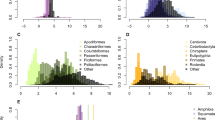
Abstract
The unified neutral theory of biodiversity states that random processes and stochastic events drive species abundance and turnover and that each lineage has an equal probability of speciation. Predictions based on this model have been tested only a few times using evolutionary rates. We used an individual-based approach to estimate the waiting times to extinction (the time between one extinction event and the next) and compare those with the neutral model. We calculated the speciation and extinction rates for all subfamilies in the primate order using a time-calibrated phylogeny and compared the estimates against those predicted by the neutral theory using taxon area distribution and population densities. In most cases, the extinction time obtained from the neutral theory exceeded that estimated using phylogenetic data by several orders of magnitude. Our main result indicates that drift is too slow to fully explain evolutionary rates of turnover in primates, suggesting that other factors besides chance may influence primate diversification rates. Although estimates of forest cover, taxon area distribution, and species densities may influence the results, the observed differences do not support the predictions of neutral theory and question the model as a reference for conservation purposes.


Similar content being viewed by others
References
Arnold, C., Matthews, L. J., & Nunn, C. L. (2010). The 10kTrees website: A new online resource for primate phylogeny. Evolutionary Anthropology, 19, 114–118.
Baker, T. R., Pennington, R. T., Magallon, S., et al. (2014). Fast demographic traits promote high diversification rates of Amazonian trees. Ecology Letters, 17(5), 527–536. doi:10.1111/ele.12252.
Bickford, D., Lohman, D. J., Sodhi, N. S., et al. (2007). Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution, 22, 148–155.
Bininda-Emonds, O. R., Cardillo, M., Jones, K. E., et al. (2007). The delayed rise of present-day mammals. Nature, 446, 507–512.
Campbell, C. J. (2011). Primates in perspective. New York: Oxford University Press.
Davies, T. J., Allen, A. P., Borda-de-Água, L., Regetz, J., & Melián, C. J. (2011). Neutral biodiversity theory can explain the imbalance of phylogenetic trees but not the tempo of their diversification. Evolution, 65(7), 1841–1850.
Defler, T. R., & Bueno, M. L. (2007). Aotus diversity and the species problem. Primate Conservation, 22, 55–70.
Drummond, A. J., Rambaut, A., Shapiro, B., & Pybus, O. G. (2005). Bayesian coalescent inference of past population dynamics from molecular sequences. Molecular Biology and Evolution, 22, 1185–1192.
Eiserhardt, W. L., Svenning, J.-C., Baker, W. J., Couvreur, T. L., & Balslev, H. (2013). Dispersal and niche evolution jointly shape the geographic turnover of phylogenetic clades across continents. Scientific Reports, 3. doi:10.1038/srep01164.
Etienne, R. S., & Apol, M. E. F. (2008). Estimating speciation and extinction rates from diversity data and the fossil record. Evolution, 63, 244–255.
Fabre, P., Rodrigues, A., & Douzery, E. (2009). Patterns of macroevolution among Primates inferred from a supermatrix of mitochondrial and nuclear DNA. Molecular Phylogenetics and Evolution, 53, 808.
Fargione, J., Brown, C. S., & Tilman, D. (2003). Community assembly and invasion: An experimental test of neutral versus niche processes. Proceedings of the National Academy of Sciences of the USA, 100, 8916–8920.
Finstermeier, K., Zinner, D., Brameier, M., Meyer, M., Kreuz, E., Hofreiter, M., & Roos, C. (2013). A mitogenomic phylogeny of living primates. PLoS ONE, 8(7), e69504.
FitzJohn, R. G. (2012). Diversitree: Comparative phylogenetic analyses of diversification in R. Methods in Ecology and Evolution, 3, 1084–1092.
Freckleton, R., Harvey, P., & Pagel, M. (2002). Phylogenetic analysis and comparative data: A test and review of evidence. The American Naturalist, 160, 712–726.
Gillespie, J. H. (2010). Population genetics: A concise guide. Baltimore: Johns Hopkins University Press.
Gómez, J. M., & Verdú, M. (2012). Mutualism with plants drives primate diversification. Systematic Biology, 61, 567–577.
Hayes, B. J., Visscher, P. M., McPartlan, H. C., & Goddard, M. E. (2003). Novel multilocus measure of linkage disequilibrium to estimate past effective population size. Genome Research, 13, 635–643.
Hubbell, S. (2001). The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press.
Jabot, F., & Chave, J. (2009). Inferring the parameters of the neutral theory of biodiversity using phylogenetic information and implications for tropical forests. Ecology Letters, 12, 239–248.
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K., & Mooers, A. O. (2012). The global diversity of birds in space and time. Nature, 491(7424), 444–448.
Johnson, D. (2012). Primate info net. In The life spans of nonhuman primates. Available at: http://pin.primate.wisc.edu/aboutp/phys/lifespan.html.
Karst, J., Gilbert, B., & Lechowicz, M. J. (2005). Fern community assembly: The roles of chance and the environment at local and intermediate scales. Ecology, 86, 2473–2486.
Kimura, M. (1985). The neutral theory of molecular evolution. Cambridge, UK: Cambridge University Press.
Leigh, G. (1981). The average lifetime of a population in a varying environment. Journal of Theoretical Biology, 90, 213–239.
MacArthur, R. (1970). Species packing and competitive equilibrium for many species. Theoretical Population Biology, 1, 1–11.
Magnuson-Ford, K., & Otto, S. P. (2012). Linking the investigations of character evolution and species diversification. The American Naturalist, 180, 225–245.
Maia, R., Rubenstein, D. R., & Shawkey, M. D. (2013). Key ornamental innovations facilitate diversification in an avian radiation. Proceedings of the National Academy of Sciences, 110(26), 10687–10692. doi:10.1073/pnas.1220784110.
Nee, S. (2001). Inferring speciation rates from phylogenies. Evolution, 55, 661–668.
Nee, S. (2005). The neutral theory of biodiversity: do the numbers add up? Functional Ecology, 19(1), 173–176. doi:10.1111/j.0269-8463.2005.00922.x.
Pagel, M. (1999). Inferring the historical patterns of biological evolution. Nature, 401, 877–884.
Paradis, E. (2011). Analysis of phylogenetics and evolution with R. New York: Springer Science+Business Media.
Perelman, P., Johnson, W. E., Roos, C., et al. (2011). A molecular phylogeny of living primates. PLoS Genetics, 7, e1001342.
Pozzi, L., Hodgson, J. A., Burrell, A. S., Sterner, K. N., Raaum, R. L., & Disotell, T. R. (2014). Primate phylogenetic relationships and divergence dates inferred from complete mitochondrial genomes. Molecular Phylogenetics and Evolution, 75, 165–183.
R Development Core Team. (2012). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Rabosky, D. L. (2010). Extinction rates should not be estimated from molecular phylogenies. Evolution, 64, 1816–1824.
Rabosky, D. L., Slater, G. J., & Alfaro, M. E. (2012). Clade age and species richness are decoupled across the eukaryotic tree of life. PLoS Biology, 10, e1001381.
Revell, L. J., Harmon, L. J., & Collar, D. C. (2008). Phylogenetic signal, evolutionary process, and rate. Systematic Biology, 57, 591–601.
Ricklefs, R. E. (2006). The unified neutral theory of biodiversity: Do the numbers add up? Ecology, 87, 1424–1431.
Ricklefs, R. E. (2007). Estimating diversification rates from phylogenetic information. Trends in Ecology & Evolution, 22, 601–610.
Ricklefs, R. E., & Renner, S. S. (2012). Global correlations in tropical tree species richness and abundance reject neutrality. Science, 335, 464–467.
Rosindell, J., Hubbell, S. P., & Etienne, R. S. (2011). The unified neutral theory of biodiversity and biogeography at age ten. Trends in Ecology & Evolution, 26, 340–348.
Rowe, N., & Myers, M. (2011). All the world’s primates. Available at: www.alltheworldsprimates.org (Accessed November 3, 2011).
Sanderson, M. J., & Donoghue, M. J. (1996). Reconstructing shifts in diversification rates on phylogenetic trees. Trends in Ecology & Evolution, 11, 15–20.
Shultz, S., Opie, C., & Atkinson, Q. D. (2011). Stepwise evolution of stable sociality in primates. Nature, 479, 219–222.
Siepielski, A. M., Hung, K.-L., Bein, E. E. B., & McPeek, M. A. (2010). Experimental evidence for neutral community dynamics governing an insect assemblage. Ecology, 91, 847–857.
Smuts, B. B., Cheney, D. L., Seyfarth, R. M., et al. (1987). Primate societies. Chicago: University of Chicago Press.
Springer, M. S., Meredith, R. W., Gatesy, J., et al. (2012). Macroevolutionary dynamics and historical biogeography of primate diversification inferred from a species Supermatrix. PLoS ONE, 7, e49521.
Stadler, T. (2011a). Mammalian phylogeny reveals recent diversification rate shifts. Proceedings of the National Academy of Sciences of the USA, 108, 6187–6192.
Stadler, T. (2011b). Simulating trees with a fixed number of extant species. Systematic Biology, 60(5), 676–684.
Stanley Harpole, W., & Tilman, D. (2006). Non-neutral patterns of species abundance in grassland communities. Ecology Letters, 9, 15–23.
Stevenson, P. R. (2001). The relationship between fruit production and primate abundance in Neotropical communities. Biological Journal of the Linnean Society, 72, 161–178. doi:10.1111/j.1095-8312.2001.tb01307.x.
Stevenson, P. R. (2010). Efectos de la fragmentación y de la producción de frutos en comunidades de primates neotropicales. Pereira-Bengoa, V., Stevenson, P. R., Bueno, M. & Nassar-Montoya, F. (Eds.), Avances en la primatología del nuevo mundo (pp. 239–257). Bogotá D.C.
Volkov, I., Banavar, J. R., He, F., Hubbell, S. P., & Maritan, A. (2005). Density dependence explains tree species abundance and diversity in tropical forests. Nature, 438(7068), 658–661.
Weiher, E., & Keddy, P. (2001). Ecological assembly rules: Perspectives, advances, retreats. Cambridge, UK: Cambridge University Press.
Weir, J. T. (2006). Divergent timing and patterns of species accumulation in lowland and highland Neotropical birds. Evolution, 60, 842–855.
Acknowledgments
We thank Sebastian Gonzalez Caro for his valuable comments and suggestions. We also thank Diana Pizano, Diana Guzmán, and Ana Aldana for their comments and corrections. An anonymous reviewer from Peerage of Science made helpful comments to the first version of this manuscript, and three anonymous referees and Dr. Joanna Setchell made insightful comments and constructive suggestions for the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 44 kb)
Rights and permissions
About this article
Cite this article
Henao-Diaz, F., Stevenson, P.R. Neutral Theory Overestimates Extinction Times in Nonhuman Primates. Int J Primatol 36, 790–801 (2015). https://doi.org/10.1007/s10764-015-9854-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-015-9854-0