex, and proximity between individuals (we grouped together individuals inhabiting overlapping areas to control for spatial autocorrelation of behaviours due to similarity of habitat). We excluded two individuals (M4 and M10) from this analysis, as data for them were only available in the wet season.
We categorized all recorded trees used by lemurs into larger trees (DBH > 5 cm) and small trees (DBH < 5 cm) and identified them to species or genus level where possible. For each individual, we determined mean values for DBH and heights of used trees and calculated the mean canopy cover of their range. We compared the mean height of individuals during behavioural observations, the mean DBH and height of used larger trees, the mean DBH and height of used small trees, and the mean canopy covers of home ranges between edge and core individuals using Student’s t-tests and the proportions of used larger trees to used small trees with a chi-square test. Throughout the analysis, we reviewed all LMMs with diagnostic plots of residuals using the R package “DHARMa” (Hartig, 2022) and adjusted p-values, where multiple comparisons where performed, per the Holm-Bonferroni-method. We set the significance level to p = 0.05.
Ethical Note
We carried out all procedures with ethical approval from the University of Bristol’s Ethical Review Group (project number UB/14/048) under the revised Animals (Scientific Procedures) Act 1986 and from Madagascar’s Ministère de l'Environnement, de l'Ecologie, de la Mer et des Forêts (MEEMF) and Madagascar National Parks (MNP) who granted permission for the research to be conducted (permit number 37/16/MEEMF/SG/DGF/DAPT/SCBT).
Data Availability
The datasets during the current study are available from the corresponding author on reasonable request.
Results
Depth of Edge Effects — Microclimate
Piecewise linear regression resulted in clear breakpoints in the microclimatic data from edge to forest interior: the temperature data showed an estimated breakpoint at 148.1 ± SE 15.1 m (± standard error) during the wet season and at 123.9 ± SE 9.6 m in the dry season (Fig. 2). Humidity breakpoints were located at 145.8 ± 10.8 m in the wet season and at 165.5 ± SE 18.8 m in the dry season (Fig. 3). Both light intensity and canopy cover showed breakpoints at 10 ± SE 1.2 m across all seasons. The mean values for temperature and humidity were significantly different before and after the calculated breakpoints (Fig. 4): in the wet season, temperature decreased after the breakpoint (paired t-test: t(154) = 2.58, p = 0.01) and humidity increased (t(118) = − 4.22, p < 0.001), whereas in the dry season temperature increased after the breakpoint (t(167) = − 4.16, p < 0.001), while humidity decreased (t(203) = 2.15, p = 0.03). The area of edge effects (area from forest edge to breakpoints) thus differed significantly in microclimate to the core area of the forest. The affected area covered 70% of the studied forest fragment (~ 35 out of 50 ha).
Calculated breakpoints for the temperature gradient from edge to core in Ankarafa Forest, Sahamalaza–Iles Radama National Park, in north-western Madagascar between October 2015 and August 2026. Data are presented for each season separately: wet season (October – January) = 148.1 m and dry season (April – July) = 123.9 m. Error bars represent the standard error. Grey shading illustrates the distances from edge to the calculated breakpoint.
Calculated breakpoints for the humidity gradient from edge to core in Ankarafa Forest, Sahamalaza–Iles Radama National Park, in north-western Madagascar between October 2015 and August 2026. Data are presented for each season separately: wet season (October – January) = 145.8 m and dry season (April – July) = 165.5 m. Error bars represent the standard error. Grey shading illustrates the distances from edge to the calculated breakpoint.
Temperature and humidity gradients within the studied forest fragment in Ankarafa Forest, Sahamalaza–Iles Radama National Park, in north-western Madagascar between October 2015 and August 2026, for the a) wet season and b) dry season.The greyscale corresponds to mean temperatures (in °C) and mean relative humidity (in %) across all transects in each season, measured along 10-m increments. Dotted lines are individual Lepilemur home ranges as minimum convex polygons (MCPs). Bold lines marked with T and H indicate breakpoints for temperature and humidity respectively.
Depth of Edge Effects — Assessment of Vegetation Structure
We found no significant MEI that differed to that of the interior in the data collected from the vegetation plots: only isolated variables at varying distances differed significantly to those in the reference plots, without a meaningful edge effect (Table II). The mean DBH (MEI = 5.85, p = 0.02) and height (MEI = 8.00, p = 0.01) of larger trees was significantly lower at the plots that were located directly at the edge of the forest than in the forest interior. There was also significantly less bamboo present in these plots compared to within the core forest (MEI = 10.00, p = 0.02). Larger trees located in the 60- and 100-m plots, however, were significantly taller than those found in the core (MEI = 9.14/9.36, p = 0.01).
Behaviour, Home Range Size and Habitat Use
As no breakpoint could be detected for vegetation data, we used the microclimatic breakpoints to determine the depth of edge effects on the forest fragment, and to determine which lemurs were ‘core’ and ‘edge’ individuals: we used the maximum breakpoint across all seasons (humidity breakpoint at 165.5 m during the dry season) as the cut-off point. The home ranges of only four individuals (F2, F3, F7, M8) were located beyond this distance in the forest fragment. However, which part of the forest an individual inhabited did not affect activity budgets: we did not find a significant difference between the activity budgets of edge and core individuals across the two seasons (feeding: X2 = 0.6, df = 1, p = 0.4; resting: X2 = 0.2, df = 1, p = 0.6; locomotion: X2 = 0.3, df = 1, p = 0.5; vigilance behaviour: X2 = 0.06, df = 1, p = 0.7; grooming: X2 = 0.0, df = 1, p = 0.9).
Home range size did not differ between edge and core individuals (t(11) = 1.9, p = 0.08) (Table III). We found no significant differences between the vegetation used by edge and that used by core individuals: neither DBH (small trees: t(3.3) = 0.2, p = 0.9; larger trees: t(11.6) = 0.04, p = 0.9), height (small trees: t(3.3) = 0.02, p = 0.8; larger trees: t(10.6) = − 1.8, p = 0.09) or canopy coverage (t(10.7) = − 0.08, p = 0.9) of used trees differed significantly. In addition, the height at which individuals were active also showed no statistically significant difference (t(11.8) = − 1.2, p = 0.2). However, there was a significant difference between the proportion of larger trees to small trees used, with edge individuals using more small trees than core animals (Χ2(1,1129) = 6.3, p = 0.01).
Discussion
Our study suggests that forest edge areas, despite harbouring a different microclimate than the forest interior for parts of the year, do not seem to impact the behaviour of folivorous and arboreal Sahamalaza sportive lemurs. We found that the microclimate at the forest edge differed to that of the forest interior, showing definite “breakpoints” (i.e., distances from the forest edge up to which the temperature decreased, and humidity increased). The interior forest harboured a different microclimate than this edge area; however, in the dry season the temperature along the transects in the forest interior increased, while humidity decreased, contrary to our expectations.
The vegetation was variable throughout the forest, possibly due to its already degraded nature (Hending, 2021; Schwitzer et al., 2007; Seiler et al., 2013a; Volampeno et al., 2013), with some interior patches showing gaps in canopy cover. Despite measurable effects of the forest edge on temperature and humidity, the gaps in the canopy cover affected the microclimate in the forest interior, resulting in decreasing temperature from edge towards the interior, which then increased again after the calculated breakpoint. Similarly, humidity increased with increasing distance from forest edge, but decreased again towards the interior. As canopy cover and light intensity along the transects did not differ between seasons, the seasonal differences in the microclimate of the core forest are therefore possibly due to cloud cover, and a generally more open structure than expected in the forest interior. During the wet season, clouds are common throughout the day, which may have produced a more stable microclimate in the forest interior during this period, while sunshine penetrated the forest during the dry season. Because of the open canopy areas, the degraded nature of the fragment, and the small size of the fragment, we cannot rule out that the entire study fragment was affected by some degree of microclimatic influence of edge effects or that the fragment was subject to additive edge effects (Malcolm, 1994). Whether the forest interior, with its higher temperatures and lower humidity during the dry season, thus represented a more challenging habitat than the direct edge of the forest, is unclear. During the dry season, we often observed the lemurs sitting at the entrance of their sleeping sites in the full sun, indicating that they may have benefited from the open structure of the forest in times when overall temperatures were generally lower (Mandl et al., 2018). However, the methodology we used, although comparable to other studies, may have been unsuitable to detect micro-level variations in the microclimate across the entire fragment. Transect placement is key when estimating depth of edge effects (Gehlhausen et al., 2000), but terrain and accessibility issues prevented us from placing more transects in the studied fragment. Because we were unable to place transects perpendicular to the north–south forest edges, the data we collected may have skewed the values and the extrapolation across the forest fragment.
We did not find that the edge effects present in the microclimate were mirrored in the vegetation structure of the studied forest fragment: contrary to our predictions, the vegetation was highly variable throughout, with some areas dominated by bamboo, and larger trees occurring predominantly around 60–100 m into the forest, not only in the core forest after the 165-m microclimate breakpoint. Because of the resolution at which we collected the data, it is possible that edge effects in vegetation, as they pertain to non-tree species, may not have been captured by this study, but it is likely that degradation due to past bushfires, anthropogenic use, and high percentages of non-native plant species (Volampeno et al., 2013) was the main driver of the studied forest’s vegetation structure, rather than edge effects. The vegetation of the forest was comparable to the structure of other Malagasy lowland forests (Grubb, 2003; Volampeno et al., 2013), but the level of tree diversity, measured by the Shannon–Wiener Index, resembled that of lowland forests which exhibit relatively “low biodiversity” (Armstrong et al., 2011; Ostertag et al., 2014). A further decline in plant diversity in Ankarafa Forest may have negative consequences on Sahamalaza sportive lemurs’ ability to survive in the area (Iason, 2005; Villalba et al., 2002).
Despite the measurable edge effects in microclimate, the studied lemurs did not avoid forest edge areas, and the behaviour of lemurs living in the edge areas did not differ from that of the individuals inhabiting the ‘core’ forest. As Sahamalaza sportive lemurs do not hibernate, the variable microclimate across the forest may not have impacted them enough to elicit behavioural responses, such as compensation in activity budgets (Dausmann et al., 2005). It has been observed that changes in vegetation cover and quality lead to adaptations in this genus that are reflected in their behaviour (Ganzhorn et al., 2004). We did not find any edge effects on the vegetation structure, and the sportive lemurs did not show any behavioural adaptations to living in forest edge areas, with most individuals’ home ranges located within 150 m of a forest edge. Because even highly degraded forests within their range seem to meet the habitat requirements of this species, and because this species shows an ability to adapt to differently degraded habitats (Seiler et al., 2013b), it is unlikely that forest edges in Ankarafa Forest provide unsuitable habitat for them, regardless of microclimatic changes.
Studies have shown that sportive lemurs are affected by the availability of resources like suitable sleeping sites and—more importantly—feeding resources: Petter's sportive lemur (Lepilemur petteri) presence is highly associated with tree species it feeds on (Hajanantenaina, 2018), and Scott's sportive lemur (L. scottorum) populations are impacted by a loss of suitable feeding trees (Sawyer et al., 2017). Forest edges often constitute areas of increased food quality for folivores such as sportive lemurs, as increased solar radiation along edge areas can cause an increase in leaf production and in the protein-to-sugars ratio, which may render leaves of higher quality (Ganzhorn, 1992). This may have influenced the ‘neutral’ edge responses in the small-toothed (L. microdon) and weasel sportive lemurs (L. mustelinus) (Lehman, 2007; Lehman et al., 2006c). Whether or not this is the case for Sahamalaza sportive lemurs is unclear. Investigating the impact of forest structure and edges on the diet and nutrition of Sahamalaza sportive lemurs is not within the scope of the present study, but is necessary to fully understand how this species copes with potentially degraded forest patches.
While both microclimatic changes and resource distributions are generally assumed to be the main drivers of edge-related differences in animal abundance (Irwin et al., 2010 ; Laurance et al., 1997; Lehtinen et al., 2003), other structure-related features may also influence population distributions: sportive lemurs are classified as clingers and leapers and as such are dependent on a certain size of tree diameter for travelling even though they are small-bodied (Crompton et al., 2010; Nash, 1998) and may not be able to travel in forests where trees are spaced too far apart (Ganzhorn et al., 2004). We recorded the study individuals using trees with a DBH as low as 1.7 cm, indicating that a less restrictive mode of locomotion is possible in this species.
The ability to adapt has allowed sportive lemurs to persist in degraded forests across Madagascar: northern sportive lemurs, L. septentrionalis, can adapt their activity budgets in differently degraded habitats (Dinsmore et al., 2016), and Fleurette’s sportive lemurs, L. fleuretae, which inhabit a degraded lowland rainforest in southern Madagascar, have larger home ranges and spend less time resting than most recorded sportive lemurs (Campera, 2018). Nosy Be sportive lemurs, L. tymerlachsoni, do not seem to be affected by vegetation structure and are recorded in high numbers throughout the island’s forest during surveys (Tinsman et al., 2022). With increasing fragmentation, however, Madagascar’s primates are facing increasing threats at forest edges: species that are sensitive to changes in microclimate may struggle as forest edge effects become additive in smaller fragments (Malcolm et al., 2017), and species whose dietary resources are clumped and rare may cease to inhabit forest edges altogether (Dinter et al., 2021). In addition, close contact to humans can elevate hunting pressure (Cullen & Bodmer, 2000; Seaman et al., 2018), and forest edges are associated with increased predation pressure in some species (Andren & Angelstam, 1988). As with birds, forest edges can become ecological traps, impacting the survival of populations in forest fragments (Ries et al., 2004).
Conclusions
In conclusion, while our results indicate that Lepilemur sahamalaza, a species belonging to a widespread genus, is capable of surviving in forest edges without obvious behavioural repercussions, there is a need to further understand how edge effects impact arboreal primates in the Anthropocene. The first aim of this study was to understand the microclimatic and vegetational responses to forest edges in a small fragment. Researching abiotic and biotic edge effects should be baseline for any study investigating primate responses, rather than defining forest edges arbitrarily (Schreier et al., 2022). In the present case, we found that microclimate did show distinct edge effects up until 165 m, a result that was not obvious from looking at vegetation structure alone, and that may affect other primate species in the same forest. The second aim of this study was to determine whether there were behavioural responses to living in forest edges. We found no measurable behavioural responses, adding to the body of knowledge about small-bodied, folivorous primates’ responses to forest edges. It is likely that edges do not impact these species, and that they can persist in small fragments as long as their ecological needs are met, highlighting that even small forest fragments are valuable targets for conservation (Marsh, 2003). This provides us with crucial information for land- and species-management, as presence/absence at forest edges may affect population density estimations and help identify potential conflict points between humans and non-human primates (Bolt et al., 2018). However, in addition to understanding whether primates tolerate forest edges, more in-depth studies into behavioural, ecological, and physiological responses are important for understanding potential trade-offs and factors affecting primate fitness in the long-term.
Abbreviations
- DBH :
-
Diameter at breast height
- MEI :
-
Magnitude of edge influence
References
Andren, H., & Angelstam, P. (1988). Elevated predation rates as an edge effect in habitat islands: Experimental evidence. Ecology, 69(2), 544. https://doi.org/10.2307/1940455
Andriaholinirina, N., Fausser, J.-L., Roos, C., Thalmann, U., Rabarivola, C. J., Ravoarimanana, I., Ganzhorn, U., Meier, B., Hilgartner, R., Walter, L., Langer, C., Hahn, T., Zimmermann, E., & Craul, M. (2017). Corrective paper concerning three new species of the genus Lepilemur Geoffroy, 1851 (Mammalia). Dumerilia, 7, 62–71.
Andriatsitohaina, B., Romero-Mujalli, D., Ramsay, M. S., Kiene, F., Rasoloharijaona, S., Rakotondravony, R., Lehman, S. M., & Radespiel, U. (2020). Effects of habitat edges on vegetation structure and the vulnerable golden-brown mouse lemur (Microcebus ravelobensis) in northwestern Madagascar. BMC Ecology, 20(1), 69. https://doi.org/10.1186/s12898-020-00337-z
Armstrong, A. H., Shugart, H. H., & Fatoyinbo, T. E. (2011). Characterization of community composition and forest structure in a Madagascar lowland rainforest. Tropical Conservation Science, 4(4), 428–444. https://doi.org/10.1177/194008291100400406
Arroyo-Rodríguez, V., & Mandujano, S. (2006). Forest fragmentation modifies habitat quality for Alouatta palliata. International Journal of Primatology, 27(4), 1079–1096. https://doi.org/10.1007/s10764-006-9061-0
Bachmann, L., Rumpler, Y., Ganzhorn, J. U., & Tomiuk, J. (2000). Genetic differentiation among natural populations of Lepilemur ruficaudatus. International Journal of Primatology, 21(5), 853–865.
Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01.
Blanchard, M. L., Furnell, S., Sellers, W. I., & Crompton, R. H. (2015). Locomotor flexibility in Lepilemur explained by habitat and biomechanics: Lepilemur locomotor comparison. American Journal of Physical Anthropology, 156(1), 58–66. https://doi.org/10.1002/ajpa.22627
Bolt, L. M., Schreier, A. L., Russell, D. G., Jacobson, Z. S., Merrigan-Johnson, C., Barton, M. C., & Coggeshall, E. M. C. (2019). Howling on the edge: Mantled howler monkey (Alouatta palliata) howling behaviour and anthropogenic edge effects in a fragmented tropical rainforest in Costa Rica. Ethology, 125(9), 593–602. https://doi.org/10.1111/eth.12886
Bolt, L. M., Schreier, A. L., Voss, K. A., Sheehan, E. A., & Barrickman, N. L. (2020). Down by the riverside: Riparian edge effects on three monkey species in a fragmented Costa Rican Forest. Biotropica, 52(3), 541–553. https://doi.org/10.1111/btp.12769
Bolt, L. M., Schreier, A. L., Voss, K. A., Sheehan, E. A., Barrickman, N. L., Pryor, N. P., & Barton, M. C. (2018). The influence of anthropogenic edge effects on primate populations and their habitat in a fragmented rainforest in Costa Rica. Primates, 59(3), 301–311. https://doi.org/10.1007/s10329-018-0652-0
Bolt, L. M., Russell, D. G., & Schreier, A. L. (2021). Anthropogenic edges impact howler monkey (Alouatta palliata) feeding behaviour in a Costa Rican rainforest. Primates, 62(4), 647–657. https://doi.org/10.1007/s10329-021-00904-y
Brodie, J. F., Giordano, A. J., & Ambu, L. (2015). Differential responses of large mammals to logging and edge effects. Mammalian Biology, 80(1), 7–13. https://doi.org/10.1016/j.mambio.2014.06.001
Burke, R. J., & Lehman, S. M. (2014). Edge effects on morphometrics and body mass in two sympatric species of mouse lemurs in Madagascar. Folia Primatologica, 85(5), 277–291. https://doi.org/10.1159/000360082
Calenge, C. (2006). The package adehabitat for the R software: Tool for the analysis of space and habitat use by animals. Ecological Modelling, 197, 1035.
Campera, M. (2018). Ecological flexibility and conservation of Fleurette’s sportive lemur, Lepilemur fleuretae, in the lowland rainforest of Ampasy, Tsitongambarika Protected Area [PhD, Oxford Brookes University]. https://radar.brookes.ac.uk/radar/file/04806ff4-ca86-42c9-93b8-4e975815e911/1/campera2018ecological.pdf.
Chen, J., Franklin, J. F., & Spies, T. A. (1992). Vegetation responses to edge environments in old-growth Douglas-fir forests. Ecological Applications, 2(4), 387–396. https://doi.org/10.2307/1941873
Chen, J., Franklin, J. F., & Spies, T. A. (1995). Growing-season microclimatic gradients from clearcut edges into old-growth Douglas-fir forests. Ecological Applications, 5(1), 74–86. https://doi.org/10.2307/1942053
Craul, M., Chikhi, L., Sousa, V., Olivieri, G. L., Rabesandratana, A., Zimmermann, E., & Radespiel, U. (2009). Influence of forest fragmentation on an endangered large-bodied lemur in northwestern Madagascar. Biological Conservation, 142(12), 2862–2871. https://doi.org/10.1016/j.biocon.2009.05.026
Crompton, R. H., Blanchard, M. L., Coward, S., Alexander, R. M., & Thorpe, S. K. (2010). Vertical clinging and leaping revisited: Locomotion and habitat use in the Western tarsier, Tarsius bancanus explored via loglinear modeling. International Journal of Primatology, 31(6), 958–979. https://doi.org/10.1007/s10764-010-9420-8
Cullen, L., Jr., & Bodmer, R. E. (2000). Effects of hunting in habitat fragments of the Atlantic forests, Brazil. Biological Conservation, 95(1), 49–56.
Dausmann, K. H., Glos, J., Ganzhorn, J. U., & Heldmaier, G. (2005). Hibernation in the tropics: Lessons from a primate. Journal of Comparative Physiology B, 175(3), 147–155. https://doi.org/10.1007/s00360-004-0470-0
Davies-Colley, R. J., & Payne, G. W. (2000). Microclimate gradients across a forest edge. New Zealand Journal of Ecology, 24(2), 11.
Denyer, K., Burns, B., & Ogden, J. (2006). Buffering of native forest edge microclimate by adjoining tree plantations. Austral Ecology, 31(4), 478–489.
de Winter, J. C. F. (2013). Using the Student’s t-test with extremely small sample sizes. Practical Assessment, Research & Evaluation, 18(10), 12.
Dinsmore, M. P., Louis, E. E., Randriamahazomanana, D., Hachim, A., Zaonarivelo, J. R., & Strier, K. B. (2016). Variation in habitat and behavior of the northern sportive lemur (Lepilemur septentrionalis) at Montagne des Français, Madagascar. Primate Conservation, 30, 73–88.
Dinter, K., Heistermann, M., Kappeler, P. M., & Fichtel, C. (2021). Life on the edge: Behavioural and physiological responses of Verreaux’s sifakas (Propithecus verreauxi) to forest edges. Primate Biology, 8(1), 1–13. https://doi.org/10.5194/pb-8-1-2021
Dumetz, N. (1999). High plant diversity of lowland rainforest vestiges in eastern Madagascar. Biodiversity and Conservation, 8, 273–315.
Estrada, A., Garber, P. A., Rylands, A. B., Roos, C., Fernandez-Duque, E., Di Fiore, A., Nekaris, K. A.-I., Nijman, V., Heymann, E. W., Lambert, J. E., Rovero, F., Barelli, C., Setchell, J. M., Gillespie, T. R., Mittermeier, R. A., Arregoitia, L. V., de Guinea, M., Gouveia, S., Dobrovolski, R., … Li, B. (2017). Impending extinction crisis of the world’s primates: why primates matter. Science Advances, 3(1), e1600946. https://doi.org/10.1126/sciadv.1600946
Fahrig, L. (2003). Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics, 34(1), 487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Ganzhorn, J. U. (1992). Leaf chemistry and the biomass of folivorous primates in tropical forests: Test of a hypothesis. Oecologia, 91(4), 540–547. https://doi.org/10.1007/BF00650329
Ganzhorn, J. U. (1995). Low-level forest disturbance effects on primary production, leaf chemistry, and lemur populations. Ecology, 76(7), 2084–2096. https://doi.org/10.2307/1941683
Ganzhorn, J. U., Pietsch, T., Fietz, J., Gross, S., Schmid, J., & Steiner, N. (2004). Selection of food and ranging behaviour in a sexually monomorphic folivorous lemur: Lepilemur ruficaudatus. Journal of Zoology, 263(4), 393–399. https://doi.org/10.1017/S0952836904005394
Gardner, T. A., Barlow, J., Chazdon, R., Ewers, R. M., Harvey, C. A., Peres, C. A., & Sodhi, N. S. (2009). Prospects for tropical forest biodiversity in a human-modified world. Ecology Letters, 12(6), 561–582. https://doi.org/10.1111/j.1461-0248.2009.01294.x
Gehlhausen, S. M., Schwartz, M. W., & Augspurger, C. K. (2000). Vegetation and microclimatic edge effects in two mixed-mesophytic forest fragments. Plant Ecology, 147, 21–35.
de Gouvenain, R. C., & Silander, J. A. (2003). Do tropical storm regimes influence the structure of tropical lowland rain forests? Biotropica, 35(2), 166–180. https://doi.org/10.1111/j.1744-7429.2003.tb00276.x
Grow, N., Gursky, S., & Duma, Y. (2013). Altitude and forest edges influence the density and distribution of pygmy tarsiers (Tarsius pumilus): Effects of altitude and forest edges in Tarsius pumilus. American Journal of Primatology, 75(5), 464–477. https://doi.org/10.1002/ajp.22123
Grubb, P. J. (2003). Interpreting some outstanding features of the flora and vegetation of Madagascar. Perspectives in Plant Ecology, Evolution and Systematics, 6(1–2), 125–146. https://doi.org/10.1078/1433-8319-00046
Hajanantenaina, H. D. (2018). Distribution et caractérisation du microhabitat de Lepilemur petteri (Louis et al., 2006) dans la partie ouest de la région Atsimo Andrefana, Madagascar [Master II]. Université d’Anatanarivo.
Harper, G. J., Steininger, M. K., Tucker, C. J., Juhn, D., & Hawkins, F. (2007). Fifty years of deforestation and forest fragmentation in Madagascar. Environmental Conservation, 34(04), 325–333. https://doi.org/10.1017/S0376892907004262
Harper, K. A., & Macdonald, S. E. (2002). Structure and composition of edges next to regenerating clear-cuts in mixed-wood boreal forest. Journal of Vegetation Science, 13(4), 535–546. https://doi.org/10.1111/j.1654-1103.2002.tb02080.x
Harper, K. A., & Macdonald, S. E. (2011). Quantifying distance of edge influence: A comparison of methods and a new randomization method. Ecosphere, 2(8), 94. https://doi.org/10.1890/ES11-00146.1.
Harper, K. A., Macdonald, S. E., Burton, P. J., Chen, J., Brosofske, K. D., Saunders, S. C., Euskirchen, E. S., Roberts, D., Jaiteh, M. S., & Esseen, P.-A. (2005). Edge influence on forest structure and composition in fragmented landscapes. Conservation Biology, 19(3), 768–782. https://doi.org/10.1111/j.1523-1739.2005.00045.x
Hartig, F. (2022). DHARMa: Residual diagnostics for hierarchical (multi-level / mixed) regression models. R package version 0.4.5.
Hending, D. (2021). Environmental drivers of Cheirogaleidae population density: Remarkable resilience of Madagascar’s smallest lemurs to habitat degradation. Ecology and Evolution, 11(11), 5874–5891. https://doi.org/10.1002/ece3.7449
Hladik, C. M., & Charles-Dominique, P. (1974). The behaviour and ecology of the sportive lemur (Lepilemur mustelinus) in relation to its dietary peculiarities. In R. D. Martin, G. A. Doyle, & A. C. Walker (Eds.), Prosimian Biology (pp. 25–37). Duckworth.
Iason, G. (2005). The role of plant secondary metabolites in mammalian herbivory: Ecological perspectives. Proceedings of the Nutrition Society, 64(1), 123–131. https://doi.org/10.1079/PNS2004415
Irwin, M. T. (2006). Ecological imacts of forest fragmentation on diademed sifakas (Propithecus diadema) at Tsinjoarivo, eastern Madagascar: Implications for conservation in fragmented landscapes. [PhD. Stony Brook University].
Irwin, M. T., Wright, P. C., Birkinshaw, C., Fisher, B. L., Gardner, C. J., Glos, J., Goodman, S. M., Loiselle, P., Rabeson, P., Raharison, J.-L., Raherilalao, M. J., Rakotondravony, D., Raselimanana, A., Ratsimbazafy, J., Sparks, J. S., Wilmé, L., & Ganzhorn, J. U. (2010). Patterns of species change in anthropogenically disturbed forests of Madagascar. Biological Conservation, 143(10), 2351–2362. https://doi.org/10.1016/j.biocon.2010.01.023
King, D. A., Leigh, E. G., Condit, R., Foster, R. B., & Hubbell, S. P. (1997). Relationships between branch spacing, growth rate and light in tropical forest saplings. Functional Ecology, 11(5), 627–635. https://doi.org/10.1046/j.1365-2435.1997.00138.x
Kun-Rodrigues, C., Salmona, J., Besolo, A., Rasolondraibe, E., Rabarivola, C., Marques, T. A., & Chikhi, L. (2014). New density estimates of a threatened sifaka species (Propithecus coquereli) in Ankarafantsika National Park: P. coquereli abundance in Ankarafantsika. American Journal of Primatology, 76(6), 515–528. https://doi.org/10.1002/ajp.22243.
Laurance, W. F., Laurance, S. G., Ferreira, L. V., Rankin-de Merona, J. M., Gascon, C., & Lovejoy, T. E. (1997). Biomass collapse in Amazonian forest fragments. Science, 278(5340), 1117–1118. https://doi.org/10.1126/science.278.5340.1117
Lehtinen, R. M., Ramanamanjato, J.-B., & Raveloarison, J. G. (2003). Edge effects and extinction proneness in a herpetofauna from Madagascar. Biodiversity and Conservation, 12, 1357–1370.
Lehman, S. M. (2007). Spatial variations in Eulemur fulvus rufus and Lepilemur mustelinus densities in Madagascar. Folia Primatologica, 78(1), 46–55. https://doi.org/10.1159/000095685
Lehman, S. M., Rajaonson, A., & Day, S. (2006a). Edge effects and their influence on lemur density and distribution in Southeast Madagascar. American Journal of Physical Anthropology, 129(2), 232–241. https://doi.org/10.1002/ajpa.20241
Lehman, S. M., Rajaonson, A., & Day, S. (2006b). Edge effects on the density of Cheirogaleus major. International Journal of Primatology, 27(6), 1569–1588. https://doi.org/10.1007/s10764-006-9099-z
Lehman, S. M., Ratsimbazafy, J., Rajaonson, A., & Day, S. (2006c). Ecological correlates to lemur community structure in Southeast Madagascar. International Journal of Primatology, 27(4), 1023–1040. https://doi.org/10.1007/s10764-006-9058-8
Lenz, B. B., Jack, K. M., & Spironello, W. R. (2014). Edge effects in the primate community of the biological dynamics of forest fragments project, Amazonas, Brazil: Primate edge effects at the BDFFP. American Journal of Physical Anthropology, 155(3), 436–446. https://doi.org/10.1002/ajpa.22590
Malcolm, J. R. (1994). Edge effects in Central Amazonian forest fragments. Ecology, 75(8), 2438. https://doi.org/10.2307/1940897
Malcolm, J. R., Valenta, K., & Lehman, S. M. (2017). Edge effects in tropical dry forests of Madagascar: Additivity or synergy? Landscape Ecology, 32(2), 327–341. https://doi.org/10.1007/s10980-016-0453-z
Mandl, I., Holderied, M., & Schwitzer, C. (2018). The effects of climate seasonality on behavior and sleeping site choice in Sahamalaza sportive lemurs, Lepilemur sahamalaza. International Journal of Primatology, 39(6), 1039–1067. https://doi.org/10.1007/s10764-018-0059-1
Marsh, L. K. (Ed.). (2003). Primates in Fragments. Springer. https://doi.org/10.1007/978-1-4757-3770-7.
McGoogan, K. C. (2011). Edge effects on the behaviour and ecology of Propithecus coquereli in Northwest Madagascar. [PhD. University of Toronto].
McLennan, M. R., Spagnoletti, N., & Hockings, K. J. (2017). The implications of primate behavioral flexibility for sustainable human–primate coexistence in anthropogenic habitats. International Journal of Primatology, 38(2), 105–121. https://doi.org/10.1007/s10764-017-9962-0
Mittermeier, R. A., Louis Jr, E. E., Richardson, M. J., Schwitzer, C., Langrand, O., Rylands, A. B., ... & MacKinnon, J. (2010). Lemurs of Madagascar, 3rd Edition. Conservation International, US.
Muggeo, V. (2008). segmented: An R package to fit regression models with broken-line relationships. R News, 8(1), 20–25.
Murcia, C. (1995). Edge effects in fragmented forests: Implications for conservation. Trends in Ecology & Evolution, 10(2), 58–62. https://doi.org/10.1016/S0169-5347(00)88977-6
Nash, L. (1998). Vertical clingers and sleepers: Seasonal influences on the activities and substrate use of Lepilemur leucopus at Beza Mahafaly Special Reserve, Madagascar. Folia Primatologica, 69(1), 204–217. https://doi.org/10.1159/000052714
Newmark, W. D. (2001). Tanzanian forest edge microclimatic gradients: Dynamic patterns. Biotropica, 33(12–11), 10.
Nickerson, D. M., Facey, D. E., & Grossman, G. D. (1989). Estimating physiological thresholds with continuous two-phase regression. Physiological Zoology, 62(4), 866–887. https://doi.org/10.1086/physzool.62.4.30157934
Ostertag, R., Inman-Narahari, F., Cordell, S., Giardina, C. P., & Sack, L. (2014). Forest structure in low-diversity tropical forests: A study of Hawaiian wet and dry forests. PLoS One, 9(8), e103268. https://doi.org/10.1371/journal.pone.0103268
Quéméré, E., Champeau, J., Besolo, A., Rasolondraibe, E., Rabarivola, C., Crouau-Roy, B., & Chikhi, L. (2010). Spatial variation in density and total size estimates in fragmented primate populations: The golden-crowned sifaka (Propithecus tattersalli). American Journal of Primatology, 72(1), 72–80. https://doi.org/10.1002/ajp.20754
Ries, L., Jr., R. J. F., Battin, J., & Sisk, T. D. (2004). Ecological responses to habitat edges: mechanisms, models, and variability explained. Annual Review of Ecology, Evolution, and Systematics, 3, 491–522.
Riley, E. P., Shaffer, C. A., Trinidad, J. S., Morrow, K. S., Sagnotti, C., Carosi, M., & Ngakan, P. O. (2021). Roadside monkeys: Anthropogenic effects on moor macaque (Macaca maura) ranging behavior in Bantimurung Bulusaraung National Park, Sulawesi, Indonesia. Primates, 62(3), 477–489. https://doi.org/10.1007/s10329-021-00899-6
Ruperti, F. S. (2007). Population density and habitat preferences of the Sahamalaza sportive lemur (Lepilemur sahamalazensis) at Ankarafa Research Site, NW Madagascar. [MSc. Oxford Brookes University].
Sawyer, R. M., Fenosoa, Z. S. E., Andrianarimisa, A., & Donati, G. (2017). The effect of habitat disturbance on the abundance of nocturnal lemur species on the Masoala Peninsula, northeastern Madagascar. Primates, 58(1), 187–197. https://doi.org/10.1007/s10329-016-0552-0
Schreier, A. L., Bolt, L. M., Russell, D. G., Readyhough, T. S., Jacobson, Z. S., Merrigan-Johnson, C., & Coggeshall, E. M. C. (2021). Mantled howler monkeys (Alouatta palliata) in a Costa Rican forest fragment do not modify activity budgets or spatial cohesion in response to anthropogenic edges. Folia Primatologica, 92(1), 49–57. https://doi.org/10.1159/000511974
Schreier, A. L., Voss, K. A., & Bolt, L. M. (2022). A mathematical modelling approach to functionally defining forest edge and its utility for primate behavioural edge effects. International Journal of Primatology, 43, 460–479. https://doi.org/10.1007/s10764-022-00289-9
Schwitzer, N., Kaumanns, W., Seitz, P., & Schwitzer, C. (2007). Cathemeral activity patterns of the blue-eyed black lemur Eulemur macaco flavifrons in intact and degraded forest fragments. Endangered Species Research. https://doi.org/10.3354/esr00045
Seaman, D., Randriahaingo, H. N. T., Randrianarimanana, H. L. L., Humle, T., & King, T. (2018). Edge effects on indri and black-and-white ruffed lemur distribution in the Andriantantely lowland rainforest, eastern Madagascar. Lemur News, 21, 51–56.
Seiler, M., Holderied, M., & Schwitzer, C. (2013a). Effects of habitat alteration on resting behaviour of the Sahamalaza sportive lemur in North West Madagascar. In L. K. Marsh & C. A. Chapman (Eds.), Primates in Fragments (pp. 259–271). Springer. https://doi.org/10.1007/978-1-4614-8839-2_18.
Seiler, M., Holderied, M., & Schwitzer, C. (2013b). Effects of habitat degradation on sleeping site choice and use in Sahamalaza sportive lemurs (Lepilemur sahamalazensis). International Journal of Primatology, 34(2), 260–280. https://doi.org/10.1007/s10764-013-9658-z
Seiler, M., Holderied, M., & Schwitzer, C. (2014). Habitat selection and use in the Critically Endangered Sahamalaza sportive lemur Lepilemur sahamalazensis in altered habitat. Endangered Species Research, 24(3), 273–286. https://doi.org/10.3354/esr00596
Spellerberg, I. F., & Fedor, P. J. (2003). A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’Index. Global Ecology and Biogeography, 12(3), 177-179.
Steury, T. D., McCarthy, J. E., Roth, T. C., Lima, S. L., & Murray, D. L. (2010). Evaluation of root-n bandwidth selectors for kernel density estimation. Journal of Wildlife Management, 74(3), 539–548. https://doi.org/10.2193/2008-327
Tinsman, J., Volampeno, S., Ganas-Swaray, J., Gann, D., Andrianirina, N., Chamizo, M., Ralazampirenena, C., Ranaivoarisoa, J. F., Ravaoarisoa, H., Rivero, J., Zamora, A., & Gomes, C. M. (2022). Habitat use by the island lemurs of Nosy Be, Madagascar. American Journal of Primatology, 84(3), e23362. https://doi.org/10.1002/ajp.23362
Turner, I. M. (1996). Species loss in fragments of tropical rain forest: A review of the evidence. The Journal of Applied Ecology, 33(2), 200. https://doi.org/10.2307/2404743
Ukizintambara, T. (2010). Forest edge effects on the behavioral ecology of L’hoest’s monkey (Cercopithecus lhoesti) in Bwindi Impenetrable National Park, Uganda. [PhD. Antioch University New England].
Villalba, J. J., Provenza, F. D., & Bryant, J. P. (2002). Consequences of the interaction between nutrients and plant secondary metabolites on herbivore selectivity: Benefits or detriments for plants? Oikos, 97(2), 282–292. https://doi.org/10.1034/j.1600-0706.2002.970214.x
Volampeno, M. S. N., Randriatahina, G., & Downs, C. T. (2013). Structure and composition of Ankarafa Forest, Sahamalaza-Iles Radama National Park, Madagascar: Implications for the frugivorous endemic blue-eyed black lemur (Eulemur flavifrons). South African Journal of Wildlife Research, 43(2), 91–102. https://doi.org/10.3957/056.043.0205
Woodroffe, R., & Ginsberg, J. R. (1998). Edge effects and the extinction of populations inside protected areas. Science, 280(5372), 2126–2128. https://doi.org/10.1126/science.280.5372.2126
Acknowledgements
The authors would like to thank the Institute for the Conservation of Tropical Environments (ICTE) for their facilitation services, as well as Madagascar National Parks (MNP) and Madagascar’s Ministère de l’Environnement, de l’Ecologie, de la Mer et des Forêts (MEEMF) for permission to conduct research in the protected area. They would also like to thank Guy Randriatahina and the Association Européenne pour l’Etude et la Conservation des Lémuriens (AEECL) for continued support during field work. Special thanks to Judith Janisch, Sian Thorn, Georgina Dunlop, Mahaut Sorlin, Andry Rakotomalala, Martin Jörgensen, and Karena Tilt, who substantially contributed to the data collection during field work. We would also like to thank three anonymous reviewers and the editors for their helpful comments and suggestions which substantially improved the original manuscript. This study was funded by the Primate Society of Great Britain, the Explorer’s Club Student Grant, Pittsburgh Zoo and PPG Aquarium Sustainability Fund, Primate Conservation Inc., Fresno Chaffee Zoo and Wildlife Conservation Fund, Minnesota Zoo’s Ulysses S. Seal Conservation Grant, and the Primate Action Fund.
Funding
Open access funding provided by University of Vienna.
Author information
Authors and Affiliations
Contributions
IM conceived and designed the study, collected, and analysed the data, and wrote the manuscript; NR collected data and provided technical input, MH and CS oversaw the study development, revised the manuscript, and provided editorial advice.
Corresponding author
Ethics declarations
Inclusion and Diversity Statement
The author list includes contributors from the location where the research was conducted, who participated in study conception, study design, data collection, analysis, and/or interpretation of the findings. We are committed to ensuring that the research results are available to those working and living in the study location.
Conflict of Interest
The authors declare they have no conflict of interest.
Additional information
Handling Editor: Addisu Mekonnen
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mandl, I., Rabemananjara, N., Holderied, M. et al. Measuring the Impact of Forest Edges on the Highly Arboreal Sahamalaza Sportive Lemur, Lepilemur Sahamalaza, in North-Western Madagascar. Int J Primatol 44, 458–481 (2023). https://doi.org/10.1007/s10764-022-00333-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-022-00333-8