Abstract
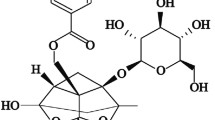
Acetyl-11-keto-beta-boswellic acid (AKBA), a potent anti-inflammatory compound purified from Boswellia species, was investigated in a preclinical study for its potential in preventing and treating non-alcoholic fatty liver disease (NAFLD), the most common chronic inflammatory liver disorder. The study involved thirty-six male Wistar rats, equally divided into prevention and treatment groups. In the prevention group, rats were given a high fructose diet (HFrD) and treated with AKBA for 6 weeks, while in the treatment group, rats were fed HFrD for 6 weeks and then given a normal diet with AKBA for 2 weeks. At the end of the study, various parameters were analyzed including liver tissues and serum levels of insulin, leptin, adiponectin, monocyte chemoattractant protein-1 (MCP-1), transforming growth factor beta (TGF-β), interferon gamma (INF-ϒ), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α). Additionally, the expression levels of genes related to the inflammasome complex and peroxisome proliferator-activated receptor gamma (PPAR-ϒ), as well as the levels of phosphorylated and non-phosphorylated AMP-activated protein kinase alpha-1 (AMPK-α1) protein, were measured. The results showed that AKBA improved NAFLD-related serum parameters and inflammatory markers and suppressed PPAR-ϒ and inflammasome complex-related genes involved in hepatic steatosis in both groups. Additionally, AKBA prevented the reduction of the active and inactive forms of AMPK-α1 in the prevention group, which is a cellular energy regulator that helps suppress NAFLD progression. In conclusion, AKBA has a beneficial effect on preventing and avoiding the progression of NAFLD by preserving lipid metabolism, improving hepatic steatosis, and suppressing liver inflammation.






Similar content being viewed by others
Availability of Data and Materials
Data are available via requests directed to the corresponding author.
References
Younossi, Z.M., A.B. Koenig, D. Abdelatif, Y. Fazel, L. Henry, and M. Wymer. 2016. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64: 73–84.
Lee, Y.A., and S.L. Friedman. 2022. Inflammatory and fibrotic mechanisms in NAFLD—Implications for new treatment strategies. Journal of Internal Medicine 291: 11–31.
Barbieri, E., N. Santoro, G.R. and Umano. 2023. Clinical features and metabolic complications for non-alcoholic fatty liver disease (NAFLD) in youth with obesity. Frontiers in Endocrinology 14.
Stefan, N., H.-U. Häring, and K. Cusi. 2019. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. The lancet Diabetes & endocrinology 7: 313–324.
He, L., G.S. Babar, J.M. Redel, S.L. Young, C.E. Chagas, W.V. Moore, and Y. Yan. 2021. Fructose intake: metabolism and role in diseases. In Sugar Intake-Risks and Benefits and the Global Diabetes Epidemic: IntechOpen.
Pereira, R.M., J.D. Botezelli, K.C. da Cruz Rodrigues, R.A. Mekary, D.E. Cintra, J.R. Pauli, A.S.R. Da Silva, E.R. Ropelle, and L.P. De Moura. 2017. Fructose consumption in the development of obesity and the effects of different protocols of physical exercise on the hepatic metabolism. Nutrients 9: 405.
Jegatheesan, P., and J.P. De Bandt. 2017. Fructose and NAFLD: The multifaceted aspects of fructose metabolism. Nutrients 9: 230.
Federico, A., V. Rosato, M. Masarone, P. Torre, M. Dallio, M. Romeo, and M. Persico. 2021. The role of fructose in non-alcoholic steatohepatitis: Old relationship and new insights. Nutrients 13: 1314.
Liu, Y., H. Lin, L. Jiang, Q. Shang, L. Yin, J.D. Lin, W.-S. Wu, and L. Rui. 2020. Hepatic Slug epigenetically promotes liver lipogenesis, fatty liver disease, and type 2 diabetes. The Journal of Clinical Investigation 130: 2992–3004.
Baboota, R.K., R. Spinelli, M.C. Erlandsson, B.B. Brandao, M. Lino, H. Yang, A. Mardinoglu, M.I. Bokarewa, J. Boucher, and C.R. Kahn. 2022. Chronic hyperinsulinemia promotes human hepatocyte senescence. Molecular Metabolism 64: 101558.
Zhang, A.M., E.A. Wellberg, J.L. Kopp, and J.D. Johnson. 2021. Hyperinsulinemia in obesity, inflammation, and cancer. Diabetes & metabolism journal 45: 285–311.
Jung, U.J., and M.-S. Choi. 2014. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. International journal of molecular sciences 15: 6184–6223.
Longo, M., F. Zatterale, J. Naderi, L. Parrillo, P. Formisano, G.A. Raciti, F. Beguinot, and C. Miele. 2019. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. International journal of molecular sciences 20: 2358.
Petrescu, M., S.I. Vlaicu, L. Ciumărnean, M.V. Milaciu, C. Mărginean, M. Florea, ȘC. Vesa, and M. Popa. 2022. Chronic inflammation—a link between nonalcoholic fatty liver disease (NAFLD) and dysfunctional adipose tissue. Medicina 58: 641.
Paradies, G., V. Paradies, F.M. Ruggiero, and G. Petrosillo. 2014. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World journal of gastroenterology: WJG 20: 14205.
Yu, L., W. Hong, S. Lu, Y. Li, Y. Guan, X. Weng, and Z. Feng. 2022. The NLRP3 inflammasome in non-alcoholic fatty liver disease and steatohepatitis: Therapeutic targets and treatment. Frontiers in Pharmacology 13: 682.
Knorr, J., A. Wree, F. Tacke, A.E. Feldstein. 2020. The NLRP3 inflammasome in alcoholic and nonalcoholic steatohepatitis. Presented at Seminars in liver disease.
Gehrke, N., and J.M. Schattenberg. 2020. Metabolic inflammation—a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology 158 (1929–47): e6.
Smith, B.K., K. Marcinko, E.M. Desjardins, J.S. Lally, R.J. Ford, and G.R. Steinberg. 2016. Treatment of nonalcoholic fatty liver disease: Role of AMPK. American Journal of Physiology-Endocrinology and Metabolism 311: E730–E740.
Zhao, P., and A.R. Saltiel. 2020. From overnutrition to liver injury: AMP-activated protein kinase in nonalcoholic fatty liver diseases. Journal of Biological Chemistry 295: 12279–12289.
Fang, C., J. Pan, N. Qu, Y. Lei, J. Han, J. Zhang, D. Han. 2022. The AMPK pathway in fatty liver disease. Frontiers in Physiology 13.
Liss, K.H., and B.N. Finck. 2017. PPARs and nonalcoholic fatty liver disease. Biochimie 136: 65–74.
Ducheix, S., A. Montagner, V. Theodorou, L. Ferrier, and H. Guillou. 2013. The liver X receptor: A master regulator of the gut–liver axis and a target for non alcoholic fatty liver disease. Biochemical pharmacology 86: 96–105.
Fleischman, M.W., M. Budoff, I. Zeb, D. Li, and T. Foster. 2014. NAFLD prevalence differs among hispanic subgroups: The Multi-Ethnic Study of Atherosclerosis. World journal of gastroenterology: WJG 20: 4987.
Xu, Y., W. Guo, C. Zhang, F. Chen, H.Y. Tan, S. Li, N. Wang, and Y. Feng. 2020. Herbal medicine in the treatment of non-alcoholic fatty liver diseases-efficacy, action mechanism, and clinical application. Frontiers in Pharmacology 11: 601.
Tian, Y., J. Ma, W. Wang, L. Zhang, J. Xu, K. Wang, and D. Li. 2016. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Molecular and cellular biochemistry 422: 75–84.
Jiang, J., L. Yan, Z. Shi, L. Wang, L. Shan, and T. Efferth. 2019. Hepatoprotective and anti-inflammatory effects of total flavonoids of Qu Zhi Ke (peel of Citrus changshan-huyou) on non-alcoholic fatty liver disease in rats via modulation of NF-κB and MAPKs. Phytomedicine 64: 153082.
Roy, N.K., D. Parama, K. Banik, D. Bordoloi, A.K. Devi, K.K. Thakur, G. Padmavathi, M. Shakibaei, L. Fan, and G. Sethi. 2019. An update on pharmacological potential of boswellic acids against chronic diseases. International journal of molecular sciences 20: 4101.
Sharma, T., and S. Jana. 2020. Boswellic acids as natural anticancer medicine: Precious gift to humankind. Journal of Herbal Medicine 20: 100313.
Siddiqui, A., Z. Shah, R.N. Jahan, I. Othman, and Y. Kumari. 2021. Mechanistic role of boswellic acids in Alzheimer’s disease: Emphasis on anti-inflammatory properties. Biomedicine & Pharmacotherapy 144: 112250.
Kawasaki, T., K. Igarashi, T. Koeda, K. Sugimoto, K. Nakagawa, S. Hayashi, R. Yamaji, H. Inui, T. Fukusato, and T. Yamanouchi. 2009. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. The Journal of nutrition 139: 2067–2071.
Upadhayay, S., S. Mehan, A. Prajapati, P. Sethi, M. Suri, A. Zawawi, M.N. Almashjary, and S. Tabrez. 2022. Nrf2/HO-1 signaling stimulation through acetyl-11-keto-beta-boswellic acid (AKBA) provides neuroprotection in ethidium bromide-induced experimental model of multiple sclerosis. Genes 13: 1324.
Li, W., J. Liu, W. Fu, X. Zheng, L. Ren, S. Liu, J. Wang, T. Ji, and G. Du. 2018. 3-O-acetyl-11-keto-β-boswellic acid exerts anti-tumor effects in glioblastoma by arresting cell cycle at G2/M phase. Journal of Experimental & Clinical Cancer Research 37: 1–15.
Meratan, A.A., and M. Nemat-Gorgani. 2012. Mitochondrial membrane permeabilization upon interaction with lysozyme fibrillation products: role of mitochondrial heterogeneity. Biochimica et Biophysica Acta (BBA)-Biomembranes 1818: 2149–57.
Sottacasa, G., B. Kuylenstierna, L. Ernster, and A. Bergstrand. 1967. Separation and some enzymatic properties of the inner and outer membrane of rat liver mitochondria. Methods in Enzymology 10: 457.
Müller-Kraft, G., and W. Babel. 1990. [53] Citrate synthases from methylotrophs. Methods in enzymology 188: 350–354.
Xu, J., J. Shen, R. Yuan, B. Jia, Y. Zhang, S. Wang, Y. Zhang, M. Liu, T. Wang. 2021. Mitochondrial targeting therapeutics: promising role of natural products in non-alcoholic fatty liver disease. Frontiers in Pharmacology 12.
Minj, E., S. Upadhayay, and S. Mehan. 2021. Nrf2/HO-1 signaling activator acetyl-11-keto-beta boswellic acid (AKBA)-mediated neuroprotection in methyl mercury-induced experimental model of ALS. Neurochemical research 46: 2867–2884.
Marefati, N., F. Beheshti, S. Memarpour, R. Bayat, M.N. Shafei, H.R. Sadeghnia, H. Ghazavi, and M. Hosseini. 2020. The effects of acetyl-11-keto-β-boswellic acid on brain cytokines and memory impairment induced by lipopolysaccharide in rats. Cytokine 131: 155107.
Ahmed, M.A., A.A. Ahmed, and E.M. El Morsy. 2020. Acetyl-11-keto-β-boswellic acid prevents testicular torsion/detorsion injury in rats by modulating 5-LOX/LTB4 and p38-MAPK/JNK/Bax/Caspase-3 pathways. Life Sciences 260: 118472.
Moussaieff, A., and R. Mechoulam. 2009. Boswellia resin: From religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. Journal of Pharmacy and Pharmacology 61: 1281–1293.
Bini Araba, A., N. Ur Rehman, A. Al-Araimi, S. Al-Hashmi, S. Al-Shidhani, R. Csuk, H. Hussain, A. Al-Harrasi, and F. Zadjali. 2021. New derivatives of 11-keto-β-boswellic acid (KBA) induce apoptosis in breast and prostate cancers cells. Natural Product Research 35: 707–716.
Taherzadeh, D., V. Baradaran Rahimi, H. Amiri, S. Ehtiati, R. Yahyazadeh, S.I. Hashemy, and V.R. Askari. 2022. Acetyl-11-Keto-β-Boswellic acid (AKBA) prevents lipopolysaccharide-induced inflammation and cytotoxicity on H9C2 cells. Evidence-based Complementary and Alternative Medicine 2022.
Nassir, F., and J.A. Ibdah. 2014. Role of mitochondria in nonalcoholic fatty liver disease. International journal of molecular sciences 15: 8713–8742.
Monzio Compagnoni, G., A. Di Fonzo, S. Corti, G.P. Comi, N. Bresolin, and E. Masliah. 2020. The role of mitochondria in neurodegenerative diseases: The lesson from Alzheimer’s disease and Parkinson’s disease. Molecular neurobiology 57: 2959–2980.
Kang, W., M. Suzuki, T. Saito, and K. Miyado. 2021. Emerging role of TCA cycle-related enzymes in human diseases. International Journal of Molecular Sciences 22: 13057.
Santamarina, A.B., M. Carvalho-Silva, L.M. Gomes, M.H. Okuda, A.A. Santana, E.L. Streck, M. Seelaender, C.M.O. do Nascimento, E.B. Ribeiro, and F.S. Lira. 2015. Decaffeinated green tea extract rich in epigallocatechin-3-gallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. The Journal of Nutritional Biochemistry 26: 1348–1356.
Ferramosca, A., A. Conte, and V. Zara. 2015. Krill oil ameliorates mitochondrial dysfunctions in rats treated with high-fat diet. BioMed Research International 2015.
Ma, Y., M. Gao, and D. Liu. 2016. Alternating diet as a preventive and therapeutic intervention for high fat diet-induced metabolic disorder. Scientific reports 6: 1–14.
Skat-Rørdam, J., D. Højland Ipsen, J. Lykkesfeldt, and P. Tveden-Nyborg. 2019. A role of peroxisome proliferator-activated receptor γ in non-alcoholic fatty liver disease. Basic & clinical pharmacology & toxicology 124: 528–537.
Zhang, Y.-L., A. Hernandez-Ono, P. Siri, S. Weisberg, D. Conlon, M.J. Graham, R.M. Crooke, L.-S. Huang, and H.N. Ginsberg. 2006. Aberrant hepatic expression of PPARγ2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. Journal of Biological Chemistry 281: 37603–37615.
Awad, A.S., E.N. Abd Al Haleem, W.M. El-Bakly, and M.A. Sherief. 2016. Thymoquinone alleviates nonalcoholic fatty liver disease in rats via suppression of oxidative stress, inflammation, apoptosis. Naunyn-Schmiedeberg’s archives of pharmacology 389: 381–391.
Chyau, C.-C., H.-F. Wang, W.-J. Zhang, C.-C. Chen, S.-H. Huang, C.-C. Chang, and R.Y. Peng. 2020. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. International Journal of Molecular Sciences 21: 360.
Gao, H., T. Guan, C. Li, G. Zuo, J. Yamahara, J. Wang, and Y. Li. 2012. Treatment with ginger ameliorates fructose-induced Fatty liver and hypertriglyceridemia in rats: modulation of the hepatic carbohydrate response element-binding protein-mediated pathway. Evidence-Based Complementary and Alternative Medicine 2012.
Li, X., Z. Xu, S. Wang, H. Guo, S. Dong, T. Wang, L. Zhang, and Z. Jiang. 2016. Emodin ameliorates hepatic steatosis through endoplasmic reticulum–stress sterol regulatory element-binding protein 1c pathway in liquid fructose-feeding rats. Hepatology Research 46: E105–E117.
Trepiana, J., I. Milton-Laskibar, S. Gómez-Zorita, I. Eseberri, M. González, A. Fernández-Quintela, and M.P. Portillo. 2018. Involvement of 5′ AMP-activated protein kinase (AMPK) in the effects of resveratrol on liver steatosis. International journal of molecular sciences 19: 3473.
Boudaba, N., A. Marion, C. Huet, R. Pierre, B. Viollet, and M. Foretz. 2018. AMPK re-activation suppresses hepatic steatosis but its downregulation does not promote fatty liver development. eBioMedicine 28: 194–209.
Shen, T., B. Xu, T. Lei, L. Chen, C. Zhang, and Z. Ni. 2018. Sitagliptin reduces insulin resistance and improves rat liver steatosis via the SIRT1/AMPKα pathway. Experimental and therapeutic medicine 16: 3121–3128.
ALTamimi JZ, Alshammari GM, AlFaris NA, Alagal RI, Aljabryn DH, Albekairi NA, Alkhateeb MA, and Yahya MA. 2022. Ellagic acid protects against non-alcoholic fatty liver disease in streptozotocin-diabetic rats by activating AMPK. Pharmaceutical Biology 60: 25–37.
Shiwa, M., M. Yoneda, H. Okubo, H. Ohno, K. Kobuke, Y. Monzen, R. Kishimoto, Y. Nakatsu, T. Asano, and N. Kohno. 2015. Distinct time course of the decrease in hepatic AMP-activated protein kinase and Akt phosphorylation in mice fed a high fat diet. PLoS ONE 10: e0135554.
Guo, H., J.B. Callaway, and J.P. Ting. 2015. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nature medicine 21: 677–687.
Gong, Y., X. Jiang, S. Yang, Y. Huang, J. Hong, Y. Ma, X. Fang, Y. Fang, and J. Wu. 2022. The biological activity of 3-O-acetyl-11-keto-β-boswellic acid in nervous system diseases. NeuroMolecular Medicine 1–11.
Majeed, M., K. Nagabhushanam, L. Lawrence, R. Nallathambi, V. Thiyagarajan, and L. Mundkur. 2021. Boswellia serrata extract containing 30% 3-acetyl-11-keto-boswellic acid attenuates inflammatory mediators and preserves extracellular matrix in collagen-induced arthritis. Frontiers in Physiology 1578.
Suther, C., L. Devon, L. Daddi, A. Matson, H. Panier, H. Yuan, K. Saar, S. Bokoliya, Y. Dorsett, and D.A. Sela. 2022. Dietary Indian frankincense (Boswellia serrata) ameliorates murine allergic asthma through modulation of the gut microbiome. Journal of Functional Foods 97: 105249.
Funding
This work was supported by Shahid Beheshti University of Medical Sciences, grant number 17283.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Reza Ataei Kachouei and Ali Jahanbazi Jahan-Abad participated in the preparation of non-alcoholic fatty liver disease rat models. Material preparation was done by Farzaneh Salmani and Somayeh Mahmoodi Baram. Data collection and analysis were performed by Reza Ataei Kachouei, Alireza Doagoo, Maral Jalilzadeh, Seyyed Hossein Khatami, Roya Pakrad, Mohammad-Amin Abdollahifar, Hojjat Allah Abbaszadeh, Meisam Mahdavi, and Mitra Rezaei. The first draft of the manuscript was written by Reza Ataei Kachouei with support from Saeed Karima and Shima Rajaei. Mitra Nourbakhsh, Mohammad Reza Shahmohammadi, and Shokoofeh Noori commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experiments were approved by the ethics committee at Shahid Beheshti University of medical sciences (Unique identifier: IR.SBMU.MCP.REC.1397.738) and were conducted in accordance with the Animal Care Committee guidelines of Shahid Beheshti University of medical sciences.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kachouei, R.A., Doagoo, A., Jalilzadeh, M. et al. Acetyl-11-Keto-Beta-Boswellic Acid Has Therapeutic Benefits for NAFLD Rat Models That Were Given a High Fructose Diet by Ameliorating Hepatic Inflammation and Lipid Metabolism. Inflammation 46, 1966–1980 (2023). https://doi.org/10.1007/s10753-023-01853-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01853-y