Abstract
Studying animal grouping behavior is important for understanding the causes and consequences of sociality and has implications for conservation. Chimpanzee (Pan troglodytes) party size is often assessed by counting individuals or extracted indirectly from camera trap footage or the number of nests. Little is known, however, about consistency across methods for estimating party size. We collected party size data for wild chimpanzees in the Issa valley, western Tanzania, using direct observations, camera traps, and nest counts over six years (2012–2018). We compared mean monthly party size estimates calculated using each method and found that estimates derived from direct observations were weakly positively correlated with those derived from camera traps. Estimates from nest counts were not significantly correlated with either direct observations or camera traps. Overall observed party size was significantly larger than that estimated from both camera traps and nest counts. In both the dry and wet seasons, observed party size was significantly larger than camera trap party size, but not significantly larger than nest party size. Finally, overall party size and wet season party size estimated from camera traps were significantly smaller than nest party size, but this was not the case in the dry season. Our results reveal how data collection methods influence party size estimates in unhabituated chimpanzees and have implications for comparative analysis within and across primate communities. Specifically, future work must consider how estimates were calculated before we can reliably investigate environmental influences on primate behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding animal grouping patterns is important for identifying causes and consequences of sociality (Hill and Dunbar 1998; Majolo et al. 2008) and has implications for conservation (Nakashima et al. 2013). Traditional approaches to monitor primates include direct observations (Goodall 1968; Mitani et al. 2002b; Nakamura et al. 2015) and nest counts for great apes (Fruth and Hohmann 1993; Anderson et al. 2002; Morgan et al. 2006; Sunderland-Groves et al. 2009). Recent technological developments have prompted application of a diversity of methods, including remote tracking via satellite telemetry (Markham and Altmann 2008), passive acoustic monitoring (Kalan et al. 2016), and motion-triggered cameras (hereafter camera traps) (Boyer-Ontl and Pruetz 2014; Krief et al. 2014; McCarthy et al. 2018; van Leeuwen et al. 2020) to reveal grouping behavior. All these methods provide data on ranging and grouping behavior, but comparisons reveal both similarities and differences between methods (McCarthy et al. 2018; van Leeuwen et al. 2020). Testing the influence of method on the resulting data is important to facilitate interstudy comparisons (Chapman et al. 1994; Kalan et al. 2016). Moreover, researcher presence may influence the natural behavior of primates (Williamson and Feistner 2003), and indirect methods such as remote sensing drones, cameras, and acoustic devices provide a practical means of collecting data for unhabituated animals. Understanding how different methods affect group size estimates may thus improve monitoring of unhabituated primates and facilitate comparisons.
In the order Primates, chimpanzees (Pan troglodytes), bonobos (P. paniscus), and spider monkeys (Ateles) show high fission–fusion dynamics (Doran 1997). In these societies, individuals belong to a permanent community but form small, temporary subgroups, or parties (Sakura 1994), that may be stable for days or only a few hours (Goodall 1968). The presence of receptive females, fruit availability, and season are known determinants of party size (Doran 1997; Matsumoto-Oda et al. 1998; Mitani et al. 2002a; Pruetz and Bertolani 2009). When measuring party size, direct observations are advantageous because researchers are less likely to miss individuals compared to other methods. With unhabituated communities, however, this method likely underestimates party size, as some individuals may avoid human observers (Boyer-Ontl and Pruetz 2014; Hicks et al. 2014; Kalan et al. 2016). In contrast, camera traps generally do not influence animal behavior (Gregory et al. 2014; cf. Meek et al. 2016), but if the entire party does not pass within view of the camera, this method also underestimates party size (Nakashima et al. 2013). Finally, counting nests is another method to assess party size (Furuichi et al., 2001; Plumptre and Reynolds 1996). Each adult chimpanzee usually builds a new nest in which to sleep each night (Goodall 1962; Hernandez-Aguilar and Reitan unpubl. data; Stewart and Pruetz unpubl. data, in this issue), occasionally re-using nests (Stewart et al. 2011). Similar to cameras, nest counts are an indirect means of assessing party size and may underestimate party size if researchers do not see nests, if individuals reuse nests, or if individuals share a single nest (e.g., dependent offspring) (Plumptre and Reynolds 1996). Differences in the results of different methods may also reflect behavior. In nesting apes, daytime party counts from observations and camera traps may be similar, whereas nest counts may reveal night-specific influences on party size, and so could be different. For example, predation by large, nocturnal carnivores may encourage larger parties during the night than during the day (Baldwin et al. 1981).
Chimpanzee grouping behavior has been studied extensively using direct observations, camera traps, and nest counts (Table I). However, relatively little is known about open-habitat chimpanzees (Marchant et al. unpubl. data), compared to forest-dwelling populations (cf. Pruetz and Bertolani 2009; Tutin et al. 1983). Factors that drive fission–fusion behavior may differ between forest-dwelling and open-habitat chimpanzees, because the latter live in dry, hot, open areas where resources are more widely distributed and scarcer compared to forests (Pruetz 2006; Wessling et al. unpubl. data). Moreover, only two studies have applied different data collection methods to calculate chimpanzee party size from the same community (Taï Forest, Ivory Coast: McCarthy et al. 2018; Nimba, Guinea: van Leeuwen et al. 2020). A study in Taï Forest, Ivory Coast, on habituated chimpanzees found an association between party size estimated from observations and camera traps, based on 12 mo of data, although estimates from camera traps were lower than those from observations (McCarthy et al. 2018). In Nimba, Guinea, however, party size estimates in unhabituated chimpanzees using observations and camera traps collected over 69 mo did not differ significantly (van Leeuwen et al. 2020). Moreover, mean party size estimated from night nest counts was significantly larger than those estimated from observations and camera traps (van Leeuwen et al. 2020).
We examined how data collection method influences party size estimates in chimpanzees (P. t. schweinfurthii) in a savanna-woodland mosaic habitat in the Issa valley in western Tanzania. We used six years of party size data (2012–2018) collected through observations, camera traps and nest counts. The multiyear data allow for comparisons across seasons and years that shorter studies cannot address, whereas habitat differences across sites provide an opportunity to explore the role habitat plays in chimpanzee grouping patterns. We first compared temporal party size patterns between the three methods and then examined whether party size estimates were similar across methods. Finally, we tested whether seasonal party size estimates varied by method. The Issa valley is rich in predators (McLester et al. 2016; Piel et al. 2018) and individuals are hypothesized to thus cluster together during the night for greater detection of and protection against nocturnal predators (Stewart and Pruetz 2013; Lindshield et al. unpubl. data). We therefore predicted that party size estimated from observations and camera traps do not differ, as both methods capture daytime party size, but party size estimated from nest counts will be larger than those estimated from either observations or camera traps.
Methods
Study Site and Subjects
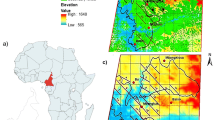
The Issa valley is ca. 85 km2 and characterized by steep hills and flat plateaus, ranging between 900 and 1800 m above sea level. The vegetation is characterized as a miombo woodland, interspersed with grasslands and thin strips of gallery forest (Fig. 1). The dry season lasts from ca. May to October and the wet season from ca. November to April (Piel et al. 2017). During the dry season, monthly rainfall can be <60 mm (Piel et al. 2017), making it one of the driest chimpanzee habitats (van Leeuwen et al. 2020).
Chimpanzees in the Issa valley have been studied since 2008, and habituation efforts began in 2012. In 2012–2016, researchers spent 10–15 days per month looking for evidence of parties and trying to follow them. The main study community consists of ca. 30 individuals (unpubl. data). We collected party size data in an area that we know covers the territories of at least two communities. All-day follows were not possible with Issa chimpanzees until September 2018, and so the observational data comprise follows of individuals that are not yet fully habituated. Besides chimpanzees, yellow baboons (Papio cynocephalus), red-tailed monkeys (Cercopithecus ascanius), vervets (Chlorocebus pygerythrus), and red colobus monkeys (Piliocolobus tephrosceles) are frequently encountered at the study site. Potential predators found in the area include spotted hyenas (Crocuta crocuta), African wild dogs (Lycaon pictus), leopards (Panthera pardus), and lions (P. leo). See Piel et al. (2018) for a full species list.
Party Size
We collected party size data using all three methods from January 2012 to June 2018.
Observations
We located chimpanzees by listening for and tracking vocalizing individuals or opportunistically encountering them through recce walks (Kalan et al. 2016). When we encountered chimpanzees, we recorded party size every 15 min. We defined party size as all individuals visible to the observer during each scan, excluding dependent infants (adapted from Chapman et al. 1994; Newton-Fisher et al. 2000; Wrangham et al. 1992). Dependent infants rely on their mother for transport and feeding (Goodall 1968) and thus were not independently socializing with the party.
Camera Traps
We deployed 28 Bushnell Trail Cam motion-triggered cameras across the study area (Fig. 1). Of these, we deployed 15 cameras on wildlife paths, seven on termite mounds and the remaining six on roads, walking paths and fruiting trees. We secured cameras ca. 1 m from the base of the tree above the ground. We set all cameras to be active 24 h per day and to record 60-s video recordings with a 1-s time interval. We defined a party as all individuals (except for dependent infants) recorded by the same camera with <15 min between consecutive recordings (adapted from McCarthy et al. 2018). In most cases, especially on wildlife paths, chimpanzees were traveling so counting individuals was straightforward. In cases in which we could not identify an individual and we were not sure if it reappeared, we counted the individual only once to avoid pseudoreplication.
Nest Counts
We collected data on party size from all fresh nest groups. We considered a nest as fresh if it had urine or feces underneath that could be attributed to a specific nest. For party size, we included all fresh nests that were constructed within a 100-m radius of each other (following Stewart and Pruetz 2013). After recording data, we marked each tree with tape around the base of the tree or under the nest to avoid double-counting.
Season
Following Piel et al. (2017), we classified season for each individual party: dry (May–October) or wet (November–April). We collected rainfall data using a HOBO rain logger, located at the research station, that recorded every 2 mm of rainfall (Piel et al. 2017).
Statistical Analysis
Because we were unable to measure party size each day using all three methods, we calculated the mean monthly party size for each method. The number of parties that we quantified varied per month for each of the three methods (observations: mean = 44, range = 2–211; camera traps: mean = 31, range 1–124; and nests: mean = 5.0, range = 1–19). We had 77 mo of party size data after we removed 3 mo that had data for fewer than two methods. As habituation status could bias our observed party size estimates, we tested the correlation between mean monthly party size estimated from observations and time (expressed as the number of our study month). To test the consistency of the party size estimates among the three methods, we conducted three pairwise correlation tests between mean monthly party size estimates for each method, i.e., observations vs. camera traps, observations vs. nest counts, and camera traps vs. nest counts. We used nonparametric Spearman’s correlation tests because the data were not normally distributed. We also calculated one overall mean party size per method (calculated from all individual parties and seasons) and used a one-way ANOVA and post hoc Tukey’s tests to test whether mean party size differed significantly between the three methods. To test whether the methodological variation in party size estimates varied per season, we calculated the mean party size per method for both the dry and wet season and used two one-way ANOVAs followed by post hoc Tukey’s tests. We conducted all analyses in R software, version 3.3.2, using the multcomp package version 1.4–8.
Ethical Note
All research complied with protocols, guidelines, ethical polices, and regulations from the Tanzanian Wildlife Research Institute (TAWIRI), Tanzania Commission for Science and Technology (COSTECH) and guidelines described by the American Society of Primatologists. The authors declare that they have no conflict of interest.
Data Availability
The datasets assembled and analyzed during the current study are available from the corresponding author on reasonable request.
Results
Monthly Party Size Patterns
In total, we assessed 3052 parties from observations, 2132 from camera traps and 332 from nest counts. The range of mean monthly party size estimates varied per method: 1.3–5.9 (mean = 3.1 ± SD = 0.94) for observations, 1.0–3.5 (mean = 2.3 ± SD = 0.59) for camera traps, and 1.0–8.5 (mean = 2.9 ± SD = 1.8) for nest counts. Observations showed moderate fluctuations in mean monthly party size estimates (Fig. 2a), while camera traps showed relatively few extremes (Fig. 2b) and nest counts showed the highest fluctuations (Fig. 2c).
Statistical Results
Monthly Party Size
There was no significant relationship between mean monthly party size estimated from direct observations and time (Spearman’s correlation test: rs = 0.10, N = 70, P = 0.43; Electronic Supplementary Material [ESM] Fig. S1). We found a significant but weak positive relationship between mean monthly party size estimated from observations and camera traps (Spearman’s correlation test: rs = 0.31, N = 61, P = 0.014; Fig. 3a). There were no significant relationships between mean monthly party size estimated from observations and nest counts (rs = 0.067, N = 59, P = 0.62; Fig. 3b) and camera traps and nest counts (rs = 0.15, N = 59, P = 0.26; Fig. 3c).
Mean Party Size
Overall mean party size differed significantly among the three methods (ANOVA: F = 60, df = 2.0, P < 0.001). Overall party size estimated from observations was significantly larger than that estimated from both camera traps (Tukey’s test, P < 0.001; Fig. 4a) and nest counts (Tukey’s test, P = 0.021; Fig. 4a). Moreover, overall party size estimated from camera traps was significantly smaller than that estimated from nest counts (Tukey’s test, P = 0.020; Fig. 4a). In both seasons, party size estimated from observations was significantly larger than estimates from camera traps (dry season: Tukey’s test, P < 0.001; Fig. 4b; wet season: Tukey’s test, P < 0.001; Fig. 4c), but not significantly larger than estimates from nest counts (dry season: Tukey’s test, P = 0.071; Fig. 4b; wet season: Tukey’s test, P = 0.30; Fig. 4c). Finally, we found that overall mean party size estimated from camera traps was significantly smaller than party size estimated from nest counts during the wet season (Tukey’s test, P = 0.040; Fig. 4c), but this was not the case during the dry season (Tukey’s test, P = 0.35; Fig. 4b).
Mean party size in chimpanzees (Pan troglodytes schweinfurthii ) from the Issa valley, Tanzania (January 2012 – June 2018) with standard deviation per method for (a) dry and wet seasons together, (b) dry season and (c) wet season. Brackets indicate pairwise Tukey’s test comparisons with asterisks indicating significance level < 0.05.
Discussion
Party Size Estimates
We found that mean monthly party size estimated using researcher observations and camera traps were weakly but significantly associated and that camera traps underestimated party size compared to observations. These results are similar to findings for chimpanzees from Taï National Park, Ivory Coast (McCarthy et al. 2018), despite methodological differences: McCarthy et al. (2018) assessed identical parties of habituated chimpanzees with both methods, whereas we calculated mean (monthly) values for each method, as we could not collect paired data because the Issa chimpanzees were not yet fully habituated during our study. Our results contrast with recent work which found no significant differences in mean party size estimated using observations and camera traps in unhabituated chimpanzees at Nimba, Guinea (van Leeuwen et al. 2020). One reason for this difference could be due to the habitat openness of Issa vs. Nimba. Issa is a savanna mosaic, and visibility can reach >150 m in some areas. Therefore, observers can count far more individuals than those that pass by a single camera, for example, and so observed party size estimates are larger than those estimated from camera traps. An alternative, and not mutually exclusive, explanation is that parties of open-habitat chimpanzees are larger and more cohesive than those at other sites (Pruetz and Bertolani 2009; Tutin et al. 1983).
Party size estimated from nest counts did not correlate strongly or significantly with party size estimated from either observations or camera traps. Overall nest party size was smaller than that estimated from observations. This may be due to differences in the methods, or to chimpanzee behavior. For example, researchers may have missed obscured nests. Alternatively, given the low tree density at Issa (Hernandez-Aguilar 2009), chimpanzees may be forced to maintain larger interindividual distances when selecting nesting trees, which may lead to smaller nesting parties. Our finding that nest party size was smaller than observed party size indicates that Issa parties are less cohesive during the night.
Party size estimates varied across methods and the methods also yielded different estimates within seasons, but the patterns were not always the same. During the wet season, mean party size estimated from camera traps was smaller than that estimated from nest counts, whereas we found no such difference during the dry season. Habitat visibility might explain some of this variation. Grass grows to >1 m during the early wet season at Issa, which may obscure individuals from camera footage, whereas this is unlikely to happen during the dry season where visibility is much higher (Piel and Stewart, pers. obs.). However, nests might also be more obscured in the wet season compared to the dry season due to an increase in foliage. Another possibility is that chimpanzees change their association patterns with seasons. In this case, chimpanzees may aggregate at nest sites in the wet season, after spending days in small parties. Overall fruit availability is lowest in the wet season (Piel et al. 2017), which suggests that group size might be low during the day to allow individuals to more efficiently locate and exploit feeding sources (Doran 1997). As such, both habitat visibility and fruit availability may explain our findings.
The poor habituation at Issa may have influenced our results. Because Issa chimpanzees were not fully habituated during our study, we may have underestimated party size due to not seeing individuals that fled before we arrived. Alternatively, because we located chimpanzees by recce walks and often from hearing vocalizing individuals, we may have overestimated party size due to oversampling larger, vocally conspicuous parties and neglecting quieter parties of fewer individuals. However, we found no significant increase in observed party size over time, suggesting that habituation status did not affect our estimates.
Implications for Assessing Party Size
Although we found significant differences between mean party size per method, the estimates derived from each method are relatively similar. As each method has its strengths and limitations, all three are applicable under certain circumstances. Observations are likely the most reliable method once chimpanzees are fully habituated. Until then, however, observations are likely the least reliable, with shy individuals fleeing researchers early in the encounter process. Nest counts are useful given the relative ease of collecting these data. However, it is not always possible to determine the age of a nest (Stewart et al. 2011; Tutin and Fernandez 1984). Moreover, our definition of a nest group required the presence of fresh urine or feces under a nest, but chimpanzees do not always urinate or defecate in the mornings, and if branches are bent, but not broken, leaves will not change color, making ageing a nest difficult. Finally, camera traps capture more data (e.g., social behavior, ranging, demography), and are useful to monitor chimpanzees in difficult terrain (Boyer-Ontl and Pruetz 2014). They cost less in money, time and research presence, but require maintenance (battery changing, imagery downloading), and are vulnerable to damage by humans and wildlife. While most chimpanzees often habituate quickly to camera traps, not all individuals do. As in other species (Meek et al. 2015, 2016), juveniles are especially interested in investigating cameras (Kalan et al. 2019), and detecting cameras can provoke shyness, thereby skewing resulting data (Wegge et al. 2004). As such, researchers must consider the strengths and limitations of each method when studying party size. Moreover, method selection may also depend on other factors such as survey time scale, spatial scope, funding, and available personnel.
Conclusion
Methodological variation in estimates of grouping behavior has implications across species. Numerous other primate species (reviewed in Amici et al. 2008) and other mammals such as elephants and giraffes (reviewed in Couzin and Laidre 2009), show high fission–fusion dynamics, and study populations are often not habituated. Biologists depend on remote monitoring to assess the influence of ecology on behavior, especially in increasingly anthropogenic landscapes. Grouping behavior patterns may reflect levels of local habitat disturbance (Constantine et al. 2004). For example, in bottlenose dolphins (Tursiops truncatus), another fission–fusion species, disturbance type influences grouping patterns, measured by group size (Constantine et al. 2004). Assessing methodological consistency when measuring grouping behavior within the same population thus has bearing on the reliability of these approaches when used with various species.
We show that data collection methods influence party size estimates in chimpanzees. It remains difficult, however, to determine whether these differences are methodological or behavioral. Our results have bearing on not only intercommunity comparisons but also intergroup comparisons across time in other great apes such as bonobos (Pan paniscus), gorillas (Gorilla), and orangutans (Pongo). Recognizing the potential methodological biases will improve monitoring efforts, which often include estimating population abundance, and thereby the conservation of great apes. Recent attempts to use camera-trap data to build occupancy models (Crunchant et al. 2017) and extract population density (Després-Einspenner et al. 2017) of wild chimpanzees are promising and provide a way forward to integrating camera trap data and conservation practice.
References
Amici, F., Aureli, F., & Call, J. (2008). Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Current Biology, 18, 1415–1419.
Anderson, D. P., Nordheim, E. V., Boesch, C., & Moermond, T. C. (2002). Factors influencing fission-fusion grouping in chimpanzees in the Taï National Park, Côte d’Ivoire. In C. Boesch, G. Hohmann, & L. Marchant (Eds.), Behavioural diversity in chimpanzees and bonobos (pp. 90–101). Cambridge: Cambridge University Press.
Baldwin, P. J., Pi, J. S., McGrew, W. C., & Tutin, C. E. (1981). Comparisons of nests made by different populations of chimpanzees (Pan troglodytes). Primates, 22, 474–486.
Boyer-Ontl, K. M., & Pruetz, J. D. (2014). Giving the forest eyes: The benefits of using camera traps to study unhabituated chimpanzees (Pan troglodytes verus) in southeastern Senegal. International Journal of Primatology, 35, 881–894.
Chapman, C., White, F. J., & Wrangham, R. W. (1994). Party size in chimpanzees and bonobos. In R. W. Wrangham (Ed.), Chimpanzee cultures (pp. 41–57). Cambridge, MA: Harvard University Press.
Constantine, R., Brunton, D. H., & Dennis, T. (2004). Dolphin-watching tour boats change bottlenose dolphin (Tursiops truncatus) behaviour. Biological Conservation, 117, 299–307.
Couzin, I. D., & Laidre, M. E. (2009). Fission–fusion populations. Current Biology, 19, R633–R635.
Crunchant, A. S., Egerer, M., Loos, A., Burghardt, T., Zuberbühler, K., et al (2017). Automated face detection for occurrence and occupancy estimation in chimpanzees. American Journal of Primatology, 79, e22627.
Després-Einspenner, M. L., Howe, E. J., Drapeau, P., & Kühl, H. S. (2017). An empirical evaluation of camera trapping and spatially explicit capture-recapture models for estimating chimpanzee density. American Journal of Primatology, 79, e22647.
Doran, D. (1997). Influence of seasonality on activity patterns, feeding behavior, ranging, and grouping patterns in Taï chimpanzees. International Journal of Primatology, 18, 183–206.
Fruth, B., & Hohmann, G. (1993). Ecological and behavioral aspects of nest building in wild bonobos (Pan paniscus). Ethology, 94, 113–126.
Furuichi, T., Hashimoto, C., & Tashiro, Y. (2001). Extended application of a marked-nest census method to examine seasonal changes in habitat use by chimpanzees. International Journal of Primatology, 22, 913–928.
Goodall, J. M. (1962). Nest building behavior in the free ranging chimpanzee. Annals of the New York Academy of Sciences, 102, 455–467.
Goodall, J. M. (1968). The behavior of free-living chimpanzees in the Gombe stream reserve. Animal Behavior Monographs, 1, 161–311.
Gregory, T., Carrasco Rueda, F., Deichmann, J., Kolowski, J., & Alonso, A. (2014). Arboreal camera trapping: Taking a proven method to new heights. Methods in Ecology and Evolution, 5, 443–451.
Hernandez-Aguilar, R. A. (2009). Chimpanzee nest distribution and site reuse in a dry habitat: Implications for early hominin ranging. Journal of Human Evolution, 57, 350–364.
Hicks, T. C., Tranquilli, S., Kuehl, H., Campbell, G., Swinkels, J., et al (2014). Absence of evidence is not evidence of absence: Discovery of a large, continuous population of Pan troglodytes schweinfurthii in the central Uele region of northern DRC. Biological Conservation, 171, 107–113.
Hill, R. A., & Dunbar, R. I. (1998). An evaluation of the roles of predation rate and predation risk as selective pressures on primate grouping behavior. Behavior, 135, 411–430.
Kalan, A. K., Hohmann, G., Arandjelovic, M., Boesch, C., McCarthy, M. S., et al (2019). Novelty response of wild African apes to camera traps. Current Biology, 29, 1211–1217.
Kalan, A. K., Piel, A. K., Mundry, R., Wittig, R. M., Boesch, C., & Kühl, H. S. (2016). Passive acoustic monitoring reveals group ranging and territory use: A case study of wild chimpanzees (Pan troglodytes). Frontiers in Zoology, 13, 1–11.
Krief, S., Cibot, M., Bortolamiol, S., Seguya, A., Krief, J. M., & Masi, S. (2014). Wild chimpanzees on the edge: Nocturnal activities in croplands. PLoS One, 9, 1–11.
van Leeuwen, K. L., Matsuzawa, T., Sterck, E. H., & Koops, K. (2020). How to measure chimpanzee party size? A methodological comparison. Primates, 1–12.
Majolo, B., de Bortoli Vizioli, A., & Schino, G. (2008). Costs and benefits of group living in primates: Group size effects on behavior and demography. Animal Behavior, 76, 1235–1247.
Markham, A. C., & Altmann, J. (2008). Remote monitoring of primates using automated GPS technology in open habitats. American Journal of Primatology, 70, 495–499.
Matsumoto-Oda, A., Hosaka, K., Huffman, M. A., & Kawanaka, K. (1998). Factors affecting party size in chimpanzees of the Mahale Mountains. International Journal of Primatology, 19, 999–1011.
McCarthy, M. S., Després-Einspenner, M. L., Samuni, L., Mundry, R., Lemoine, S., et al. (2018). An assessment of the efficacy of camera traps for studying demographic composition and variation in chimpanzees (Pan troglodytes). American Journal of Primatology, e22904.
McLester, E., Stewart, F. A., & Piel, A. K. (2016). Observation of an encounter between African wild dogs (Lycaon pictus) and a chimpanzee (Pan troglodytes schweinfurthii) in the Issa Valley, Tanzania. African Primates, 11, 27–36.
Meek, P. D., Ballard, G. A., & Fleming, P. J. (2015). The pitfalls of wildlife camera trapping as a survey tool in Australia. Australian Mammalogy, 37, 13–22.
Meek, P., Ballard, G., Fleming, P., & Falzon, G. (2016). Are we getting the full picture? Animal responses to camera traps and implications for predator studies. Ecology and Evolution, 6, 3216–3225.
Mitani, J. C., Watts, D. P., & Lwanga, J. S. (2002a). Ecological and social correlates of chimpanzee party size and composition. In C. Boesch, G. Hohmann, & L. Marchant (Eds.), Behavioural diversity in chimpanzees and bonobos (pp. 102–111). Cambridge: Cambridge University Press.
Mitani, J. C., Watts, D. P., & Muller, M. N. (2002b). Recent developments in the study of wild chimpanzee behavior. Evolutionary Anthropology: Issues, News, and Reviews, 11, 9–25.
Morgan, D., Sanz, C., Onononga, J. R., & Strindberg, S. (2006). Ape abundance and habitat use in the Goualougo triangle, republic of Congo. International Journal of Primatology, 27, 147–179.
Nakamura, M., Hosaka, K., Itoh, N., & Zamma, K. (2015). Mahale chimpanzees, 50 years of research (p. 797). Cambridge: Cambridge University Press.
Nakashima, Y., Iwata, Y., Ando, C., Nze Nkoguee, C., Inoue, E., et al (2013). Assessment of landscape-scale distribution of sympatric great apes in African rainforests: Concurrent use of nest and camera-trap surveys. American Journal of Primatology, 75, 1220–1230.
Newton-Fisher, N. E., Reynolds, V., & Plumptre, A. J. (2000). Food supply and chimpanzee (Pan troglodytes schweinfurthii) party size in the Budongo Forest reserve, Uganda. International Journal of Primatology, 21, 613–628.
Piel, A. K., Bonnin, N., RamirezAmaya, S., Wondra, E., & Stewart, F. A. (2018). Chimpanzees and their mammalian sympatriates in the Issa Valley, Tanzania. African Journal of Ecology, 57, 31–40.
Piel, A. K., Strampelli, P., Greathead, E., Hernandez-Aguilar, R. A., Moore, J., & Stewart, F. A. (2017). The diet of woodland-mosaic chimpanzees (Pan troglodytes schweinfurthii) in the Issa valley, western Tanzania. Journal of Human Evolution, 112, 57–69.
Plumptre, A. J., & Reynolds, V. (1996). Censusing chimpanzees in the Budongo forest, Uganda. International Journal of Primatology, 17, 85–99.
Pruetz, J. D. (2006). Feeding ecology of savanna chimpanzees (Pan troglodytes verus) at Fongoli, Senegal. In G. Hohmann, M. M. Robbins, & C. Boesch (Eds.), Feeding ecology in apes and other primates: Ecological, physiological and behavioural aspects (pp. 161–182). Cambridge: Cambridge University Press.
Pruetz, J. D., & Bertolani, P. (2009). Chimpanzee (Pan troglodytes verus) behavioral responses to stresses associated with living in a savanna-mosaic environment: implications for hominin adaptations to open habitats. PaleoAnthropology, 252–262.
Sakura, O. (1994). Factors affecting party size and composition of chimpanzees (Pan troglodytes verus) Bossou, Guinea. International Journal of Primatology, 15, 167–183.
Stewart, F. A., Piel, A. K., & McGrew, W. C. (2011). Living archaeology: Artefacts of specific nest site fidelity in wild chimpanzees. Journal of Human Evolution, 61, 388–395.
Stewart, F. A., & Pruetz, J. D. (2013). Do chimpanzee nests serve an anti-predatory function? American Journal of Primatology, 75, 593–604.
Sunderland-Groves, J. L., Ekinde, A., & Mboh, H. (2009). Nesting behavior of Gorilla gorilla diehli at Kagwene Mountain, Cameroon: Implications for assessing group size and density. International Journal of Primatology, 30, 253–266.
Tutin, C. E., & Fernandez, M. (1984). Nationwide census of gorilla (Gorilla g. gorilla) and chimpanzee (Pan t. troglodytes) populations in Gabon. American Journal of Primatology, 6, 313–336.
Tutin, C. E., McGrew, W. C., & Baldwin, P. J. (1983). Social organization of savanna-dwelling chimpanzees, Pan troglodytes verus, at Mt. Assirik, Senegal. Primates, 24, 154–173.
Wegge, P., Pokheral, C. P., & Jnawali, S. R. (2004). Effects of trapping effort and trap shyness on estimates of tiger abundance from camera trap studies. Animal Conservation, 7, 251–256.
Williamson, E. A., & Feistner, A. T. (2003). Habituating primates: Processes, techniques, variables and ethics. In J. M. Setchell & D. J. Curtis (Eds.), Field and laboratory methods in primatology: A practical guide (pp. 25–39). Cambridge: Cambridge University Press.
Wrangham, R. W., Clark, A. P., & Isabirye-Basuta, G. (1992). Female social relationships and social organization of Kibale Forest chimpanzees. In T. Nishida, W. C. McGrew, P. Marler, M. Pickford, & F. B. M. de Waal (Eds.), Topics in primatology, Vol. I: Human origins (pp. 81–98). Tokyo: University of Tokyo Press.
Acknowledgements
We would like to thank all researchers and field assistants from the Greater Mahale Ecosystem Research and Conservation (GMERC) Project. Permission to work in Tanzania was granted by the Tanzania Wildlife Research Institute (TAWIRI), Tanzania Commission for Science and Technology (COSTECH), and Mpanda District. Long-term support for GMERC is provided by the UCSD/Salk Center for Academic Research and Training in Anthropogeny (CARTA). Additional support for this work was also generously provided by Harold Hyam Wingate Foundation, L. S. B. Leaky Foundation, National Science Foundation, Royal Anthropological Institute, UCSD, and Wenner-Gren Foundation. Finally, we would like to thank Stacy M. Lindshield, Joanna M. Setchell, and three anonymous reviewers for their helpful feedback on previous versions of this article.
Author information
Authors and Affiliations
Contributions
AKP originally formulated the idea; DNV and AKP developed methodology; DNV conducted fieldwork; DNV performed statistical analyses; and DNV, AKP, and FAS wrote the manuscript.
Corresponding author
Additional information
Handling Editor: Stacy Lindshield
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vink, D.N., Stewart, F.A. & Piel, A.K. Comparing Methods for Assessing Chimpanzee (Pan troglodytes schweinfurthii) Party Size: Observations, Camera Traps, and Bed Counts from a Savanna–Woodland Mosaic in the Issa Valley, Tanzania. Int J Primatol 41, 901–915 (2020). https://doi.org/10.1007/s10764-020-00142-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-020-00142-x