Abstract
In theory, practical work is an established part of university-level chemistry courses. However, mainly due to budget constraints, large class size, time constraints and inadequate teacher preparations, practical activities are frequently left out from chemistry classroom instruction in most developing countries. Small-scale chemistry (SSC) experimentation in which one uses miniature chemical equipment can drastically reduce quantities of chemicals used during experimentation, which can help overcome some of the barriers preventing practical work in the chemistry classroom. This study evaluated the effectiveness of using miniature chemical equipment at the undergraduate level in increasing students’ understanding of chemistry concepts as well as in improving their attitude towards chemistry practical work. Two comparable groups of first-year students who enrolled for a Practical General Chemistry course participated. A quasi-experimental design was employed in which the experimental group (N = 49) used the SSC approach while the control group (N = 52) followed the traditional approach for over 8 weeks. Data were gathered using chemistry tests, attitude and perception questionnaires and interviews. Findings showed that the SSC approach was as effective as the traditional laboratory approach in improving students’ attitude towards practical work, but more effective in enhancing students’ understanding of chemistry concepts. More interestingly, SSC was positively accepted by both students and instructors as an effective strategy for teaching first-year undergraduate chemistry practicals. Some shortcomings of the approach were also identified.

Similar content being viewed by others
References
Abdullah, M., Mohamed, N. & Ismail, Z. H. (2007). The effect of microscale chemistry experimentation on students’ attitude and motivation towards chemistry practical work. Journal of Science and Mathematics Education in Southeast Asia, 30(2), 44–72. Retrieved from http://www.recsam.edu.my/R&D_Journals/.
Abdullah, M., Mohamed, N. & Ismail, Z. H. (2009). The effect of an individualized laboratory approach through microscale chemistry experimentation on students’ understanding of chemistry concepts, motivation and attitudes. Chemistry Education Research and Practice, 10(1), 53–61. doi:10.1039/B901461F.
Abdullah, M., Mohamed, N. & Ismail, Z. H. (2013). Introducing microscale experimentation in volumetric analysis for pre-service teachers. In M.-H. Chiu et al. (Eds.), Chemistry education and sustainability in the global age (pp. 311–320). Dordrecht, The Netherlands: Springer.
Acharry, S. & Suwannathada, J. (2010). The development of microscale laboratory: Titration. International Journal of Arts And Sciences, 3(9), 296–305. Retrieved from http://www.universitypublications.net/ijas/.
Asfaw, E., Otore, D., Ayele, T. & Gebremariam, Z., (2009). Science and mathematics secondary education in Ethiopia. An Option paper presented at the Technical Workshop on Science and Mathematics Secondary Education in Africa (SEIA), Tunis. Retrieved from http://info.worldbank.org/etools/docs/library/245746/day9%207b.%20Ethiopia%20Options%20Paper.pdf
Bekalo, S. & Welford, G. (2000). Practical activity in Ethiopian secondary physical sciences: Implications for policy and practice of the match between the intended and the implemented curriculum. Research Papers in Education, 15(2), 185–212. doi:10.1080/026715200402498.
Bradley, J. D. (1999). Hands-on practical chemistry for all. Pure and applied chemistry, 71(5), 817-823.
Bradley, J. D. (2000). The microscience project and its impact on pre-service and in-service teacher education. Washington, DC: The World Bank. Retrieved from http://www1.worldbank.org/education/scied/Training/training.htm.
Bradley, J. D. (2001). UNESCO/IUPAC-CTC global program in microchemistry. Pure Applied Chemistry, 73(7), 1215–1219. Retrieved from http://www.iupac.org/publications/pac/73/7/1215/.
Durbach, S. & Bradley, J. D. (1998). Hands-on practical chemistry for all—why and how? Journal of Chemical Education, 75(11), 1406–1409.
FDRE (1994). Education and training policy. Federal Democratic Republic of Ethiopia: Addis Ababa.
Gros, N. (2012). Small-Scale, Low-Cost Analytical Instruments: Extended opportunities for learning analytical chemistry. Paper presented at the 1st edition of the international conference on new perspectives in science education. Florence, Italy. Retrieved from http://conference.pixel-online.net/science/conferenceproceedings.php
Hanson, R. (2014). Using small scale chemistry equipment for the study of some organic chemistry topics—a case study in an undergraduate class in Ghana. Journal of Education and Practice, 5(18), 59–63. Retrieved from http://www.iiste.org/Journals/index.php/JEP.
Hanson, R. & Acquah, S. (2014). Enhancing concept understanding through the use of micro chemistry equipment and collaborative activities. Journal of Education and Practice, 5(12), 120–130. Retrieved from http://www.iiste.org/Journals/index.php/JEP.
Hofstein, A. (2004). The laboratory in chemistry education: Thirty years of experience with developments, implementation, and research. Chemistry Education Research and Practice, 5(3), 247–264. doi:10.1039/B4RP90027H.
Hofstein, A. & Lunetta, V. N. (1982). The role of the laboratory in science teaching: Neglected aspects of research. Review of Educational Research, 52(2), 201–217.
Hofstein, A. & Lunetta, V. N. (2004). The laboratory in science education: Foundations for the twenty‐first century. Science Education, 88(1), 28–54.
Jenkins, E. W. (2000). Constructivism in school science education: powerful model or the most dangerous intellectual tendency? Science & Education, 9, 599–610.
Kelkar, S. L. & Dhavale, D. D. (2000). Microscale experiments in chemistry: The need of the new millennium. Resonance, 5(10), 24–31. Retrieved from http://www.ias.ac.in/resonance/.
Lewin, K. (1992). Science education in developing countries: Issues and perspectives for planners. Paris, Unesco: International institute for educational planning. Retrieved from http://www.unesco.org/education/ed_publications/Detailed/228.shtml
Lunetta, V. N., Hofstein, A. & Clough, M. P. (2007). Learning and teaching in the school science laboratory: An analysis of research, theory, and practice. In S. K. Abell & N. G. Lederman (Eds.), Handbook of research on science education (pp. 393–441). Mahwah, NJ: Erlbaum.
Mafumiko, F. S. M. (2006). Micro-scale experimentation as a catalyst for improving the chemistry curriculum in Tanzania (Doctoral dissertation). Retrieved from http://doc.utwente.nl/55448/
Mamlok-Naaman, R. & Barnea, N. (2012). Laboratory activities in Israel. Eurasia Journal of Mathematics, Science & Technology Education, 8(1), 49–57. Retrieved from http://www.ejmste.com/.
Mekelle University (n.d.). Practical general chemistry (Chem.203) manual (Unpublished working paper). Department of chemistry. Mekelle, Ethiopia
Ministry of Education (MoE). (2009). Harmonized curriculum for B.Sc. degree program in chemistry. Addis Ababa, Ethiopia: Ministry of education.
Mohamed, N., Abdullah, M. & Ismail, Z. (2012). Ensuring sustainability through microscale chemistry. In R. Sanghi & V. Singh (Eds.), Green chemistry for environmental remediation (pp. 119–136). Hoboken, NJ: Wiley.
Musar, A. (1993). Equipment for science education constraints and opportunities (ESP discussion paper series; no. 11). Washington, DC: The World Bank. Retrieved from http://documents.worldbank.org/curated/en/1993/10/698835/equipment-science-education-constraints-opportunities
MyLab (2012). Small-scale chemistry grade 10 –12 worksheets. Potchefstroom, South Africa: Northwest University.
Sane, K. V. (1999). Cost-effective science education: The role of educational technology. Staff and Educational Development International, 3(1), 49-60.
Singh, M. M., Szafran, Z. & Pike, R. M. (1999). Microscale chemistry and green chemistry: Complementary pedagogies. Journal of Chemical Education, 76, 1684–1686.
Skinner, J. (Ed.). (1997). Microscale chemistry. Cambridge, England: Royal Society of Chemistry.
Tantayanon, S. (2010). Small-scale chemistry: Experiences for developing countries. Paper presented at the microscale chemistry symposium, Mexico. Retrieved from http://www.uia.mx/investigacion/cmqvm/files/simposio2010/Tantayanon-1.pdf.
Tesfamariam, G., Lykknes, A., & Kvittingen, L. (2014a). Small-scale chemistry for a hands-on approach to chemistry practical work in secondary schools: Experiences from Ethiopia. African Journal of Chemical Education, 4(3), 48–94.
Tesfamariam, G. M., Lykknes, A., & Kvittingen, L. (2014b). Teachers’ perception of the use of small-scale chemistry experimentation. Eilks, S. Markic & B. Ralle (eds.), Science education research and education for sustainable development (pp. 237–242). Aachen, Germany: Shaker.
Tobin, K. (1990). Research on science laboratory activities: In pursuit of better questions and answers to improve learning. School Science and Mathematics, 90(5), 403–418.
Vermaak I. & Bradley J. D. (2003, September). New technologies for effective science education break the cost barrier. Paper presented at the British Educational Research Association Annual Conference. Heriot-Watt University, Edinburg, Scotland. Retrieved from http://www.leeds.ac.uk/educol/documents/00003349.htm
Wood, C. G. (1990). Microchemistry. Journal of Chemical Education, 67(7), 596–597.
Worley B. (n.d.). Microscale and reduced scale chemistry; notes and suppliers. [Brochure] Retrieved from http://www.cleapss.org.uk
Zakaria, Z., Latip, J. & Tantayanon, S. (2012). Organic chemistry practices for undergraduates using a small lab kit. Procedia-Social and Behavioral Sciences, 59, 508–514. doi:10.1016/J.SBSPRO.2012.307.
Zymelman, M. (1990). Science, education and development in sub-Saharan Africa. Washington, DC: The World Bank. Retrieved from http://go.worldbank.org/K9L6M6TT20.
Author information
Authors and Affiliations
Corresponding author
Appendix: SSC Experiment, Sample (Source: MyLab, 2012)
Appendix: SSC Experiment, Sample (Source: MyLab, 2012)
-
Experiment 6: Effect of concentration on reaction rate
-
Objective: to observe how the changing concentration of a reactant will affect the speed of a reaction.
-
Theory: The rate of a chemical reaction can be measured in many ways. In reactions were gases are involved the rate can be monitored by measuring the pressure change in the system. Measuring how fast the colour of a solution changes is another method. Colorimeters are capable of measuring the amount of light passing through a solution containing colour absorbing species. What other methods do you know? In this experiment, hydrochloric acid reacts with sodium thiosulphate (Na2S2O3) to produce colloidal sulphur as one of the products.
$$ 2\mathrm{H}\mathrm{C}\mathrm{l} + {\mathrm{Na}}_2{\mathrm{S}}_2{\mathrm{O}}_3\to {\mathrm{S}\mathrm{O}}_2\left(\mathrm{g}\right) + \mathrm{S}\left(\mathrm{s}\right) + 2{\mathrm{Na}}^{+} + 2{\mathrm{Cl}}^{-} + {\mathrm{H}}_2\mathrm{O} $$ -
Apparatus: MYLAB apparatus stand, 10 test tubes, test tube holder, spatula, thermometer, water bowl, glass beaker
-
Materials: stopwatch, and two sheets of graph paper
-
Chemicals: Hydrochloric acid (6 mol.dm−3), sodium thiosulphate and water
-
Safety: Hydrochloric acid can cause burns and gives off a vapour which is irritating to the respiratory system. Be careful when you work with glass apparatus
-
Procedure: Set up the apparatus as shown in the sketch below
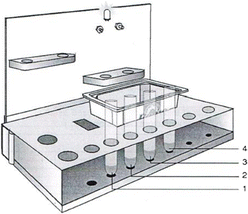
-
1.
Put four test tubes in the apparatus stand as shown on the sketch. Dissolve 5 spatulas of sodium thiosulphate in 10 ml water in the glass beaker
-
2.
Prepare four diluted solutions of sodium thiosulphate as follows in the four test tubes. Use the syringe or the pipette to measure the volume accurately.
Test tube
Sodium thiosulphate, Na2S2O3
water
1
2 ml
0 ml
2
1.5 ml
0.5 ml
3
1 ml
1 ml
4
0.5 ml
1.5 ml
-
3.
Make four dark pencil marks (X) on a paper and put it beneath each of the four test tubes.
-
4.
Use a stopwatch to time the disappearance of the black mark under the test tube. While looking from the top of the test tube through the solution in the test tube, start the stop watch immediately after you have added 2 drops of diluted HCl with a propette to test tube one. Stop the watch when the mark disappears completely.
-
5.
Now, repeat step 4 with test tubes 2, 3, and 4 respectively and tabulate your data.
Post-lab exercise
-
1.
What is the significance of adding varying amounts of water (1 ml to 4 ml) to the four test tubes?
-
2.
Write the reaction of this experiment. What type of reaction is this?
-
3.
Which one of the products of this reaction can be used as an indicator of the rate of the reaction (explain why the other factors cannot be used)
-
4.
How do you prepare a 10 ml and 6 mol.dm−3 solution of HCl from an 11.65 mol.dm−3 stock solution of HCl.
-
5.
Write the possible sources of error in this experiment?
-
6.
What do you learn from this experiment?
-
7.
Suggest any other method to do this experiment or suggest a similar experiment with different reagents, involving the factors that influence the reaction rate.
Rights and permissions
About this article
Cite this article
Tesfamariam, G.M., Lykknes, A. & Kvittingen, L. ‘Named Small but Doing Great’: An Investigation of Small-Scale Chemistry Experimentation for Effective Undergraduate Practical Work. Int J of Sci and Math Educ 15, 393–410 (2017). https://doi.org/10.1007/s10763-015-9700-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10763-015-9700-z





