Abstract
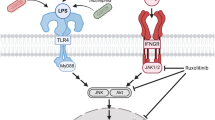
The development of microglial endotoxin tolerance (ET) is a critical event in protecting neurons against excessive immune responses when microglia are administered two consecutive lipopolysaccharide (LPS) challenges. However, the intrinsic mechanisms of microglia shape ET programs and protect neurons remain unclear. This study aimed to determine whether extracellular autocrine cascades or intracellular signaling pathways are involved in ET microglia-mediated tumor necrosis factor-alpha (TNF-α) reduction and neuroprotection. Neuron-glia cultures composed of astroglia, neurons, and microglia were performed in different conditions: with or without serum or LPS-binding proteins (LBP), along with an induction approach of ET. Enzyme-linked immunosorbent assay results revealed that LPS induced TNF-α tolerance of microglia in an LBP-dependent manner. Furthermore, we determined whether the early pro-inflammatory cytokines induced by LPS might contribute to the development of microglial ET. Our data showed that the neutralization of TNF-α using an anti-TNF-α antibody had no change in the TNF-α tolerance of microglia during the ET challenge. Furthermore, pre-incubation of TNF-α, interleukin-1 beta, and prostaglandin E2 failed to induce any TNF-α tolerance in microglia after LPS treatment. Moreover, using three specific chemical inhibitors that respectively blocked the activities of the mitogen-activated protein kinases (MAPKs) namely p38, c-Jun N-terminal kinase and extracellular signal-related kinases revealed that inhibition of p38 MAPK by SB203580 disrupted the tolerated microglia-mediated TNF-α reduction and neuroprotection. In summary, our findings demonstrated that the LPS pre-treatment immediately programmed the microglial ET to prevent endotoxin-induced TNF-α production and neuronal damage through the intracellular p38 MAPK signaling pathway.







Similar content being viewed by others
AVAILABILITY OF DATA AND MATERIALS
All data and materials as well as software application information are available in the manuscript or are available from the corresponding author upon reasonable request.
References
Block, M.L., L. Zecca, and J.S. Hong. 2007. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nature Reviews Neuroscience 8: 57–69.
Hansen, D.V., J.E. Hanson, and M. Sheng. 2018. Microglia in Alzheimer's disease. Journal of Cell Biology 217: 459–472.
Wood, H. 2022. alpha-Synuclein-activated microglia are implicated in PD pathogenesis. Nature Reviews. Neurology 18: 188.
Clarke, B.E., and R. Patani. 2020. The microglial component of amyotrophic lateral sclerosis. Brain 143: 3526–3539.
Yong, V.W. 2022. Microglia in multiple sclerosis: Protectors turn destroyers. Neuron 110: 3534–3548.
Chu, C.H., S.H. Chen, Q. Wang, R. Langenbach, H. Li, D. Zeldin, et al. 2015. PGE2 Inhibits IL-10 Production via EP2-Mediated beta-arrestin signaling in neuroinflammatory condition. Molecular Neurobiology 52: 587–600.
Rosenblum, M.D., K.A. Remedios, and A.K. Abbas. 2015. Mechanisms of human autoimmunity. The Journal of Clinical Investigation 125: 2228–2233.
Rodriguez, A.M., J. Rodriguez, and G.H. Giambartolomei. 2022. Microglia at the crossroads of pathogen-induced neuroinflammation. ASN Neuro 14: 17590914221104566.
Gao, Y., D. Tu, R. Yang, C.H. Chu, J.S. Hong, and H.M. Gao. 2020. Through reducing ROS production, IL-10 suppresses caspase-1-dependent IL-1beta maturation, thereby preventing chronic neuroinflammation and neurodegeneration. International Journal Molecular Sciences 21.
Burguillos, M.A., T. Deierborg, E. Kavanagh, A. Persson, N. Hajji, A. Garcia-Quintanilla, et al. 2011. Caspase signalling controls microglia activation and neurotoxicity. Nature 472: 319–324.
Hilliard, A., P. Mendonca, and K.F.A. Soliman. 2020. Involvement of NFkB and MAPK signaling pathways in the preventive effects of Ganoderma lucidum on the inflammation of BV-2 microglial cells induced by LPS. Journal of Neuroimmunology 345.
Xing, B., A.D. Bachstetter, and L.J. Van Eldik. 2011. Microglial p38alpha MAPK is critical for LPS-induced neuron degeneration, through a mechanism involving TNFalpha. Molecular Neurodegeneration 6: 84.
Kang, J.B., D.J. Park, M.A. Shah, M.O. Kim, and P.O. Koh. 2019. Lipopolysaccharide induces neuroglia activation and NF-kappaB activation in cerebral cortex of adult mice. Laboratory Animal Research 35: 19.
Kwon, J., C. Arsenis, M. Suessmilch, A. McColl, J. Cavanagh, and B.J. Morris. 2022. Differential effects of toll-like receptor activation and differential mediation by MAP kinases of immune responses in microglial cells. Cellular and Molecular Neurobiology 42: 2655–2671.
Chu, C.H., S. Wang, C.L. Li, S.H. Chen, C.F. Hu, Y.L. Chung, et al. 2016. Neurons and astroglia govern microglial endotoxin tolerance through macrophage colony-stimulating factor receptor-mediated ERK1/2 signals. Brain, Behavior, and Immunity 55: 260–272.
Zhang, X., L. Kracht, A.M. Lerario, M.L. Dubbelaar, N. Brouwer, E.M. Wesseling, et al. 2022. Epigenetic regulation of innate immune memory in microglia. Journal of Neuroinflammation 19: 111.
Zhang, Q., and X. Cao. 2021. Epigenetic remodeling in innate immunity and inflammation. Annual Review of Immunology 39: 279–311.
Kuo, H.C., K.F. Lee, S.L. Chen, S.C. Chiu, L.Y. Lee, W.P. Chen, et al. 2022. Neuron-microglia contacts govern the PGE(2) tolerance through TLR4-mediated de novo protein synthesis. Biomedicines 10.
Bras, J.P., J. Bravo, J. Freitas, M.A. Barbosa, S.G. Santos, T. Summavielle, et al. 2020. TNF-alpha-induced microglia activation requires miR-342: Impact on NF-kB signaling and neurotoxicity. Cell Death & Disease 11: 415.
Riazi, K., M.A. Galic, J.B. Kuzmiski, W. Ho, K.A. Sharkey, and Q.J. Pittman. 2008. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proceedings of the National Academy of Sciences of the United States of America 105: 17151–17156.
Qin, L., X. Wu, M.L. Block, Y. Liu, G.R. Breese, J.S. Hong, et al. 2007. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55: 453–462.
Nimmervoll, B., R. White, J.W. Yang, S. An, C. Henn, J.J. Sun, et al. 2013. LPS-induced microglial secretion of TNFalpha increases activity-dependent neuronal apoptosis in the neonatal cerebral cortex. Cerebral Cortex 23: 1742–1755.
Takeuchi, H., S. Jin, J. Wang, G. Zhang, J. Kawanokuchi, R. Kuno, et al. 2006. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. Journal of Biological Chemistry 281: 21362–21368.
Liu, B., L. Du, and J.S. Hong. 2000. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. Journal of Pharmacology and Experimental Therapeutics 293: 607–617.
Ryu, J.K., S.J. Kim, S.H. Rah, J.I. Kang, H.E. Jung, D. Lee, et al. 2017. Reconstruction of LPS transfer cascade reveals structural determinants within LBP, CD14, and TLR4-MD2 for efficient LPS recognition and transfer. Immunity 46: 38–50.
Kim, S.J., and H.M. Kim. 2017. Dynamic lipopolysaccharide transfer cascade to TLR4/MD2 complex via LBP and CD14. BMB Reports 50: 55–57.
Shi, J., Y. Zhao, Y. Wang, W. Gao, J. Ding, P. Li, et al. 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514: 187–192.
Youn, J.H., Y.J. Oh, E.S. Kim, J.E. Choi, and J.S. Shin. 2008. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. The Journal of Immunology 180: 5067–5074.
Jack, R.S., X. Fan, M. Bernheiden, G. Rune, M. Ehlers, A. Weber, et al. 1997. Lipopolysaccharide-binding protein is required to combat a murine gram-negative bacterial infection. Nature 389: 742–745.
Wurfel, M.M., B.G. Monks, R.R. Ingalls, R.L. Dedrick, R. Delude, D. Zhou, et al. 1997. Targeted deletion of the lipopolysaccharide (LPS)-binding protein gene leads to profound suppression of LPS responses ex vivo, whereas in vivo responses remain intact. Journal of Experimental Medicine 186: 2051–2056.
Esen, N., and T. Kielian. 2006. Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. The Journal of Immunology 176: 6802–6811.
O’Neill, L.A., D. Golenbock, and A.G. Bowie. 2013. The history of toll-like receptors - redefining innate immunity. Nature Reviews Immunology 13: 453–460.
Lu, Y.C., W.C. Yeh, and P.S. Ohashi. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42: 145–151.
Cargnello, M., and P.P. Roux. 2011. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases.Microbiology and Molecular Biology Reviews 75: 50–83.
Kawasaki, T., and T. Kawai. 2014. Toll-like receptor signaling pathways. Frontiers in Immunology 5: 461.
Wen, A.Y., K.M. Sakamoto, and L.S. Miller. 2010. The role of the transcription factor CREB in immune function. The Journal of Immunology 185: 6413–6419.
Yang, S.H., A. Galanis, and A.D. Sharrocks. 1999. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Molecular and Cellular Biology 19: 4028–4038.
Han, J., J. Wu, and J. Silke. 2020. An overview of mammalian p38 mitogen-activated protein kinases, central regulators of cell stress and receptor signaling. F1000Research 9.
Maphis, N., S. Jiang, G. Xu, O.N. Kokiko-Cochran, S.M. Roy, L.J. Van Eldik, et al. 2016. Selective suppression of the alpha isoform of p38 MAPK rescues late-stage tau pathology. Alzheimer’s Research & Therapy 8: 54.
Asih, P.R., E. Prikas, K. Stefanoska, A.R.P. Tan, H.I. Ahel, and A. Ittner. 2020. Functions of p38 MAP kinases in the central nervous system. Frontiers in Molecular Neuroscience 13.
Cuadrado, A., and A.R. Nebreda. 2010. Mechanisms and functions of p38 MAPK signalling. The Biochemical Journal 429: 403–417.
Da Silva, J., B. Pierrat, J.L. Mary, and W. Lesslauer. 1997. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. Journal of Biological Chemistry 272: 28373–28380.
Lee, S.H., J. Park, Y. Che, P.L. Han, and J.K. Lee. 2000. Constitutive activity and differential localization of p38alpha and p38beta MAPKs in adult mouse brain. Journal of Neuroscience Research 60: 623–631.
Nito, C., H. Kamada, H. Endo, K. Niizuma, D.J. Myer, and P.H. Chan. 2008. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A2 signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion. Journal of Cerebral Blood Flow and Metabolism 28: 1686–1696.
Fitzsimmons, B.L., M. Zattoni, C.I. Svensson, J. Steinauer, X.Y. Hua, and T.L. Yaksh. 2010. Role of spinal p38alpha and beta MAPK in inflammatory hyperalgesia and spinal COX-2 expression. NeuroReport 21: 313–317.
Lawson, S.K., E.Y. Dobrikova, M. Shveygert, and M. Gromeier. 2013. p38alpha mitogen-activated protein kinase depletion and repression of signal transduction to translation machinery by miR-124 and -128 in neurons. Molecular and Cellular Biology 33: 127–135.
Haines, J.D., D.L. Fulton, S. Richard, and G. Almazan. 2015. p38 mitogen-activated protein kinase pathway regulates genes during proliferation and differentiation in oligodendrocytes. PLoS One1 10.
Bachstetter, A.D., R.K. Rowe, M. Kaneko, D. Goulding, J. Lifshitz, and L.J. Van Eldik. 2013. The p38alpha MAPK regulates microglial responsiveness to diffuse traumatic brain injury. Journal of Neuroscience 33: 6143–6153.
Morganti, J.M., D.S. Goulding, and L.J. Van Eldik. 2019. Deletion of p38alpha MAPK in microglia blunts trauma-induced inflammatory responses in mice. Journal of Neuroinflammation 16: 98.
He, Y., H. She, T. Zhang, H. Xu, L. Cheng, M. Yepes, et al. 2018. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. Journal of Cell Biology 217: 315–328.
Huang, Q., X.F. Mao, H.Y. Wu, H. Liu, M.L. Sun, X. Wang, et al. 2017. Cynandione A attenuates neuropathic pain through p38beta MAPK-mediated spinal microglial expression of beta-endorphin. Brain, Behavior, and Immunity 62: 64–77.
Wendeln, A.C., K. Degenhardt, L. Kaurani, M. Gertig, T. Ulas, G. Jain, et al. 2018. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556: 332–338.
ACKNOWLEDGEMENTS
We thank Professor Jau-Shyong Hong and the National Laboratory Animal Center (NLAC), NARLabs, Taiwan for supporting this work and technical support in contract breeding and testing services, respectively.
Funding
This work was supported by grants MOST 107–2320-B-006–046-MY3, MOST 110–2320-B-255–005 -MY3, and NSTC 111–2320-B-006–018 from the Taiwan Ministry of Science and Technology, and National Science and Technology Council. This study was supported by the grants CMRPF6J0081, CMRPF6J0082, CMRPF6J0083, CMRPF6M0051, ZRRPF6L0011 and ZRRPF6M0011 from Chang Gung Memorial Hospital, Chiayi, Taiwan, and Chang Gung University of Science and Technology, Chia-Yi Campus, Taiwan.
Author information
Authors and Affiliations
Contributions
HC.K. and SC.C. prepared and analyzed the data for Figs. 1–4; SL.C. prepared and analyzed the data for Figs. 5–7; KF. L. prepared and analyzed the data for Figure S1. HC.K. and CH.C. wrote the main manuscript text. CH.C. designed the research protocol and supervised the research. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Animal Care and Use Committee of Chang Gung University of Science and Technology.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuo, HC., Chen, SL., Chiu, SC. et al. Tolerized Microglia Protect Neurons Against Endotoxin-Induced TNF-α Production via an LBP-Dependent Intracellular p38 MAPK Signaling Pathway. Inflammation 46, 2011–2023 (2023). https://doi.org/10.1007/s10753-023-01858-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01858-7