Abstract
The effectiveness of seed dispersal by frugivorous primates may vary between seasons and plant species, depending on foraging strategies. We investigated how foraging strategies of an invasive frugivorous primate (the long-tailed macaque, Macaca fascicularis) affect seed dispersal effectiveness (SDE) between native and invasive plants in Mauritius’ native remnant forests. By collecting behavioural data on a group of partially habituated macaques via scan sampling from December 2019 until December 2020 (mean 19.2 ± SD 7.3 hours per month), we investigated seasonal patterns in diet, home range, and fruit availability to identify foraging strategies and determine fruit preference. We simultaneously assessed SDE for invasive vs native plants by quantifying native and invasive fruits consumed or dropped intact by macaques during feeding bouts (n = 114). Macaques fed increasingly on ripe invasive fruits and less on other food items as fruit availability increased, due to preference for invasive fruits and disproportionate availability of invasive vs native fruits. When fruit became scarcer, macaques had larger home ranges, increasingly fed on scarce unripe native and invasive fruits, and expanded their diet by eating orchard crops, indicating use of energy-maximizing strategies. Macaques consumed more native than invasive fruits when unripe and commonly destroyed seeds of native fruits, indicating higher SDE for invasive vs native plants. Higher discard rates of unripe compared to ripe fruits further reinforced these differences in SDE. Our results highlight potential facilitation of plant invasion by an invasive primate, due to foraging strategies shaped by the availability of invasive fruits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optimal foraging theory predicts that animals consume low-quality food items increasingly as the abundance of preferred high-quality food items decreases (MacArthur & Pianka, 1966; Pyke, 1984). Therefore, seasonal changes in availability of preferred foods often influence foraging strategies of animals, including frugivorous primates (Garber, 1987). Ripe fruits are typically preferred foods for frugivorous primates (Marshall & Wrangham, 2007), as they are high-quality and high-energy food sources that are easy to digest (Garber, 1987; Harrison, 1984; Lambert, 2007). Consequently, frugivorous primates commonly adjust foraging strategies depending on fruit availability. When fruit becomes scarcer, frugivorous primates usually increasingly consume mature leaves, flowers, immature fruits, and seeds (Harrison, 1984; Nagy-Reis & Setz, 2017; Terborgh, 1983). These foods are often considered ‘fallback foods’ (Marshall et al. 2009), because they are less profitable than ripe fruits (e.g., due to lower digestibility, presence of toxins, or longer handling time) (Garber, 1987; Lambert, 2007). Furthermore, when fruits become scarcer, primates may range further to obtain a sufficient amount of their base diet due to low concentrations or patchy distribution of suitable food sources (Hall, 1962), an example of an energy-maximizing strategy (Harrison, 1984). They may also use energy-minimizing strategies in which animals range less far and increasingly forage on abundant low-quality ‘fallback foods’ (Nagy-Reis & Setz, 2017).
Such foraging strategies may cause seasonal variation in seed dispersal effectiveness (SDE) of frugivorous primates (Chapman & Russo, 2006), both in terms of quantity (number of seeds dispersed) and quality (probability that a viable dispersed seed survives handling, germinates and produces a new adult) (Schupp et al., 2010). Quantity is affected by the number of consumed fruits (Schupp et al., 2010), which often varies seasonally in frugivorous primates (Terborgh, 1983). Quality is affected in part by the type of fruit-handling behaviour and seed-dispersal distance. For example, seed swallowing or seed spitting are more likely to contribute to successful seed dispersal (Gross-Camp & Kaplin, 2011), whereas dropping intact fruits without removing the pulp (increasing the risk of fruit rot) (Traveset, 1998) and crushing seeds will probably hinder seed dispersal (Schupp et al., 2010). Moreover, seed dispersal over bigger distances reduces distance- and density-dependent mortality and may thus increase establishment and survival success of seeds (Schupp et al., 2010). The ripeness stage at which the fruit is handled can also affect dispersal quality, as ripeness is generally an indicator of seed germination success (Sumner & Mollon, 2000). Therefore, frugivorous primates may be less effective dispersers during periods of fruit scarcity, because the frequency of unripe fruit/seed predation can increase (Chapman & Russo, 2006). In contrast, primates may become more effective seed dispersers when fruit is scarce if they increase their home range, potentially increasing seed dispersal distances (Chapman & Russo, 2006). However, seed dispersal distance will largely depend on the fruit-handling behaviour (Chapman & Russo, 2006).
Foraging strategies may also lead to variation in SDE between plant species, as different fruiting species have an unequal chance of selection: fruit choice of vertebrate frugivores generally depends on fruit traits (Crestani et al., 2019), crop size (Ortiz-Pulido et al., 2007), spatial aggregation of fruiting trees (Manasse & Howe, 1983), and the timing of fruiting of a tree relative to conspecifics or other plant species (Gleditsch et al., 2017). Variation in SDE between plant species may be problematic in areas where frugivorous primates are invasive (Jones et al., 2018). Even though in some cases invasive frugivores can provide important dispersal services to native plants (Vizentin-Bugoni et al., 2019), invasive frugivores often mainly disperse invasive plants and/or predate seeds of native plants (López-Darias & Nogales, 2008; Martin-Albarracin et al., 2018), due to factors such as disproportionate availability of invasive compared to native fruits, or attractive fruit traits of invasive plants (Bitani et al., 2020; Chimera & Drake, 2010). This may be especially common on oceanic islands, where invasive plants are typically numerous and highly abundant (Denslow, 2003) and may produce more profitable fruits than native plants (e.g., higher energy, protein, or lipid content), potentially due to the limited spectrum of fruit traits in island floras (Kueffer et al., 2009). Consequently, invasive primates may use foraging strategies that promote plant invasion on islands. Remarkably, seed dispersal by invasive primates has barely been addressed (Oliveira-Silva et al., 2018), let alone on islands (Kemp & Burnett, 2003).
The long-tailed macaque, Macaca fascicularis (Cercopithecidae), is a primate native to Southeast Asia (IUCN status: Vulnerable) (Eudey et al., 2020), where it is often highly frugivorous and conforms to predictions from optimal foraging theory (Ruslin et al., 2019; Yeager, 1996). It is also an example of a primate that has been introduced to oceanic islands globally (Global Invasive Species Database, 2019), including Mauritius, where it was introduced around the seventeenth century (Sussman & Tattersall, 1986). Even though Macaca spp. are considered valuable seed dispersers in their native range (Albert et al., 2013b; Sengupta et al., 2020; Tsuji, & Su, H. –H., 2018), in Mauritius long-tailed macaques may mainly disperse invasive plants. Invasive plants dominate most forest habitats and are a major threat to native forests (Florens et al., 2016; Florens et al., 2017; Strahm, 1993), which now cover only 4.4% of their original extent (Hammond et al., 2015). The macaques are known to feed on the fruits of abundant invasive plants (Sussman et al., 2011), which often have fruit traits that are generally preferred by long-tailed macaques (sweet and acidic, Ungar, 1995) (e.g., Psidium cattleyanum) and/or are consumed by long-tailed macaques in their native range (e.g., Litsea glutinosa; Azzahra, 2017). In contrast, the macaques appear to act mainly as a seed predator for native plants (Baider & Florens, 2006; Krivek, 2017; Reinegger et al., 2021), indicating that native fruits may mainly serve as ‘fallback food’.
These foraging patterns would create quantitative and qualitative differences in SDE between invasive and native plants that could facilitate plant invasion. Therefore, we aimed to investigate foraging strategies used by macaques in Mauritius and how these strategies affect SDE of macaques for invasive and native plants in a degraded native remnant forest. We first identified foraging strategies by evaluating the relationships between seasonal fruit availability, diet composition, and ranging patterns of macaques. As ripe fleshy fruits are a high-quality and often preferred food source for frugivorous primates (Marshall & Wrangham, 2007), we first hypothesized that macaques adjust foraging strategies depending on seasonal availability of fleshy fruit (Lucas & Corlett, 1991; Yeager, 1996). We predicted that during high fruit availability, macaques would feed on more clumped/abundant ripe fleshy fruits and consequently would have a smaller home range size. Conversely, during low fruit availability, macaques would increasingly consume more scattered ‘fallback’ foods (e.g., flowers, unripe fruits, and leaves) and increase their home range size as a result. Thereafter, we measured seasonal SDE of macaques for native and invasive plants in both qualitative and quantitative terms: determining the fruit handling methods used by macaques, and quantifying the consumption and drop of invasive and native ripe vs unripe fruits. We hypothesized that macaques disperse invasive plants more effectively than native plants, because invasive plants are disproportionally abundant in Mauritius (Florens et al., 2016) and may possess fruits that are more attractive or profitable to macaques than native fruits (Kueffer et al., 2009). Therefore, we predicted macaques would consume more ripe fruits of invasive vs native plants. Additionally, we hypothesized that the probability of fruit rejection (drop of intact fruit) by macaques is affected by fruit maturity and availability, because primates are considered ‘choosy’ feeders that are more likely to reject unripe fruit when more appealing ripe fruit is available (Janson, 1996). We predicted that macaques would drop a higher proportion of unripe than ripe fruits intact during high fruit availability compared to low fruit availability.
Methods
Study Site
We conducted our study on Mt. Calebasses (-20.181203°S, 57.584498°E), a forest remnant in the north of Mauritius (Fig. 1) that contains a relatively rich native plant community (Reinegger, 2018) and is inhabited by four to five macaque groups (RR pers. obs.). The site is between 420 and 580 m asl and receives 1800–2200 mm of rainfall per year (Willaime, 1984). The forest consists of semi-dry vegetation at lower elevations and sub-humid vegetation at higher elevations. Moreover, the forest is extremely degraded at lower elevations, and mostly consists of a remnant pine plantation (non-native Pinus elliottii) and dense thickets comprising invasive Flacourtia indica, Hiptage benghalensis, Ligustrum robustum, Litsea spp., Psidium cattleyanum, Rhamnus nepalensis and Syzygium jambos. Most of these species were introduced for gardening and horticulture by the French during the eighteenth century (Cheke, 1987; Cheke & Hume, 2008). Except for F. indica, P. cattleyanum, all species occur in the native range of the long-tailed macaque.
Study Group and Habituation Process
We habituated a group of macaques occupying a steep, densely vegetated, northwest-facing slope on Mt. Calebasses during October–November 2019 (Fig. S1). We could not fully habituate the group to human observers during this period, but by December 2019 we could approach to within 5–20 m of them and follow them consistently for 2–6 hours at a time. At this stage of the habituation process, we were able to collect data (cf. ‘partial habituation stage’; Gazagne, Hambuckers, et al., 2020a). We could identify the group by three immature individuals that commonly foraged together and were the first to approach within 10 m of the researcher in the first 2 months and < 2 m after 4 months. Additionally, the group reused the same three sleeping sites from December 2019 until September 2020. On days that we lost our study group during the first 4 months, we revisited the sleeping sites in the early evening to confirm they had not changed sleeping sites. Individuals from the group also increasingly tolerated and ignored the researcher as our study progressed, eventually enabling us to follow the group in dense stunted vegetation at a 5–10 m distance from March 2020. In contrast, individuals from neighbouring groups (n = 3) remained very skittish and ran as soon as they noticed the researcher, making it impossible to follow these groups. Our group consisted of 19–23 individuals, made up of 1–2 adult males, 3–5 adult females and 12–16 immature individuals (sub-adult, juveniles, and infants). We could identify adult males and females based on differences in their physical features: adult females have ‘beards’ and protruding teats, whereas adult males are larger than adult females, have prominent genitalia and an often upwards curling moustache (lacking the ‘beard’ that is present in adult females) (Brotcorne, 2014; Jamieson, 1998). These features are much less pronounced in immature individuals (largely absent in infants and juveniles) (Brotcorne, 2014) and thus we were not able to clearly identify sex for most immature individuals. Group size may have slightly varied during our study due to migration of males. However, we could not detect migrations, because we had few opportunities to observe the complete group and it was difficult to distinguish between individuals in the same age–sex class.
Data Collection
We followed the group for half a day 4 times a week from December 2019 until December 2020: either from the moment the macaques left their sleeping site (05:30–6:00) until 13:30, or from 11:00 until the macaques returned to their sleeping site (18:00–18:30), or until we lost the group for longer than 1.5 hours. We usually followed the group for 2 consecutive half-days in a row twice a week, spreading sampling effort equally across times of day (2 days 5:30–13:30, 2 days 11:00–18:30). We chose half-day intervals over full days (05:30–18:30) because the high topographical relief in the area combined with steep slopes made following the group very physically demanding. Furthermore, activity patterns of macaques in the southwest of Mauritius were very consistent throughout the day (Sussman et al., 2011), suggesting that our chosen half-day interval can represent the whole day.
We recorded the study group’s geographical position every 30 minutes with a handheld Global Positioning System (GPS) (eTrex 30x, Garmin; Olathe, KS) using the degrees and decimal minutes (DMM) format projection. Simultaneously, we collected dietary and behavioural data by scan sampling the group at 5-min intervals (Altmann, 1974), typically at 5–20 m distance from the group. We collected a total of 2764 scans over 75 days (mean = 3.1 ± SD 1.8 hours per day) from December 2019 until December 2020 (19.2 ± 7.3 hours per month), excluding January 2020 (due to frequent cyclones and torrential storms). During scans, we observed each visible group member for 10–15 seconds to record its behaviour (2 ± 1 individuals per scan; range = 1–9 individuals). We noted different behaviours (moving, resting, feeding, and social interactions), but we only used feeding behaviour in our analyses. Feeding behaviour corresponded to the manual handling of food items and then putting them into the mouth, or oral handling of food items when they were directly taken by the mouth. When an individual was feeding, we recorded the first item that was consumed (pine cone, insect, fruit, leaf, bark, flower, egg, seed, grass, or crop). Whenever we could not clearly identify food items or a plant species (because of poor visibility), we recorded them as unknown.
Availability of Fruit and Other Plant Foods
To assess fruit availability within the home range of our study group, we first estimated tree density by marking all stems with diameter at breast height (DBH) ≥ 5 cm along four vegetation transects. Two were oriented in the north–south direction and two were oriented east–west. Each transect was 2 m wide and 200 m to 400 m in length, covering a total area of 0.24 ha within the group home range, but outside of the pine plantation (Fig. 1b). Because the long-tailed macaque is an opportunistic feeder in its native range (Gumert, 2011) as well as in Mauritius (Sussman et al., 2011), we included all tree and liana species present in the vegetation transects. However, we grouped invasive Litsea glutinosa and Litsea monopetala together as Litsea sp., because it was difficult to distinguish between the two species (similar leaves, fruits, flowers, and fruiting periods). At the start of every month, we recorded the phenological state of all stems (1288 stems and 39 plant species, Table S1) along three transects (presence/absence of flowers and ripe and unripe fruits). Based on the percentage of crown area covered by fruits (visual estimation), we ranked trees on a 5-point scale where a score of 0 implies no fruits and scores of 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (≥76%) imply increasing percentages of crown area covered by fruits (Albert et al. 2013a). We also recorded flower cover using the same 5-point scale. The total fruit availability index (FAI) was then calculated for each month:
Here, Di is the mean density of species i in the home range (stems/ha), Bi is the mean basal area of trees of species i (cm2/ha) and Pim is the mean score of fruit cover in species i in month m. This is then totalled for all species (n) in the phenology transects. We defined high versus low fruit availability periods as months with FAI values twice the standard error (SE) above versus below the mean FAI value across the entire study period. Months with FAI values in between the high and low values were classified as the medium fruit availability period. We used the same method to calculate FAI scores for invasive and native plant species independently. We also calculated an availability index for flowers (FlAI), where Pim was replaced with the mean score of flower cover in species i in month m. Furthermore, during the first 2 months of the study it became apparent that pine cone was an important resource for the study group. Therefore, we also calculated a pine cone availability index (PcAI), by establishing a vegetation transect (2 m by 300 m) in the pine plantation (Fig. 1b) and recording monthly pine cone cover on trees using the same 5-point scale as for the FAI and FlAI. We calculated the PcAI by multiplying Bpine by Ppine for every month. We could only record pine cone availability from March 2020 until December 2020.
Diet Composition
We obtained monthly diet composition by calculating the weighted monthly proportions of dietary scans macaques spent feeding on every food item and plant species to account for unequal daily sampling effort, using the formula from Harrison et al. (2009):
Where Fij is the daily number of scans for feeding on a particular food item or plant species i on day j, and Wj is the total number of dietary scans on day j.
Fruit Preference
We calculated fruit preference indices (FPIi) for every plant species that macaques consumed fruits of to investigate preference among different fruits using the formula from Sengupta and Radhakrishna (2015):
Here, fi is the weighted ratio of dietary scans that macaques spent feeding on the fruits of species i to the total number of fruit feeding scans in the period that species i was available, and ai is the ratio of fruiting stems of species i to the total number of fruiting stems during the fruiting period of species i. A preference index of > 1 indicated that macaques preferred the fruit of the plant species.
Home Range
We used R to perform least square cross-validated fixed kernel density estimation (LSCV KDE) to estimate the monthly and annual home ranges (using package adehabitatHR — Calenge, 2021), because it is one of the most widely used methods for home-range estimation and enables comparison with other studies (Albert et al. 2013a; Hanya et al., 2020; Seaman & Powell, 1996). Based on the group location every 30 min, we used the 95% confidence region of the KDE to represent the home range and reflect the area associated with a 95% probability of finding the animal. Moreover, we calculated the 50% confidence region, reflecting the core area that is used disproportionally more by the animals than other areas of the home range (Rühmann et al., 2019). We determined whether spatial autocorrelation of GPS locations was a problem by following the workflow from Calabrese et al. (2016) (using package ctmm — Fleming, 2021), because LSCV KDE relies on spatial independence of GPS locations. The results indicated some autocorrelation for locations separated by short time lags (0.5–6 hours). Therefore, we carried out autocorrelated kernel density estimation (AKDE, Fleming et al., 2015) and compared its performance to the LSCV KDE by following the workflow from Noonan et al. (2019). The performances differed very little, but the AKDE estimate extended into open areas that our study group avoided (including motorways). Therefore, we concluded that LSCV KDE provided more accurate estimates of monthly home ranges and core areas.
We also assessed how well our GPS data represented the monthly home ranges by bootstrapping monthly estimates (as estimated by LSCV KDE) for different sample sizes (using package rhr — Signer & Balkenhol, 2015), because we collected an unequal number of GPS locations every month (range: 24–79). We plotted the bootstrapped home-range estimates against sample size to determine whether the sampling imbalance was a problem. Home- range estimates roughly approached an asymptote at around 30–40 GPS points for all months (Fig. S2). Therefore, a larger number of GPS points barely affected monthly home range estimates, so we included all GPS points. We exported the final monthly and annual home ranges (including both the 95% and 50% KDE’s) as shapefiles and mapped them in QGIS (QGIS Development Team, 2020).
We removed November 2020 from our dataset, because the group migrated to the north-western forest edges near the orchards in Vallée du Paradis during October 2020 (when forest fruit was scarce), where it remained until the end of our study period. The group did not travel to this area prior to October 2020. In November 2020 our group primarily fed on mango (Mangifera indica) around the orchards and agricultural fields, a resource that was not available in the area the group used prior to October 2020. Consequently, monthly home range in November was much smaller than in the other months outside of the high fruit availability period. Mean monthly home range outside of the high fruit availability period (December 2019 and July–December 2020) was 15.1 ha (SD = 7.1) and November was the only month under 12 ha (4.3 ha).
Seed Dispersal Effectiveness
We assessed SDE of macaques by opportunistically quantifying consumption and drop of ripe vs unripe fruits by macaques during feeding bouts using all-occurrence sampling (Lehner, 1992). We defined the start of a feeding bout as the moment that a macaque or a group of macaques started feeding in a tree (or clump of small trees of the same species, e.g., P. cattleyanum). We recorded the exact time when the macaques started feeding and the number of feeding individuals. We then randomly selected one feeding individual and observed it for as long as the individual fed on the same tree (or clump of small trees) to determine the fruit parts it was consuming (whole fruit, pericarp, seed). We also determined the state of ripeness of fruits (ripe/unripe), defined the size of the seed by length to allow comparison to other studies (small: maximum length < 5 mm; medium: ≥ 5 mm but < 10 mm; and large: ≥ 10 mm) (Traveset, 1998) and the type of fruit handling method used by macaques: swallowed (when the entire fruit was ingested), spat out (when the fruit was taken into the mouth, mostly stored in cheek pouches, cleaned of the pulp, and the seeds spat out), partly eaten (when portions of the fruit, e.g., epicarp, were fed upon and then discarded), destroyed (when seeds were crunched by macaques) and dropped (when fruits were picked and discarded without being fed upon or accidentally dropped). We also recorded exits and entries of individuals during group feeding bouts to determine the total number of individuals that had fed in the same feeding tree. We defined the end of the feeding bout as the moment when all macaques had left the feeding tree.
At the end of the feeding bout, we counted the discarded partly eaten pericarp of ripe and unripe fruits beneath the feeding tree to determine the number of consumed fruits. For plant species with small fruits that only contained a single seed, we counted spat-out or discarded seeds instead of discarded pericarp (e.g., Litsea spp.). We also counted the number of fruits dropped by macaques intact (either picked and discarded or accidentally dropped). To avoid counting fruits that had been dropped (both partly eaten and intact) during earlier feeding bouts, we distinguished between fresh and old dropped fruits. Older dropped fruits (0.5–2 hours after the end of the feeding bout) were either discoloured (enzymic browning), had a sour and pungent smell (fresh fruit of most plant species have a neutral or sweet smell) and/or lost their original shape (became soft and wet), whereas fresh fruit that was counted or gathered shortly after the feeding bout (mean feeding bout duration = 7.4 ± SD 5.6 minutes) had barely browned and/or remained crisp. We determined these changes in fruit shape, colour, and smell by revisiting the feeding tree 0.5–2 hours after the feeding bout whenever the group remained close to the feeding tree after the feeding bout to check the dropped fruits again. When counting fruits after a feeding bout, we either counted fruits on site (only when possible within 2–3 minutes) or collected and stored in zip-lock bags so that they could be counted after field activities on the same day (Fig. S3). Since we could often not distinguish between fruits handled by different individuals on the same tree in dense vegetation, we divided the total number of consumed and dropped fruits by the duration of the feeding bout and the total number of feeding macaques recorded during the feeding bout, to estimate fruit consumption and dropped fruit per macaque per minute of the bout. This method was suitable for most canopy species with medium to large-seeded fruits. However, very small seeded fruits, such as those of Ficus reflexa and F. rubra, were sometimes swallowed whole, so quantification was limited for these species. Furthermore, seeds of Litsea sp. were too big to be swallowed, but the small fruits were often stored in the cheek pouches (Fig. S4). Therefore, for these species we could not count all eaten fruits.
Statistical Analyses
We used R (R Core Team, 2020) for all statistical analyses. To identify foraging strategies, we first examined whether the monthly weighted percentage of scans spent feeding on different food items (e.g., fruits, flowers, and crops) was related to monthly availability of different food items (e.g., FAI and FlAI) by calculating Spearman’s correlation coefficients. We used the same method to examine whether the monthly home range and core area sizes were affected by FAI. We adjusted for multiple comparisons using the Benjamini and Hochberg method (Benjamini & Hochberg, 1995), because it provides a better compromise between type I and type II errors than traditional Bonferroni methods (Nakagawa, 2004; Pike, 2011). This is more appropriate in our case due to small sample size (n = 12) (Jennions & Møller, 2003; Nakagawa, 2004). We also provided bootstrapped confidence intervals for every tested relationship to provide additional information about their biological relevance and statistical plausibility, as suggested by Nakagawa (2004).
To test our hypothesis that macaques are more effective seed dispersers for invasive than native plant species, we used a model selection approach based on the second-order Akaike information criterion (AICc). We first calculated the proportion of ripe vs unripe fruits consumed by macaques per feeding bout and used it as a measure for SDE. We fitted generalized linear models (GLMs) using the proportion of ripe vs unripe fruits consumed by macaques as response variable and ‘Species’ (consisting of all species macaques fed on) and monthly FAI as predictors. We fitted the GLMs with binomial error distributions, as our response variables were proportions derived from counts (Douma & Weedon, 2019). Because we had an unequal number of samples in different months — during some months macaques did not feed on native fruit at all, and only three invasive species (F. indica, Litsea sp. and P. cattleyanum) made up nearly all of the ripe fruit macaques ate — we collapsed predictor ‘Species’ into two categories. The first category contained the three invasive species (referred to as ‘main invasive plants’) and the second contained the other plant species (referred to as ‘other’). We examined a set of models with all possible combinations of the explanatory variables, including a two-way interaction between the explanatory variables. We ranked the models based on AICc to find the most parsimonious models. We defined the most parsimonious models as those with a ∆AICc score ≤ 2 (Symonds & Moussalli, 2011). When models with a ∆AICc score ≤ 2 were more complex versions of the top-ranked model (with additional predictors or interactions), we eliminated them as recommended by Richards (2008) to avoid retaining overly complex models with potentially spurious covariates. We also ranked model weights for every predictor and two-way interactions, by summing the Akaike weights for each model in which that predictor or two-way interaction appeared. This weight is equivalent to the probability that that predictor is a component of the best model, with a summed weight close to 1 meaning that the predictor is highly likely to be part of the best model (Symonds & Moussalli, 2011). We used a similar model selection procedure to test the hypothesis that the probability of fruit rejection by macaques is affected by fruit maturity and availability, using GLMs with the proportion of fruits consumed vs dropped by macaques for both ripe and unripe fruits for every feeding bout as response variable and ‘Species’, monthly FAI, and ‘Fruit Maturity’ (ripe/unripe) as predictors. As we only include plausible predictors and interactions in our set of alternative models, our model selection procedure is appropriate for inference (Burnham et al., 2011). In our case, model selection procedures also yield similar results to null-hypothesis significance testing (NHST) methods, due to the limited number of predictors (Castilho & Prado, 2021).
We assessed the fit of the most parsimonious models with residual diagnostic plots from package DHARMa (Hartig, 2021). Because the top-ranked models showed signs of under-dispersion, we refitted the models with quasi-binomial error distribution. Furthermore, the top-ranked model explaining variation in ripe vs unripe fruit consumption included a two-way interaction between ‘Species’ and monthly FAI and thus we calculated regression coefficients and SEs for the simple slopes of FAI for the two levels of ‘Species’ (using function emtrends from package emmeans – Lenth et al., 2021). The top-ranked model explaining consumed vs dropped fruit also included a two-way interaction (between ‘Fruit Maturity’ and monthly FAI), and therefore we calculated pairwise comparisons between predicted marginal means of ‘Fruit Maturity’ levels (ripe/unripe) for low (mean FAI minus one SD), intermediate (mean FAI), and high (mean FAI plus one SD) FAI values.
Ethical Note
This research was non-invasive. The study conforms to the Code of Best Practices for Field Primatology for the Ethical Treatment of Non-Human Primates (International Primatological Society). Additionally, our research complied with the conditions set by the National Parks and Conservation Service, Ministry of Agro Industry and Food Security (MOAFS) and was conducted under research permit No. RRRS1912359. The research was also approved by the Forestry Service, MOAFS in order to work on state land.
Data Availability
The datasets collected and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
Seasonality in Food Availability
We defined the high fruit availability period as February–June 2020, when monthly fruit availability index (FAI) scores were at least twice the SE (2110) above the mean (Fig. 2). The majority of stems along vegetation transects comprised invasive species (86%; Table S1) and invasive species accounted for the majority of the total FAI (70%) and FAI during the high fruit availability period (72%). Only three invasive species (F. indica, Litsea sp., and P. cattleyanum) accounted for the bulk of total FAI (63%; Table S1) and invasive FAI (90%). The native proportion of total FAI mostly comprised three native species (24% by Canarium paniculatum, P. mauritianum, and S. glomeratum; Table S1). Native species produced little fruit compared to invasives during our study period: only 15 out of 29 native species produced fruits (only eight developed ripe fruits), whereas eight out of ten invasive species produced fruits during our study period (seven with ripe fruits; Table S1). Additionally, only 45% of native stems produced fruits, whereas 73% of invasive stems produced fruits (Table S1). Development of ripe fruit coincided with the high fruit availability period: out of all plant species that produced ripe fruits, 86% developed ripe fruits in the high fruit availability period (Table S1).
Weighted monthly percentages of dietary scans long-tailed macaques (Macaca fascicularis) spent feeding on various food items (left y-axis) and the monthly fruit availability index (FAI) scores for all plant species (totalFAI), invasive plant species (invFAI), and native plant species (nativeFAI) (right axis) in Mt. Calebasses (Mauritius) from December 2019 until December 2020.
There was a clear peak and trough in FAI, due to the timing of fruiting of the disproportionally abundant F. indica, Litsea sp. and P. cattleyanum: F. indica fruited during the period December 2019–September 2020, Litsea sp. during December 2019–May 2020, and P. cattleyanum during December 2019–October 2020 (Table S1). Several native plant species remained available during the low fruit availability period (Table S1), but they contributed little to total FAI (~10%). Overall, native FAI was more stable than invasive FAI, showing a less clear peak than invasive FAI and a slower decline during the low fruit availability period (Fig. 2). The monthly flower availability index (FlAI) also showed a clear peak and trough during our study period (67 in June 2020 to 42,543 in October 2020). Invasive species also accounted for the majority of total FlAI (81%), mostly made up of only two species: Litsea sp. and S. jambos (93% of invasive FlAI). The pine cone availability index scores (PcAI) ranged from 20,655 in March 2020 to 9914 in August 2020, indicating that pine cone was a less variable resource compared to flowers and fruits.
Effects of Food Availability on Diet
The macaques spent the largest percentage of activity scans feeding (37%). The majority of these dietary scans was made up of fruit (40.4%), followed by pine cones (26.3%), leaves (15.5%), invertebrates (6.1%), flowers (4.1%), and crops (2.7%; Fig. 2). Pine cones were only consumed when unripe. Other food items included tree bark (0.5%), grasses (0.3%), and eggs (0.2%). A small proportion of the food items could not be identified during field observation due to poor visibility (3.9%). The percentage of dietary scans that macaques fed on fruit was strongly positively correlated with FAI (Table I; Fig. 2). We also found moderately strong negative correlations between FAI and the percentage of dietary scans that macaques fed on crops, flowers, and invertebrates (Table I), indicating that macaques increasingly fed on fruits as fruit became more abundant, whereas it increasingly fed on crops, flowers, and invertebrates as fruit became scarcer. We did not find statistically significant correlations between the percentages of time macaques fed on other food items and FAI, FlAI, and PcAI (Table I).
Macaques consumed plant parts of 36 plant species and the fruits of 17 plant species (Table S2). Feeding on invasive species comprised the majority of dietary scans when macaques fed on both all plant foods (98%) and fruit specifically (97%; Table S2). Of the dietary scans when macaques fed on fruit, the majority consisted of invasive Litsea sp. (40.2%) and P. cattleyanum (39.3%, Table S2), followed by invasive F. indica (9.5%), and non-native Mangifera indica (4.9%). Out of the small percentage of fruit feeding scans when macaques fed on native fruits (3%), E. pyxidata made up the largest percentage (24.1%), followed by F. rubra (20.7%) and F. mauritiana (13.8%). The availability of invasive (invasive FAI) and native fruits (native FAI) peaked during the same period (February–July 2020) and invasive FAI was strongly positively correlated with the percentage of dietary scans when macaques fed on invasive fruits, whereas we did not find statistically significant correlations between invasive FAI and consumption of native fruit, or native FAI and consumption of invasive and native fruits (Table I). The plant species with the highest fruit preference index (FPIi) scores (> 1) were all invasive: Litsea sp. (4.0), S. jambos (1.2), and P. cattleyanum (1.1). All other plant species had FPIi scores ≤ 0.8 (Table S2), indicating that macaques only preferred fruits of invasive plant species, especially Litsea sp., over other fruits during the period that they were available.
Effects of Fruit Availability on Home Range
Our study group had a total home range of 34.9 ha, mean monthly home range of 14.5 ± SD 5.8 ha (range: 6.3–28.4 ha) and mean monthly core area size of 3.2 ± SD 1.7 ha (range: 1.2–6.8 ha) (Fig. 3). During the high fruit availability period (February–June 2020), the mean monthly home range was 10.6 ± SD 3.0 ha and the mean monthly core area size 2.2 ± SD 0.7 ha. Outside of the high fruit availability period, mean monthly home range was 17.8 ± SD 5.6 ha and the mean monthly core area size 3.95 ± SD 2.0 ha. Monthly home range and FAI were significantly negatively correlated (|r| = -0.65, Padjusted = 0.037, CI low = −0.96, CI up = −0.26), indicating that home range increased as fruit became less abundant. Core area size and FAI were negatively associated, but not statistically significant (|r| = -0.41, Padjusted = 0.21, CI low = −0.85, CI up = 0.15).
Monthly home ranges of long-tailed macaques (Macaca fascicularis) in Mt. Calebasses (Mauritius) from December 2019 until December 2020, represented by the 95% kernel density estimate (KDE). Core areas are represented by the 50% KDE. Forest Grade 2–3 consists of at least ≥ 50% but ≤ 75% native plant cover.
Seed Dispersal Effectiveness
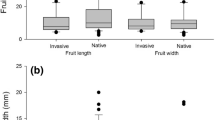
We quantified fruit consumption and drop for a total of 114 feeding events (total feeding time = 14 hours) spread across 39 days from December 2019 until December 2020 (mean = 11 ± SD 9 events per month). The majority of fruits consumed and dropped by macaques were invasive (105 out of 114 total feeding events), and we mainly recorded consumption and drop of native fruits outside of the high fruit availability period (seven out of nine native fruit feeding events). Overall, macaques consumed more ripe than unripe fruits (61% ripe), but dropped less ripe than unripe fruits intact (20% ripe, Table II). Out of all the ripe fruits macaques handled, macaques consumed (94%) and dropped (73%) the majority during the high fruit availability period (February–June 2020). The percentages of ripe vs unripe fruits that macaques consumed were much smaller for native species (2%) than for invasive species (72%, Table II). Macaques consumed ripe fruits of only one native species (Table II). The percentages of ripe vs unripe fruits consumed by macaques were largest for invasive F. indica (95%), P. cattleyanum (84%) and Litsea sp. (74%). Combined, these three species made up 98% of ripe fruits consumed by macaques. Moreover, the most common types of fruit handling by macaques during the feeding events were: partly eating the fruit pulp and discarding the rest, plucking and dropping intact fruits, and destroying seeds (Table II). We only recorded seed spitting for invasive Litsea sp. (Table II), and we only observed seed destruction when fruits were unripe. Additionally, macaques consumed less fruit than it dropped overall (29%), but consumption vs drop varied greatly between species (mean = 50 ± SD 31%, range = 0–100%; Table II). Fruit consumption vs drop by macaques was especially low for Citrus sp., F. indica, F. reflexa, F. rubra, and Litsea sp. (< 40%, Table II), indicating that the long-tailed macaque is a wasteful consumer for these species. For other species, macaques consumed large percentages of fruit, especially E. pyxidata and F. mauritiana, (> 75%, Table II).
The top-ranked GLM explaining the proportion of ripe vs unripe fruits consumed by macaques contained a two-way interaction between FAI and ‘Species’ (Δ AICc ≤ 2, Table III), indicating a strong positive effect of FAI on the proportions of ripe vs unripe fruits of the main invasive species (F. indica, Litsea sp., and P. cattleyanum) consumed by macaques (Fig. 4; Table S3). The inclusion of the two-way interaction also indicated that FAI had a weak negative effect on the proportions of ripe vs unripe fruits of other plant species consumed by macaques, but the high SE relative to the regression coefficient indicated that this estimate was highly imprecise (Fig. 4; Table S3). The summed predictors weights for each predictor and interaction included in the top-ranked model were high (> 0.85; Table S4), indicating that it is highly likely these predictors are part of the best model. Therefore, our findings suggested macaques only increasingly consumed ripe fruits of F. indica, Litsea sp., and P. cattleyanum, and not for other plant species as fruit became more abundant.
a Predicted means and 95% confidence intervals for the proportion of fruits consumed (cons.) vs dropped (drop.) by long-tailed macaques (Macaca fascicularis) per feeding bout in relation to ‘Species’ (‘main inv. plants’ = invasive Flacourtia indica, Litsea sp., and Psidium cattleyanum ; ‘other’ = other native and invasive plant species) as estimated by the most parsimonious binomial generalized linear model (GLM) b Line of best fit with 95% confidence intervals (indicated by dashed lines) estimated by the most parsimonious binomial GLM expressing the relationship between monthly fruit availability index (FAI) scores and the proportion of ripe vs unripe fruits consumed by macaques per feeding bout for ‘main inv. plants’ and ‘other’. c Predicted marginal means and 95% confidence intervals for the proportion of fruits consumed vs dropped by macaques per feeding event in relation to ‘Fruit Maturity’ (ripe/unripe) for low (FAI: −1 SD), intermediate (FAI: mean) and high (FAI: +1 SD) FAI scores as estimated by the most parsimonious GLM. We collected the data used in these figures during December 2019–December 2020 in Mt. Calebasses, Mauritius.
The top-ranked model explaining the proportion of fruits consumed vs dropped by macaques included predictor ‘Species’ and a two-way interaction between FAI and ‘Fruit Maturity’. There were two more models with ΔAICc ≤ 2, but these were more complex versions of the top-ranked model and thus we discarded them (Table III). The top-ranked model indicated that macaques dropped a significantly bigger proportion of fruit for F. indica, Litsea sp., and P. cattleyanum than other plant species (Fig. 4; Table S3) and that macaques dropped a higher proportion of unripe than ripe fruits during times of intermediate and high fruit availability and not during low fruit availability (Fig. 4; Table S3). The summed predictors weights for each predictor and interaction in the top-ranked model were high (> 0.95), whereas summed weights for interactions not included in the top-ranked model were low (< 0.5; Table S4). The regression coefficients and SEs for all predictors are summarized in Table S3.
Discussion
Seasonal Patterns in Diet Composition
Fruit was the most dominant component of our study group’s diet, similar to previous studies in Mauritius (Sussman et al., 2011). Additionally, we found that macaques increasingly consumed ripe fruits as these became more abundant, contrary to flowers, invertebrates, crops, and unripe fruit, supporting our hypotheses. Therefore, our findings suggest long-tailed macaques in Mauritius have a similar dietary response to seasonal variation in fruit availability as their counterpart in South-East Asia (Lucas & Corlett, 1991; Ruslin et al., 2019; Sha & Hanya, 2013; Yeager, 1996). Macaques probably preferred ripe fruits over unripe fruits, because both unripe and ripe fruits were available during high fruit availability. Macaques may also prefer ripe fruits over flowers and invertebrates: we did not find a relationship between flower consumption and flower availability, flowers were available during the high fruit availability period and we assume that invertebrates were available year-round. Therefore, flowers, unripe fruits, and invertebrates were probably ‘fallback’ foods for macaques during our study period.
After fruit, macaques spent most time feeding on unripe pine cones of P. elliottii, especially at the start of the high fruit availability period. One of the two birth peaks in Mauritian long-tailed macaques coincides with the high fruit availability period (March) (Jamieson, 1998). During pregnancy and lactation, female primates require a lot of protein (McCabe & Ferdigan, 2007), and unripe pine seeds are probably a better source of protein than ripe fruit pulp (White, 2011). Additionally, pine cones of Pinus spp. are high in condensed tannins (Eberhardt & Young, 1994), which females in some primate species increasingly consume during the birth season as a form of self-medication (Carrai et al., 2003), because their immune system may be weakened due to increased allocation of protein to reproductive vs immune functions (Houdijk et al., 2001). However, pine cones are also high in cellulose and should be more difficult to digest and thus less nutritious than fleshy fruit for macaques, because macaques are hindgut-fermenters (Chivers, 1994). Nevertheless, unripe pine cones may be an important source of protein/tannins for macaque females, but additional research is required to confirm this, as we did not distinguish between males and females during feeding bouts.
Seasonal Ranging Patterns
Ranging patterns were also driven by fruit availability: home range increased as fruit availability decreased, aligning with our predictions. Most species that fruited during the low fruit availability period also occurred at low densities, probably forcing the macaques to range further. This increase in home range was also accompanied by a group migration to the orchards in October 2020. Ranging patterns and dietary patterns are often on an annual cycle for Macaca spp. (Hanya et al., 2003), and macaque females are usually philopatric (Noordwijk & Van Schaik, 1999). Therefore, we consider the ranging behaviour in October 2020 a group migration or ‘expedition’ (Hanya et al., 2002). From October until December 2020, the group spent most of its time feeding on mango (M. indica) on the forest edges and in the adjacent mango orchards. Mangifera indica was abundant near the forest edges that bordered the mango orchards, and very rare in the area that the group used prior to October 2020 (also not present on the vegetation transects). Therefore, the increase in home range enabled the group to access a new fruit source and maintain a relatively high proportion of fruit in their diet, despite low fruit availability within their original home range, indicating that macaques used energy-maximizing strategies.
The availability of commercial orchard fruits, such as mango (M. indica) and lychee (Litchi chinensis), may also have influenced patterns in diet composition and home range. Crops were increasingly consumed as forest fruit became less abundant, and the start of the fruiting season of the main orchard fruits (M. indica and L. chinensis) coincides with the end of the low fruit availability period (September–October). Anthropogenic foods, such as crops, may offer energetic/nutritional advantages to primates compared to wild fruits (Riley et al., 2013; Strum, 2010), and the accessibility of these foods typically influences foraging patterns of primates (Cancelliere et al. 2018), including Macaca spp. (Gazagne, José-Domínguez, et al., 2020b; Sha & Hanya, 2013b).
The ongoing capture of macaques at the forest edges near the orchards may be another important factor that can explain the observed ranging patterns. Macaques in Mauritius are captured year-round by various local monkey breeders that export the animals for medical research and by locals that are encouraged to capture and sell macaques to the monkey breeders. We were informed by orchard farmers from Vallée du Paradis that macaques were captured year-round in this area. We confirmed this, because we found several cage traps with minor rust stains near the new sleeping site of our group. As far as we are aware, nobody carried out capture/trapping activities when our group moved into this area, but capture by humans may have potentially dissolved a group that occupied the area before our group (Sugiyama & Ohsawa, 1982). This may have provided our study group with an opportunity to range outside of their original home range in October 2020. For example, food scarcity, macaque capture by humans, and/or lack of adjacent groups often drive group migrations in Japanese macaques (Macaca fuscata) that occupy habitat heavily modified by humans (Hanya et al., 2002; Sugiyama & Ohsawa, 1982).
Foraging Strategies and Seed Dispersal Effectiveness
Macaques predominantly fed on the fruits of three invasive species (F. indica, Litsea sp., and P. cattleyanum, all highly invasive) and increasingly fed on ripe vs unripe fruits of these species as fruit became more abundant. These three invasive species also made up nearly all ripe fruit consumed by macaques, agreeing with our prediction that macaques mainly feeds on ripe invasive vs native fruits. In contrast, the vast majority of native fruits consumed by macaques were unripe, aligning with previous studies (Baider & Florens, 2006; Krivek, 2017; Reinegger et al., 2021). Four native species (E. pyxidata, F. mauritiana, N. broomeana, S. dupontii) were even exclusively consumed when unripe. Even though in some cases fruit does not have to be ripe in order for seeds to germinate (Cruz-Tejada et al., 2018), generally ripeness of a fruit is an indicator for seed germination success (Sumner & Mollon, 2000). Therefore, our findings indicate macaques may potentially be a poor seed disperser for many native plants and a major seed disperser for three of the most invasive woody plants in Mauritius: F. indica, Litsea sp., and P. cattleyanum.
We also found differences in fruit-handling behaviour between native and invasive plants, indicating additional differences in SDE between invasive and native plants. We only recorded seed spitting for invasive Litsea sp., which is considered an effective seed dispersal mechanism (Gross-Camp & Kaplin, 2011), and we often found mature seeds of invasive F. indica and P. cattleyanum in the faeces of macaques (RR pers. obs.). In contrast, macaques typically destroyed the soft seeds within unripe native fruits, indicating that macaques may mostly act as seed predator instead of seed disperser for native species. However, as macaques consumed most native species during the low fruit availability period when home ranges were larger, there is potential for higher SDE, as the frequency of long-distance dispersal events may be higher (Chapman & Russo, 2006). Nevertheless, the negative effects of exclusive consumption of unripe fruit and/or seed predation may largely outweigh the benefits of larger dispersal distances for the plants consumed.
The macaques probably further enhance differences in SDE between invasive and native plants by discarding more fruit when foraging on unripe fruit. In line with our predictions, macaques rejected more unripe than ripe fruit when fruit was abundant. Conversely, during low fruit availability, there was no difference in rejection by macaques between ripe and unripe fruit. According to optimal foraging theory, primates are expected to increasingly reject fruits of lower quality when the chances of obtaining higher quality fruits increase during high fruit availability, and may thus become increasingly ‘choosy’ (Janson, 1996). Consequently, (unripe) native fruits handled by macaques have a high chance of being rejected, probably leading to dispersal failure. However, we also found that macaques rejected significantly more fruit of F. indica, Litsea sp., and P. cattleyanum (especially F. indica and Litsea sp.) than other species, presumably because these species have the largest fruit crops out of all the species macaques ate, giving the macaques more fruit to choose from. Nevertheless, unlike native species, macaques compensate for wasted fruit of these invasive species by consuming more ripe fruit when fruit availability is high, and seed dispersal limitation is probably also largely avoided due to large fruit crops.
Factors Explaining Observed Foraging Strategies and SDE
The general preference for invasive fruits can partly explain the observed foraging patterns of macaques and the resulting differences in SDE between native and invasive plants: macaques disproportionally selected three invasive plant species relative to their availability (Si – scores > 1: Litsea sp., S. jambos, and P. cattleyanum), suggesting that these species were preferred. The availability of Litsea sp. may be particularly important in explaining foraging patterns of the macaques, because its preference index score was nearly 4 times higher than the other two preferred species.
Morphological traits may partly explain preference for these fruits. All three preferred species have juicy pulp and external covers that are not difficult to pierce: traits preferred by some Macaca spp. (Sengupta & Radhakrishna, 2015). However, non-preferred native species that fruited simultaneously also possess these traits (e.g., E. pyxidata and P. mauritianum). Only P. cattleyanum appears to really stand out from the native fruits in our study site morphologically: the ripe fruits are very sweet, slightly acidic, and bright red/yellow. The long-tailed macaque is known to prefer juicy, acidic, and sweet fruits (Ungar, 1995) and primates are known to select bright yellow, orange, and sometimes red colours (Gautier-Hion et al., 1985; Skalníkova et al., 2020; Terborgh, 1983), attributed to their trichromatic vision (Mollon, 1991). Litsea sp. and S. jambos do not possess these traits, but the long-tailed macaque also consumes L. glutinosa in its native range (Azzahra, 2017), and Macaca spp. generally prefer food plants in the genera Litsea and Syzygium (Sengupta et al., 2020). Fruit nutrient and energy content analysis may provide insights into why these species are preferred over native fruits by long-tailed macaques in Mauritius. For example, the high lipid, protein, and energy content of invasive plants (including Litsea sp.) largely explains the preference of native frugivores for invasive over native fruits in the Seychelles (Kueffer et al., 2009), preference for invasive over native fruits by frugivores in California (Vilá & D’Antonio, 1998), and the attractiveness of cultivated cacao to Tonkean macaques (Macaca tonkeana) in Indonesia (Riley et al., 2013).
Imbalance in availability between invasive and native fruits may be another important factor in explaining the observed differences in consumption of ripe vs unripe invasive and native fruits. The fruiting success of the relatively few invasive species in our study area was greater than of the much greater number of native species. Invasive species also occurred at much higher densities than native species. Only three invasive species (F. indica, Litsea sp. and P. cattleyanum) accounted for the bulk of all fruit combined, and there was high overlap between their fruiting periods, resulting in a clear peak and trough in fruit availability. Even though the fruits of several native species remained available during the low fruit availability period, they were scarce and contributed little to total FAI. Development of ripe fruit of most species coincided with the high fruit availability period (February–June 2020), and macaques consumed the vast majority of ripe fruit during the high fruit availability period as a result. Macaques mostly consumed native fruits outside of the high fruit availability period, partly explaining why macaques consumed these almost exclusively when unripe. Nevertheless, the high preference index score of some invasive species, especially Litsea sp., indicates that macaques may disproportionally feed on the ripe fruits of these species regardless of high relative abundance compared to native species.
To some extent, the adaptations of many large-seeded native canopy trees to seed dispersal by fruit bats (e.g., Pteropus niger) may also explain the consumption of primarily unripe native fruits. The ripe fruits of these trees predominantly have inconspicuous colours (green/ brown) and strong odours (Nyhagen, 2004). Just like other primates, the long-tailed macaque probably depends on its trichromatic vision to detect and select ripe fruits (Onstein et al., 2020; Skalníková et al. 2020), which is not useful when ripe fruits remains brown or green. Primates also use olfactory cues to detect ripe fruit (Nevo & Valenta, 2018), but their sense of smell may be less developed than that of fruit bats (e.g., more suitable for short distances) (Nevo & Heymann, 2015). Additionally, the odour of ripe native fruits in Mauritius may be less recognizable for macaques than they are for the endemic fruit bats, as the specific bouquet of volatile organic compounds (VOCs) emitted by fruits to signal ripeness can vary geographically and between plants that rely on different seed disperser guilds (Nevo & Valenta, 2018). Therefore, the long-tailed macaque may have difficulty selecting ripe native fruits in Mauritius.
Implications for Conservation and Future Directions
The contrasting SDE of macaques for invasive and native plant species as a result of their foraging strategies may promote plant invasion: regeneration of invasive plants may be enhanced, whereas regeneration of native plants may be halted. Additionally, waste and consumption of unripe native fruits may further halt native plant regeneration by making fruit unavailable to endemic seed dispersers that mostly consume ripe native fruits, such as the endemic bat P. niger (Reinegger et al., 2021). We argue the macaques have similar impacts elsewhere on the island, because the imbalance in fruit production is a common phenomenon across all remnant forests. Invasive plants, such as F. indica, Litsea sp., and P. cattleyanum, dominate native remnant forests across the island (Florens et al., 2016; Florens et al., 2017; Strahm, 1993), where they inhibit growth (Florens, 2008) and flower/fruit production of native trees (Krivek et al., 2020; Monty et al., 2013).
To confirm that foraging strategies of the macaques promote plant invasion, it is necessary to assess all aspects of SDE. Ideally, SDE is measured as the number of new adult plants produced by dispersal activities of an animal (Schupp et al., 2010). To confirm that the observed patterns in fruit consumption lead to disproportional recruitment of invasive plants, it would be necessary to also quantify seeds dispersed through faeces and carry out seed germination/seedling recruitment experiments. Moreover, it would be valuable to monitor seedling recruitment before and after macaque exclusion to better understand SDE of macaques and how macaques induce changes in vegetation composition. Complete exclusion is not realistic on a large scale, but local control of macaques in isolated forest fragments that are poorly connected to other forest patches may be feasible with assistance from local monkey breeding companies.
To better understand the potential contribution of the macaques to plant invasion at an island scale, it is necessary to conduct a population census. The last census was conducted in the 1980s and it is currently unknown how large the macaque population is or how densities vary between habitats and regions. Population growth has probably been limited by capture and export to some extent (Sussman et al., 2011), but the population may still be as large as 40,000 individuals or higher (Bertram, 1994). Reports of macaques moving into urban areas and harassing residents have become common (L’Express, 2017, 2020a, 2020b), suggesting that insufficient suitable forest habitat is available. Therefore, it is essential to estimate the population size to develop a deeper understanding of the magnitude of the potential impacts of macaques on plant communities at an island scale. Nevertheless, our study was an essential first step in understanding the effects of the macaques on native plant communities.
Conclusion
Here, we presented an example of how foraging patterns of an invasive primate may promote plant invasion, due to imbalance in availability between invasive and native fruits and general preference for invasive fruits. Our example may be uncommon among invasive macaques and other invasive primates, because there are few regions with invasive primates where plant invasion has progressed to the point where invasive plants have largely replaced native plants (e.g., Angaur; Kemp & Burnett, 2003). However, the growing anthropogenic pressure on insular habitats (Kueffer & Kinney, 2017) and the resulting continued spread of invasive plants (Seebens et al., 2015) may increase the potential for negative impacts of invasive primates on islands. Therefore, similar differences in SDE of invasive primates between native and invasive plants may be expected on islands elsewhere. Nevertheless, there are still only a few examples of impacts of invasive primates as seed dispersers, and further research is required to make valid generalizations.
References
Albert, A., Huynen, M.-C., Savini, T., & Hambuckers, A. (2013a). Influence of food resources on the ranging patterns of Northern pig-tailed macaques (Macaca leonina). International Journal of Primatology, 34, 696–713. https://doi.org/10.1007/s10764-013-9690-z.
Albert, A., Savini, T., & Huynen, M.–C. (2013b). The role of Macaca spp. (Primates: Cercopithecidae) in seed dispersal networks. The Raffles Bulletin of Zoology, 61, 423–434
Altmann, J. (1974). Observational study of behaviour: sampling methods. Behaviour, 49(227), 267.
Azzahra, B. W. (2017). Potential feeding plants of long-tail monkeys (Macaca fascicularis Raffles) in Suranadi Natural Tourism Park, West Lombok. B.Sc. thesis. Mataram City, Indonesia: Mataram University
Baider, C. and Florens, F. B. V. (2006). Current decline of the ‘Dodo-tree’: a case of broken-down interactions with extinct species or the result of new interactions with alien invaders? In W. F. Laurance & C. A. Peres (Eds.), Emerging threats to tropical forests (pp. 199–214). : University of Chicago Press
Brotcorne, F. (2014). Behavioral ecology of commensal long-tailed macaque (Macaca fascicularis) populations in Bali, Indonesia: impact of anthropic factors. Ph.D. thesis. Liège, Belgium: University of Liège
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B, 57(289), 300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
Bertram, B. (1994). Monkeys in Mauritius: potential for humane control. London UK: Conservation and Consultancy Division, Zoological Society of London
Bitani, N., Ehlers Smith, D. A., Ehlers Smith, Y. C., & Downs, C. T. (2020). Functional traits vary among fleshy-fruited invasive plant species and their potential avian dispersers. Acta Oecologic, 108, 103651. https://doi.org/10.1016/j.actao.2020.103651.
Burnham, K. P., Anderson, D. R., & Huyvaert, K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology, 65, 23–35. https://doi.org/10.1007/s00265-010-1029-6.
Calabrese, J. M., Fleming, C. H., & Gurarie, E. (2016). Ctmm: an R package for analyzing animal relocation data as a continuous-time stochastic process. Methods in Ecology and Evolution, 7(1124), 1132. https://doi.org/10.1111/2041-210X.12559.
Calenge, C. (2021). adehabitatHR, version 0.4.19. R package. https://cran.r-project.org/. Accessed 12 December 2021
Cancelliere, E. C., Chapman, C. A., Twinomugisha, D., & Rothman, J. M. (2018). The nutritional value of feeding on crops: Diets of vervet monkeys in a humanized landscape. African Journal of Ecology, 56, 160–167. https://doi.org/10.1111/aje.12496
Carrai, V., Borgognini-Tarli, S. M., Huffman, M. A., & Bardi, M. (2003). Increase in tannin consumption by sifaka (Propithecus verreauxi verreauxi) females during the birth season: a case for self-medication in prosimians? Primates, 44(61), 66. https://doi.org/10.1007/s10329-002-0008-6.
Castilho, L. B., & Prado, P. I. (2021). Towards a pragmatic use of statistics in ecology. PeerJ, 9, e12090. https://doi.org/10.7717/peerj.12090.
Chapman, C. A. & Russo, S. E. (2006). Primate seed dispersal: linking behavioral ecology with forest community structure. In C. J. Campbell, A. Fuentes, K. C. MacKinnon, M. Panger & K. Bearder (Eds.), Primates in perspective (pp. 510–525 ). Oxford, United Kingdom: Oxford University Press
Cheke, A. S. (1987). An ecological history of the Mascarene Islands, with particular reference to extinctions and introductions of land vertebrates. In A. W. Diamond (Ed.) Studies of Mascarene Island birds (pp. 5–89). Cambridge UK: Cambridge University Press https://doi.org/10.1017/CBO9780511735769.003
Chimera, C. G., & Drake, D. (2010). Patterns of seed dispersal and dispersal failure in a Hawaiian dry forest having only introduced birds. Biotropica, 42(493), 502. https://doi.org/10.1111/j.1744-7429.2009.00610.x.
Chivers, D. J. (1994). Functional anatomy of the gastrointestinal tract. In A. Davies & J. Oates (Eds.), Colobine monkeys: their ecology, behaviour and evolution (pp. 205–227). Cambridge University Press.
Cheke, A., & Hume, J. (2008). Lost land of the dodo: an ecological history of the Mascarene Islands. T and AD Poyser.
Crestani, A. C., Mello, M. A. R., & Cazetta, E. (2019). Interindividual variations in plant and fruit traits affect the structure of a plant–frugivore network. Acta Oecologica, 95(120), 127. https://doi.org/10.1016/j.actao.2018.11.003.
Cruz-Tejada, D. M., Acosta-Rojas, D. C., & Stevenson, P. R. (2018). Are seeds able to germinate before fruit color ripening? Evidence from six Neotropical bird-dispersed plant species. Ecosphere, 9, e02174. https://doi.org/10.1002/ecs2.2174.
Denslow, J. S. (2003). Weeds in paradise: thoughts on the invisibility of tropical islands. Annals of the Missouri Botanical Garden, 90(119), 127. https://doi.org/10.2307/3298531.
Douma, J. C., & Weedon, J. T. (2019). Analysing continuous proportions in ecology and evolution: a practical introduction to beta and Dirichlet regression. Methods in Ecology and Evolution, 10(1412), 1430. https://doi.org/10.1111/2041-210X.13234.
Eberhardt, T. L., & Young, R. A. (1994). Conifer seed cone proanthocyanidin polymers: characterization by 13C NMR spectroscopy and determination of antifungal activities. Journal of Agricultural and Food Chemistry, 42(1704), 1708. https://doi.org/10.1021/jf00044a023.
Eudey, A., Ang, A. & Ong, P. (2020). Macaca fascicularis ssp. fascicularis. The IUCN Red List of Threatened Species 2020: e.T39768A17985511. https://doi.org/10.2305/IUCN.UK.2020-3.RLTS.T39768A17985511.en. Accessed on 29 December 2021.
Fleming, C. H. (2021). Ctmm, version 0.6.1. R package. https://cran.r-project.org/. Accessed 27 October 2021
Fleming, C. H., Fagan, W. F., Mueller, T., Olson, K. A., Leimgruber, P., & Calabrese, J. M. (2015). Rigorous home range estimation with movement data: a new autocorrelated kernel density estimator. Ecology, 96(1182), 1188. https://doi.org/10.1890/14-2010.1.
Florens, F. B. V. (2008). Ecologie des forêts tropicales de l’Ile Maurice et impact des espèces introduites envahissantes. PhD thesis. Réunion, France : Université de la Réunion
Florens, F. B. V., Baider, C., Martin, G. M. N., Seegoolam, N. B., Zmanay, Z. & Strasberg, D. (2016). Invasive alien plants progress to dominate protected and best-preserved wet forests of an oceanic island. Journal for Nature Conservation, 34, 93–100. Réunion, France https://doi.org/10.1016/j.jnc.2016.09.006
Florens, F. B. V., Baider, C., Seegoolam, N. B., Zmanay, Z., & Strasberg, D. (2017). Long-term declines of native trees in an oceanic island's tropical forests invaded by alien plants. Applied Vegetation Science, 20, 94–105. https://doi.org/10.1111/avsc.12273.
Garber, P. A. (1987). Foraging strategies among living primates. Annual Review of Anthropology, 16(339), 364. https://doi.org/10.1146/annurev.an.16.100187.002011.
Gautier-Hion, A., Duplantier, J. – M., Quris, R., Feer, F., Sourd, C., Decoux, J.-P., Dubost, G., Emmons, L., Erard, C., Hecketsweiler, P., Moungazi, A., Roussilhon, C. & Thiollay, J.-M. (1985). Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia, 65, 324–337. https://doi.org/https://doi.org/10.1007/BF00378906
Gazagne, E., Hambuckers, A., Savini, T., Poncin, P., Huynen, M.-C., & Brotcorne, F. (2020a). Toward a better understanding of habituation process to human observer: a statistical approach in Macaca leonina (Primates: Cercopithecidea). Raffles Bulletin of Zoology, 68, 735–749. https://doi.org/10.26107/RBZ-2020-0085.
Gazagne, E., José-Domínguez, J. M., Huynen, M. C., Hambuckers, A., Poncin, P., Savini, T., & Brotcorne, F. (2020b). Northern pigtailed macaques rely on old growth plantations to offset low fruit availability in a degraded forest fragment. American Journal of Primatology, 80, e23117. https://doi.org/. https://doi.org/10.1002/ajp.23117.
Gleditsch, J. M., Hruska, A. M., & Foster, J. T. (2017). Connecting resource tracking by frugivores to temporal variation in seed dispersal networks. Frontiers in Ecology and Evolution, 5, 98. https://doi.org/. https://doi.org/10.3389/fevo.2017.00098.
Global Invasive Species Database (GISD). (2019). 100 of the world’s worst invasive alien species. Available at: http://www.iucngisd.org/gisd/100_worst.php. Accessed 1 November 2019
Gross-Camp, N., & Kaplin, B. A. (2011). Differential seed handling by two African primates affects: Seed fate and establishment of large-seeded trees. Acta Oecologica, 37, 578–586. https://doi.org/10.1016/j.actao.2011.04.003.
Gumert, M. D. (2011). The common monkey of Southeast Asia: Long-tailed macaque populations, ethnophoresy, and their occurrence in human environments. In M. D. Gumert, A. Fuentes, & L. Jones-Engel (Eds.), Monkeys on the edge: ecology and management of long-tailed macaques and their interface with humans (pp. 3–44). Cambridge University Press. https://doi.org/10.1017/CBO9780511974434.003.
Hall, K. R. L. (1962). Numerical data, maintenance activities and locomotion of the wild chacma baboon, Papio ursinus. Journal of Zoology, 139, 181–220. https://doi.org/10.1111/j.1469-7998.1962.tb01827.x.
Hammond, D. S., Gond, V., Baider, C., Florens, F. B. V., Persand, S., & Laurance, S. G. W. (2015). Threats to environmentally sensitive areas from peri-urban expansion in Mauritius. Environmental Conservation, 42(256), 267. https://doi.org/10.1017/S0376892914000411.
Hanya, G., Yamada, H., & Arakane, T. (2002). Expeditionary ranging by a Japanese macaque troop in Hieizan. Anthropological Science, 110(415), 420. https://doi.org/10.1537/ase.110.415.
Hanya, G., Noma, N., & Agetsuma, N. (2003). Altitudinal and seasonal variations in the diet of Japanese macaques in Yakushima. Primates, 44(51), 59. https://doi.org/10.1007/s10329-002-0007-7.
Hanya, G., Yoshihiro, S.-I., Hayaishi, S., & Takahata, Y. (2020). Ranging patterns of Japanese macaques in the coniferous forest of Yakushima: home range shift and travel rate. American Journal of Primatology, 82, e23185. https://doi.org/10.1002/ajp.23185.
Harrison, M. E., Vogel, E. R., Morrogh-Bernard, H. C., & Van Noordwijk, M. A. (2009). Methods for calculating activity budgets compared: a case study using orangutans. American Journal of Primatology, 71(353), 358. https://doi.org/10.1002/ajp.20655.
Harrison, M. J. S. (1984). Optimal foraging strategies in the diet of the green monkey, Cercopithicus sabaeus, at Mt. Assirik, Senegal. International Journal of Primatology, 5, 435–471. https://doi.org/10.1007/BF02692269.
Hartig, F. (2021). DHARMa, version 0.4.4. R package. https://cran.r-project.org/. Accessed 27 October 2021
Houdijk, J. G. M., Jessop, N. S., & Kyriazakis, I. (2001). Nutrient partitioning between reproductive and immune functions in animals. Proceedings of the Nutrition Society, 60(515), 525. https://doi.org/. https://doi.org/10.1079/pns2001114.
Jamieson, R. W. (1998). The effects of seasonal variation in fruit availability on social and foraging behaviour in Macaca fascicularis in Mauritius. PhD thesis. St. Louis, MO: Washington University
Janson, C. H. (1996). Towards an experimental socioecology of primates: examples from Argentine brown capuchin monkeys (Cebus apella nigritus). In M. A. Norconck, A. L. Rosenberger & P. A. Garber (Eds.), Adaptive radiations of Neotropical primates (pp. 309–325). Boston, MA: Springer. https://doi.org/10.1007/978-1-4419-8770-9
Jennions, M. D., & Møller, A. P. (2003). A survey of the statistical power of research in behavioral ecology and animal behavior. Behavioral Ecology, 14(438), 445. https://doi.org/10.1093/beheco/14.3.438.
Jones, H. P., Campbell, K. J., Burke, A. M., Baxter, G. S., Hanson, C. C., & Mittermeier, R. A. (2018). Introduced non-hominid primates impact biodiversity and livelihoods: management priorities. Biological Invasions, 20, 2329–2342. https://doi.org/10.1007/s10530-018-1704-5.
Kemp, N. J. & Burnett, J. B. (2003). A biodiversity risk assessment and recommendations for risk management of long-tailed macaques Macaca fascicularis in New Guinea. Washington, DC: Indo-Pacific Conservation Alliance. http://www.indopacific.org/wp-content/uploads/2017/02/papuamacaques-English-Version.pdf. Accessed 15 October 2021
Krivek, G. (2017). The influence of invasive plant control on the foraging habitat quality of the Mauritian flying fox Pteropus niger. M.Sc. thesis. As, Norway: Norges Miljo-og Biovitenskapelige Universitet
Krivek, G., Florens, F. B. V., Baider, C., Seegobin, V. O., & Haugaasen, T. (2020). Invasive alien plant control improves foraging habitat quality of a threatened island flying fox. Journal for Nature Conservation, 54, 125805. https://doi.org/10.1016/j.jnc.2020.125805.
Kueffer, C., Kronauer, L., & Edwards, P. J. (2009). Wider spectrum of fruit traits in invasive than native floras may increase the vulnerability of oceanic islands to plant invasions. Oikos, 118(1327), 1334. https://doi.org/10.1111/j.1600-0706.2009.17185.x.
Kueffer, C., & Kinney, K. (2017). What is the importance of islands to environmental conservation? Environmental Conservation, 44(311), 322. https://doi.org/10.1017/S0376892917000479.
Lambert, J. E. (2007). Seasonality, fallback strategies, and natural selection: a chimpanzee and Cercopithecoid model for interpreting the evolution of the hominin diet. Pp. 324 – 343 In P. S. Ungar (Ed.), Evolution of the human diet: the known, the unknown, and the unknowable (pp. 324–343). Oxford UK: Oxford University Press
Lehner, P. N. (1992). Sampling methods in behavior research. Poultry Science, 71(643), 649.
Lenth, R. V., Buerkner, B., Herve, M., Love, J., Miguez, F., Riebl, H. & Singmann, H. (2021). emmeans, version 1.7.1-1. R package. https://cran.r-project.org/web/packages/emmeans/index.html. Accessed 15 October 2021.
L’Express (2017). Récentes attaques: à cause des singes, les humains font la grimace. https://www.lexpress.mu/article/314014/recentes-attaques-cause-singes-humains-font-grimace. Accessed 27 October 2020
L’Express (2020a). Invasion de singes au Jardin Balfour: A malin, malin et demi. https://www.lexpress.mu/video/379125/invasion-singes-au-jardin-balfour-malin-malin-et-demi. Accessed 27 October 2020
L’Express (2020b). Beau-Bassin: les singes font la loi, les habitants font la grimace. https://www.lexpress.mu/article/383888/beau-bassin-singes-font-loi-habitants-font-grimace. Accessed 27 October 2020
López-Darias, D., & Nogales, M. (2008). Effects of the invasive Barbary ground squirrel (Atlantoxerus getulus) on seed dispersal systems of insular xeric environments. Journal of Arid Environments, 72(926), 939. https://doi.org/10.1016/j.jaridenv.2007.12.006.
Lucas, P. W., & Corlett, R. T. (1991). Relationship between the diet of Macaca fascicularis and forest phenology. Folia Primatologica, 57(201), 215. https://doi.org/10.1159/000156587.
MacArthur, R. H., & Pianka, E. R. (1966). An optimal use of a patchy environment. The American Naturalist, 100(609), 609. https://doi.org/10.1086/282454.
Manasse, R. S., & Howe, H. (1983). Competition for dispersal agents among tropical trees: influences of neighbours. Oecologia, 59(184), 190. https://doi.org/10.1007/BF00378836.
Marshall, A. J., Boyko, C. M., Feilen, K. L., Boyko, R. H., & Leighton, M. (2009). Defining fallback foods and assessing their importance in primate ecology and evolution. American Journal of Physical Anthropology, 140, 603–614. https://doi.org/10.1002/ajpa.21082.
Marshall, A. J., & Wrangham, R. W. (2007). Evolutionary consequences of fallback foods. International Journal of Primatology, 28, 1219. https://doi.org/10.1007/s10764-007-9218-5.
Martin-Albarracin, Nunez, M. A., & Amica, G. C. (2018). Non-redundancy in seed dispersal and germination by native and introduced frugivorous birds: implications of invasive bird impact on native plant communities. Biodiversity and Conservation, 27(3793), 3806. https://doi.org/10.1007/s10531-018-1629-4.
McCabe, G. M., & Ferdigan, L. M. (2007). Effects of reproductive status on energy intake, ingestion rates and dietary composition of female Cebus capucinus at Santa Rosa. Costa Rica. International Journal of Primatology, 28(837), 851. https://doi.org/10.1007/s10764-007-9159-z.
Mollon, J. D. (1991). Uses and evolutionary origins of primate colour vision. In J. R. Cronly-Dillon & R. L. Gregory (Eds.), Evolution of the eye and visual system (pp. 306–319). Macmillan.
Monty, M. L. F., Florens, F. B. V., & Baider, C. (2013). Invasive alien plants elicit reduced production of flowers and fruits in various native forest species on the tropical island of Mauritius (Mascarenes, Indian Ocean). Tropical Conservation Science, 6(35), 49. https://doi.org/10.1177/194008291300600107.
Nagy-Reis, M. B., & Setz, E. Z. F. (2017). Foraging strategies of black-fronted titi monkeys (Callicebus nigrifons) in relation to food availability in seasonal tropical forest. Primates, 58(149), 158. https://doi.org/10.1007/s10329-016-0556-9.
Nakagawa, S. (2004). A farewell to Bonferroni: the problems of low statistical power and publication bias. Behavioural Ecology, 15(1044), 1045. https://doi.org/10.1093/beheco/arh107.
Nevo, O., & Heymann, E. W. (2015). Led by the nose: olfaction in primate feeding ecology. Evolutionary Anthropology, 24(137), 148. https://doi.org/10.1002/evan.21458.
Nevo, O., & Valenta, K. (2018). The ecology and evolution of fruit odor: implications for primate seed dispersal. International Journal of Primatology, 39(338), 355. https://doi.org/10.1007/s10764-018-0021-2.
Noonan, M. J., Tucker, M. A., Fleming, C. H., Akre, T. S., Alberts, S. C., Ali, A. H., Altmann, J., Castro Antunes, P., Belant, J. L., Beyer, D., Blaum, N., Böhning-Gaese, K., Cullen Jr., L., et al (2019). A comprehensive analysis of autocorrelation and bias in home range estimation. Ecological Monographs, 89, e01344. https://doi.org/10.1002/ecm.1344.
Noordwijk, M. A., & Van Schaik, C. P. (1999). The effects of dominance rank and group size on female lifetime reproductive success in wild long-tailed macaques. Macaca fascicularis. Primates, 40(105), 30. https://doi.org/10.1007/BF02557705.
Nyhagen, D. F. (2004). A study of the bat–fruit syndrome on Mauritius. Indian Ocean. Phelsuma, 12(118), 125.
Oliveira-Silva, L. R. B., Campelo, A. C., Lima, I. M. S., Araújo, A. C. L., Bezerra, B. M., & Souza-Alves, J. P. (2018). Can a non-native primate be a potential seed disperser? A case study on Saimiri sciureus in Pernambuco State. Brazil. Folia Primatologica, 89(138), 149. https://doi.org/10.1159/000486413.
Onstein, R. E., Vink, D. E., Veen, J., Barratt, C. D., Flantua, S. G. A., Wich, S. A., & Kissling, W. D. (2020). Palm fruit colours are linked to the broad-scale distribution and diversification of primate colour vision systems. Proceedings of the Royal Society B: Biological Sciences, 287, 20192731. https://doi.org/10.1098/rspb.2019.2731.
Ortiz-Pulido, R., Alborez-Barajas, Y. V., & Díaz, S. A. (2007). Fruit removal efficiency and success: influence of crop size in a neotropical treelet. Plant Ecology, 189(147), 154. https://doi.org/10.1007/s11258-006-9175-7.
Pike, N. (2011). Using false discovery rates for multiple comparisons in ecology and evolution. Methods in Ecology and Evolution, 2(278), 282. https://doi.org/10.1111/j.2041-210X.2010.00061.x.
Pyke, G. H. (1984). Optimal foraging theory: a critical review. Annual Review of Ecology and Systematics, 15(523), 575. https://doi.org/10.1146/annurev.es.15.110184.002515.
QGIS Development Team (2020). QGIS Geographic Information System, Open Source Geospatial Foundation Project, Version 3.4.15. http://qgis.osgeo.org. Accessed 16 February 2022
R Core Team (2020). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/. Accessed 15 October 2021
Reinegger, R. D. (2018). The feeding competition between the invasive crab-eating macaque Macaca fascicularis and the Mauritian flying fox Pteropus niger. The Rufford Foundation. https://ruffordorg.s3.amazonaws.com/media/project_reports/23082-1%20Detailed%20Final%20Report.pdf. Accessed 28 October 2021
Reinegger, R. D., Oleksy, R. Z., Bissessur, P., Naujeer, H., & Jones, G. (2021). First come, first served: fruit availability to keystone bat species is potentially reduced by invasive macaques. Journal of Mammalogy, 102(428), 439. https://doi.org/10.1093/jmammal/gyaa182.
Richards, S. A. (2008). Dealing with overdispersed count data in applied ecology. Journal of Applied Ecology, 45(218), 227. https://doi.org/10.1111/j.1365-2664.2007.01377.x.
Riley, E. P., Tolbert, B., & Farida, W. R. (2013). Nutritional content explains the attractiveness of cacao to crop raiding Tonkean macaques. Current Zoology, 59(160), 169. https://doi.org/10.1093/czoolo/59.2.160.
Rühmann, J., Soler, M., Pérez-Contreras, T., & Ibáñez-Álamo, J. D. (2019). Territoriality and variation in home range size through the entire annual range of migratory great spotted cuckoos (Clamator glandarius). Scientific Reports, 9, 6238. https://doi.org/10.1038/s41598-019-41943-2.
Ruslin, F., Matsuda, I., & Md-Zain, B. M. (2019). The feeding ecology and dietary overlap in two sympatric primate species, the long-tailed macaque (Macaca fascicularis) and dusky langur (Trachypithecus obscurus obscurus), in Malaysia. Primates, 60(41), 50. https://doi.org/10.1007/s10329-018-00705-w.
Schupp, E. W., Jordano, P., & Gómez, J. M. (2010). Seed dispersal effectiveness revisited: a conceptual review. New Phytologist, 188(333), 353. https://doi.org/. https://doi.org/10.1111/j.1469-8137.2010.03402.x.
Seaman, D. E., & Powell, R. A. (1996). An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology, 77(2075), 2085. https://doi.org/10.2307/2265701.
Seebens, H., Essl, F., Dawson, W., Fuentes, N., Moser, D., Pergl, J., Pyšek, P., Van Kleunen, M., Weber, E., Winter, M., & Blasius, B. (2015). Global trade will accelerate plant invasions in emerging economies under climate change. Global Change Biology, 21(4128), 4140. https://doi.org/10.1111/gcb.13021.
Sengupta, A., & Radhakrishna, S. (2015). Fruit trait preference in rhesus macaques (Macaca mulatta) and its implications for seed dispersal. International Journal of Primatology, 36(999), 1013. https://doi.org/10.1007/s10764-015-9869-6.
Sengupta, A., Gazagne, E., Albert-Daviaud, A., Tsuji, Y., & Radhakrishna, S. (2020). Reliability of macaques as seed dispersers. American Journal of Primatology, 82, e23115. https://doi.org/10.1002/ajp.23115.
Sha, J. C. M., & Hanya, G. (2013b). Temporal food resource correlates to the behavior and ecology of food-enhanced long-tailed macaques (Macaca fascicularis). Mammal Study, 38(163), 175. https://doi.org/10.3106/041.038.0305.
Signer, J., & Balkenhol, N. (2015). Reproducible home ranges (rhr): a new, user-friendly R package for analyses of wildlife telemetry data. Wildlife Society Bulletin, 39(358), 363. https://doi.org/10.1002/wsb.539.
Skalníkova, P., Frynta, D., Abramjan, A., Rokyta, R., & Nekovářová, T. (2020). Spontaneous color preferences in rhesus monkeys: what is the advantage of primate trichromacy? Behavioural Processes, 174, 104084. https://doi.org/10.1016/j.beproc.2020.104084.
Strahm, W. A. (1993). The conservation and restoration of the flora of Mauritius and Rodrigues. PhD thesis. University of Reading.
Strum, S. C. (2010). The development of primate raiding: implications for management and conservation. International Journal of Primatology, 31(133), 156. https://doi.org/10.1007/s10764-009-9387-5.
Sugiyama, Y., & Ohsawa, H. (1982). Population dynamics of Japanese macaques at Ryozenyama: III. Female desertion of the troop. Primates, 23(31), 44. https://doi.org/10.1007/BF02381436.
Sumner, P., & Mollon, J. D. (2000). Chromaticity as a signal of ripeness in fruits taken by primates. Journal of Experimental Biology, 203(987), 2000. https://doi.org/10.1242/jeb.203.13.1987.
Sussman, R. W., & Tattersall, I. (1986). Distribution, abundance and putative ecological strategy of Macaca fascicularis on the island of Mauritius. Southwestern Indian Ocean. Folia Primatologica, 46(28), 43. https://doi.org/10.1159/000156234.
Sussman, R. W., Schaffer, C. A. & Guidi, L. (2011). Macaca fascicularis in Mauritius: Implications for macaque-human interactions and for future research on long-tailed macaques. In M. Gumert, A. Fuentes, L. Jones-Engel, (Eds.), Monkeys on the edge: ecology and management of long-tailed macaques and their interface with humans (pp. 207–235). Cambridge UK: Cambridge University Press. https://doi.org/10.1017/CBO9780511974434.010
Symonds, M. R. E., & Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioural Ecology and Sociobiology, 65(13), 21. https://doi.org/10.1007/s00265-010-1037-6.
Terborgh, J. (1983). Five new world primates. Princeton University Press.
Traveset, A. (1998). Effect of seed passage through vertebrate frugivores’ guts on germination: a review. Perspectives in Plant Ecology, Evolution and Systematics, 1, 151–190. https://doi.org/10.1078/1433-8319-00057.
Tsuji, Y., & Su, H. –H. (2018). Macaques as seed dispersal agents in Asian forests: a review. International Journal of Primatology, 39(356), 376. https://doi.org/10.1007/s10764-018-0045-7.
Ungar, P. S. (1995). Fruit preferences of four sympatric primate species at Ketambe, northern Sumatra, Indonesia. International Journal of Primatology, 16(221), 245. https://doi.org/10.1007/BF02735479.
Vilá, M., & D’Antonio, C. (1998). Fruit choice and seed dispersal of invasive vs. noninvasive Carpobrotus (Aizoaceae) in coastal California. Ecology, 79, 1053–1060. https://doi.org/10.2307/176600.
Vizentin-Bugoni, J., Tarwater, C. E., Foster, J. T., Drake, D. R., Gleditsch, J. M., Hruska, A. M., Kelley, J. P., & Sperry, J. H. (2019). Structure, spatial dynamics, and stability of novel seed dispersal mutualistic networks in Hawai’i. Science, 364(78), 82. https://doi.org/10.1126/science.aau8751.
White, T. C. R. (2011). The significance of unripe seeds and animal tissues in the protein nutrition of herbivores. Biological Reviews, 86(217), 224. https://doi.org/10.1111/j.1469-185X.2010.00143.x.
Willaime, P. (1984). Carte Pédologique de l’ile Maurice 1/50 000. Mauritius Sugar Industry Research Institute occasional paper. In Office de la recherche scientifique et technique outre-mer (Vol. no. 33). MSIRI and ORSTOM.
Yeager, C. P. (1996). Feeding ecology of the long-tailed macaque (Macaca fascicularis) in Kalimantan Tengah, Indonesia. International Journal of Primatology, 17(51), 62. https://doi.org/10.1007/BF02696158.
Acknowledgements
We thank the Rufford Small Grants Foundation (grant 27571-2 and 31861-B) for their financial support. We also thank the National Parks and Conservation Service, the Forestry Service, and the Ministry of Agro Industry and Food Security for providing necessary residential permits and permits to work on state land. We are particularly grateful to Noveprim Ltd. for their support, recommendations and assistance with locating our study group in Mont Calebasses. We also thank C. Baider of The Mauritius Herbarium for her plant identification support, I. Janoo of the Ecosystem Restoration Alliance, Indian Ocean (ERA) for his assistance with vegetation transect sampling, and I. Sheik Abass for his contribution to field activities during the early stages of our study. We thank two anonymous reviewers and Prof. J. M. Setchell (editor-in-chief) for their helpful comments. The authors declare that they have no conflict of interest.
Funding
This research was funded by the Rufford Small Grants Foundation (grant 27571-2 and 31861-B).
Author information
Authors and Affiliations
Contributions
RDR and RZO formulated the idea. RDR designed the study, collected and analysed the data. GJ, RZO and EG reviewed methodology/data analyses and provided editorial advice. RDR wrote the manuscript with input from GJ, RZO, and EG.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Joanna M. Setchell
Supplementary Information
ESM 1
(DOCX 491 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reinegger, R.D., Oleksy, R.Z., Gazagne, E. et al. Foraging Strategies of Invasive Macaca fascicularis may Promote Plant Invasion in Mauritius. Int J Primatol 44, 140–170 (2023). https://doi.org/10.1007/s10764-022-00324-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-022-00324-9