Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is considered a critical hepatic manifestation of metabolic syndrome. Berberine (BBR) exerts anti-hyperglycemic and anti-dyslipidemic effects and can also ameliorate NAFLD. Thus, BBR might exert its therapeutic effect on NAFLD by improving glucolipid metabolism. Here, we investigated the aspects and extent to which glucolipid metabolism were affected by BBR in rats with NAFLD.
Methods
Three groups of Sprague–Dawley rats were studied: a control group (n = 6) fed a normal chow diet and a NAFLD group (n = 6) and a NAFLD + BBR group (n = 6) fed a high-fat diet. Normal saline and BBR (150 mg/kg body weight/day for 16 weeks) were administered by gavage. All rats were infused with isotope tracers. The rates of glucose appearance (Raglu), gluconeogenesis (GNG) and glycerol appearance (Ragly) were assessed with 2H and 13C tracers, whereas the rates of hepatic lipogenesis and fatty acid β oxidation were measured using the 3H tracer.
Results
When the NAFLD model was successfully induced by administering a high-fat diet, body weight, insulin resistance and dyslipidemia were significantly increased. After the BBR treatment, weight loss, decreased lipid profiles and HOMA-IR, and increased ISI were observed. Meanwhile, BBR reduced Raglu, GNG and hepatic lipogenesis, whereas the rate of fatty acid β oxidation in skeletal muscle showed an increasing trend. Ragly showed a decreasing trend. Based on the results of the histological analysis, BBR obviously attenuated the ectopic liver fat accumulation.
Conclusions
BBR improved NAFLD by inhibiting glucogenesis and comprehensively regulating lipid metabolism, and its effect on inhibiting hepatic lipogenesis was much stronger. The improvement may be partly mediated by weight loss. Berberine might be a good choice for patients with NAFLD and glucose metabolic disorder. Future clinical trials need to be conducted to confirm these effects.
Similar content being viewed by others
Background
NAFLD has become the second most common liver disease in China after viral hepatitis [1]. Due to the alterations in lifestyle and the epidemic of obesity, the prevalence of NAFLD is increasing worldwide [2]: up to 30% in developed countries and nearly 10% in developing nations [3]. NAFLD encompasses the whole spectrum of liver diseases that are not associated with significant alcohol consumption, including simple hepatic steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis, liver failure and even hepatocellular carcinoma (HCC) [4]. However, the precise mechanisms underlying the pathogenesis of NAFLD remain unclear. A well-known “two-hit” hypothesis has been proposed for NAFLD progression [5]. The “first hit” is the development of hepatic steatosis as a result of insulin resistance, whereas in the “second hit”, proinflammatory mediators induce hepatic inflammation, hepatocellular injury, fibrosis and cirrhosis. Furthermore, lifestyle modifications are the only suggested remedy to avoid progression of benign steatosis to NASH and fibrosis [6]. With the exception of preventive lifestyle intervention, anti-diabetic, lipid-lowering or anti-hypertensive agents may be used to control NAFLD comorbidities. Currently, no approved pharmacological agents are available for NAFLD [5]. Recently, Chinese herbs, including berberine (BBR), have received more attention as treatments for NAFLD.
BBR, an isoquinoline alkaloid, is a natural compound in numerous Chinese herb plants such as Berberisaristata, Coptischinensis, Coptis rhizome, etc. [7]. Low doses of BBR have been widely used to treat intestinal bacteria-related diarrhea with good safety for thousands of years. Over the last few decades, many animal studies and clinical trials have reported the anti-hyperglycemic and anti-dyslipidemic effects of BBR [8–10]. Interestingly, in these investigations, BBR was also reported to have a potent effect on reducing hepatic steatosis [8, 10]. Although the bioavailability of BBR was reported to be less than 1% in some studies [11, 12], other studies indicated that BBR was typically concentrated in the liver (at levels 50–70 times higher than the plasma levels) after oral administration [10, 13]. Moreover, in some animal experiments, BBR was shown to alleviate TG deposition in the liver following an intraperitoneal injection or oral gavage [6]; therefore, BBR is indeed suitable as a treatment for NAFLD [13]. However, the mechanism underlying its therapeutic effect is still unclear. Many possible metabolic pathways have been suggested, such as decreasing the severity of liver and adipose tissue inflammation [4], modulating ER stress [6], regulating the expression of hepatic genes related to glucolipid metabolism [10] and gut microbiota [14], etc. However, comprehensive studies on the aspects and extent to which glucolipid metabolism in visceral and peripheral tissues in NAFLD were affected by BBR are lacking.
In the present study, we used isotope tracers to explore the effect of BBR on hepatic and extra hepatic glucolipid metabolism in rats with NAFLD.
Methods
Animal model establishment and experimental design
Eighteen male Sprague-Dawley rats (8 weeks old) were obtained from Shanghai Laboratory Animal Center in China. The animals were maintained on a 12/12-h light/dark cycle in a temperature-controlled room (22 ± 2 °C) and given free access to food and water. After 1 week of acclimation, the animals were randomly divided into two groups: a control group (NCD group, n = 6) receiving a normal chow diet (NCD:65.5% carbohydrate, 20% protein and 10.3% fat) and a HFD group (n = 12) fed a high-fat diet (HFD:40% carbohydrate, 20% protein and 40% fat). After 16 weeks, the NAFLD Model was successfully induced in these 12 rats (verified by ultrasound diagnosis), which were further subdivided into two groups (6 rats per group): (i) the NAFLD group, which was administered an equal volume of normal saline, and (ii) the NAFLD + BBR group, which was treated with 150 mg BBR/kg body weight/day (Sigma-Aldrich, USA) by gavage for 16 weeks.
Body weights and fasting blood glucose (FBG) levels were measured every 2 weeks. Glucose levels were immediately measured using an electronic glucometer (Terumo, Tokyo, Japan). Lipid profiles, including the total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterol (LDL) and free fatty acid (FFA) and insulin levels, were assayed at the 0th, 16th, and 32nd weeks using Siemens Dimension MAX (Siemens Healthcare Diagnostics Inc.) and ELISA kits (Shibayaji, Japan). Then, the HOMA-IR and insulin sensitivity index (ISI) were calculated from the FBG and fasting insulin (FINS) levels using the following detailed formulas: HOMA-IR = FBG*FINS/22.5; ISI = Ln[1/(FBG*FINS)] [15]. All experimental procedures were conducted in accordance with the ethical principles in animal research adopted by the Department of Laboratory Animal Science and approved by the Animal Experimental Ethical Committee of Jiaotong University School of Medicine, Shanghai, China.
Isotope infusion
All rats were fasted overnight and studied the following morning. After local anesthesia was induced with lidocaine, the lateral tail vein was catheterized for the infusion of tracers and the tail artery was catheterized for blood sampling using previously described methods [16]. The animals were conscious and relaxed throughout the experiments; in addition, the animals could groom themselves normally, drink water freely or sit calmly to minimize experimentally induced stress (Fig. 1). When the FBG levels returned to baseline (usually within 30 min), 6,6-2D-glucose (2 μmol/kg/ min) and U-13C-glycerol (0.84 μmol/kg/min) were constantly infused through the i.v. infusion line driven by a Harvard mini-infusion pump (Harvard Apparatus, Holliston, MA, USA) for 90 min. At 60 min, 1 μCi of 9,10-3H-palmitic acid was injected. During the final 10 min, three arterial blood samples (0.5 ml each) were collected at 5 min intervals and were used to quantify steady state glucose and glycerol metabolism (Fig. 2). Then, the animals were euthanized by opening the heart under anesthesia with pentobarbital (50 mg/kg). A strip of gastrocnemius muscle (approximately 13*3*1 mm) was promptly obtained to examine β oxidation of 9,10-3H-palmitic acid (1 μCi) in vitro. Livers were also harvested swiftly, immersed in liquid nitrogen and stored at −80 °C. Plasma samples were prepared on ice, centrifuged at 4 °C, separated and stored at −80 °C until further analysis.
Experimental conditions used for tracer perfusion. The lateral tail vein was catheterized for tracer infusion and the tail artery was catheterized for blood collection. Throughout the experiments, all rats were conscious and relaxed, with the ability to groom themselves normally and drink water freely. Thus, the experimentally induced stress was minimized
Measurement of isotope tracers
Plasma samples were processed with methoxyamine-HCl and BSTFA to obtain the trimethylsilyl derivatives of 6, 6-2D-glucose and U-13C-glycerol. Hydroxylamine hydrochloride was used for derivation to avoid interference from the reaction of 6,6-2D-glucose to 1,2,3-13C-glucose formed in GNG via U-13C-glycerol. Then, the enrichment of the derivatives was measured using gas chromatography/mass spectrometry (GC-MS, Agilent 5975C, Agilent Technologies). Ions with mass-to-charge ratios (m/z) of 319 (unlabeled glucose) and 321 (labeled glucose) were monitored. The peak area 321/319 ratio was calculated, and the corresponding enrichment was determined from standard curves. A similar method was used to obtain the m/z 221/218 U-13C-glycerol and m/z 215/212 1,2,3-13C-glucose ratios and determine their corresponding enrichment.
Lipids were extracted from the liver using the Folch method [17], and triglyceride concentrations were assayed using an ELISA kit (Jiancheng, Nanjing, China). Then, pure triglycerides were isolated using thin layer chromatography (TLC). In addition, 3H2O converted from 9,10-3H-palmitic acid during the process of β oxidation was obtained by removing the lipids with chloroform. 3H radioactivity from both liver triglycerides and 3H2O was determined using liquid scintillation counting (LS6500 Multipurpose Scintillation Counter, Beckman, USA) as previously described [16].
Calculations
The appearance rates (Ra) of glucose and glycerol were calculated with the steady-state equation from the respective tracer infusion rates (F) and mole percent excess (MPE). The infusion rate of 6,6-2D-glucose was divided by the MPE of plasma glucose to yield Raglu [18]. The detailed formula for Ragly was described previously [16]. The glycerol gluconeogenesis rates were calculated using the following formula: 1,2,3-13 C-glucose MPE*Raglu /U-13C-glycerol MPE [18]. The percent of glycerol converted to glucose was calculated using the following formula: Ragly*13C-glucose MPE/Fgly. Fatty acid β oxidation rates were deduced by measuring the specific activity of 3H2O. Moreover, the hepatic fat synthesis rate was calculated by dividing the total concentrations of triglycerides by the radioactivity of the corresponding labeled triglycerides [19].
Liver pathomorphology
After the rats were sacrificed, part of their livers was fixed in 4% paraformaldehyde, dehydrated with ethanol and xylene, embedded in paraffin, and sliced into 5 μm sections on a microtome (SLEE, Germany). The sections were stained with hematoxylin and eosin (H&E) and analyzed under an optical microscope (CKX41, Olympus, Japan) to examine the pathologic structures of the livers.
Statistical analysis
The software package SPSS version 17.0 was used for data analysis. All data are presented as the means ± standard deviations (SD) and statistical significance was assessed by one-way ANOVA followed by LSD for multiple comparisons as appropriate. P < 0.05 was considered statistically significant.
Results
Berberine reduced body weight gain and regulated the FBG levels
Prior to the experiment, no differences were observed in body weights or FBG levels between the three groups. When the NAFLD model was successfully established, a significant increase in body weight was observed (NAFLD: 648 ± 69.95; NAFLD + BBR: 641.67 ± 35.90; NCD: 568.5 ± 36.09, P < 0.05), but the FBG levels remained unchanged. After the 16-week BBR intervention, the NAFLD + BBR group (736 ± 20.80) exhibited a significant reduction in body weight compared with the NAFLD group (828.67 ± 86.78) (P < 0.05, Fig. 3a). However, the FBG levels were obviously decreased during the first 8-week treatment period (P < 0.05, NCD vs NAFLD vs NAFLD + BBR: 3.60 ± 0.59 vs 4.12 ± 0.28 vs 2.74 ± 0.78, respectively), whereas similar FBG levels were observed in the three groups (NCD vs NAFLD vs NAFLD + BBR: 4.77 ± 1.44 vs 5.35 ± 0.42 vs 4.98 ± 0.84, respectively) at the end of the experiment (Fig. 3b).
Changes in body weights, FBG levels, FINS levels, HOMA-IR and ISI in the three groups before and after the BBR intervention. a A significant increase in body weight was observed at the 16th week after the successful establishment of the NAFLD model. After the 16 week BBR intervention, the NAFLD + BBR group exhibited a significant reduction in body weight compared with NAFLD group.b During the first 8-week treatment period, the FBG levels were obviously decreased, but differences were not observed between the three groups at the end of the treatment. After the NAFLD model was successfully established, the FINS concentrations (c) and HOMA-IR (d) increased significantly and the ISI (e) decreasing dramatically. BBR reduced the FINS concentrations, HOMA-IR and increased the ISI at the 32nd week, although the differences were not significant. The data are presented as the means ± SEM. *P < 0.05, NCD vs NAFLD + BBR; &P < 0.05, NCD vs NAFLD; #P < 0.05, NAFLD vs NAFLD + BBR
Berberine improves insulin resistance
After 16 weeks of feeding the HFD, the FINS concentrations (NAFLD: 2.44 ± 0.87; NAFLD + BBR: 2.75 ± 0.85) and HOMA-IR (NAFLD: 0.53 ± 0.14; NAFLD + BBR: 0.54 ± 0.17) in the two HFD groups were significantly higher than the values in the NCD group (FINS: 1.36 ± 0.48; HOMA-IR: 0.23 ± 0.07, P < 0.05). However, no differences were observed between NAFLD group and NAFLD + BBR group (Fig. 3c and d). In contrast, the NCD group (−1.46 ± 0.50) had a higher ISI than the two HFD groups (NAFLD: −2.46 ± 0.25; NAFLD + BBR: −2.46 ± 0.32, P < 0.05), but no difference was observed between the latter two groups (Fig. 3e). However, after the 16 weeks intervention with BBR, the NAFLD + BBR group showed an obvious improvement in the FINS concentrations (1.57 ± 0.39), HOMA-IR (0.37 ± 0.15) and ISI (−2.07 ± 0.39) compared with the NAFLD group (2.73 ± 1.18, 0.65 ± 0.22, −2.65 ± 0.31, respectively), although the differences were not statistically significant.
Berberine attenuated the plasma lipid profiles
Compared to the NCD group (TG: 0.80 ± 0.33; TC: 1.56 ± 0.18; LDL: 0.19 ± 0.02), the two HFD groups had higher TG (NAFLD: 1.16 ± 0.43; NAFLD + BBR: 1.30 ± 0.38), TC (NAFLD: 1.67 ± 0.13; NAFLD + BBR: 1.76 ± 0.17) and LDL (NAFLD: 0.24 ± 0.03; NAFLD + BBR: 0.21 ± 0.06) levels beginning at the 16th week, but the differences between groups were not significant (P > 0.05). Over the next 16 weeks, the rats with NAFLD had the highest plasma TG (1.11 ± 0.05), TC (1.92 ± 0.44) and LDL (0.41 ± 0.09) levels. BBR reversed the elevated plasma lipid profiles of the NAFLD + BBR group (TG: 0.70 ± 0.16; TC: 1.55 ± 0.21; LDL: 0.25 ± 0.07) to levels similar to the NCD group (TG: 0.72 ± 0.05; TC: 1.66 ± 0.06; LDL: 0.24 ± 0.03), particularly the TG and LDL levels (Fig. 4a and b). During the entire trial, differences were not observed in the FFA levels between the three groups (P > 0.05, data not shown).
Changes in the TG and LDL concentrations in the three groups at the 0th, 16th, and 32nd weeks. The TG (a) and LDL (b) levels were higher in the two HFD groups at the 16th week, but the differences between the groups were not significant. Over the next 16 weeks, BBR reversed the elevated TG and LDL levels to levels similar to the NCD group. The data are expressed as the means ± SEM. &P < 0.05, NCD vs NAFLD; #P < 0.05, NAFLD vs NAFLD + BBR
Berberine decreased Raglu, Ragly, GNG from glycerol and the percent of glycerol converted to glucose (%)
Although significant differences in Ragly were not observed between the three groups, Ragly was still the highest in the NAFLD group (69.74 ± 27.95) and was similar in the NCD (47.64 ± 6.36) and NAFLD + BBR (49.36 ± 18.60) groups (Fig. 5a). Raglu was significantly increased in the NAFLD group (111.32 ± 51.88, P < 0.05, vs NCD or NAFLD + BBR), whereas the NCD (67.24 ± 12.68) and NAFLD + BBR (57.97 ± 10.44) groups had similar Raglu values (Fig. 5b). The GNG from glycerol primarily increased in the NAFLD group (16.64 ± 7.93, P < 0.05, vs NCD or NAFLD + BBR), whereas it was comparable in the NCD (3.63 ± 1.44) and NAFLD + BBR (5.09 ± 2.82) groups (Fig. 5c). Similarly, the percent of glycerol converted to glucose displayed the same trend (P < 0.05, NAFLD: 25.82 ± 13.03%; NCD: 7.81 ± 3.49%; NAFLD + BBR: 10.32 ± 5.57%) (Fig. 5d).
Changes in Ragly, Raglu, GNG from glycerol, the percent of glycerol converted to glucose, rates of hepatic lipogenesis and rates of fatty acid β oxidation in skeletal muscle. Ragly (a) showed a decreasing trend after the BBR treatment. BBR dramatically decreased Raglu (b), GNG from glycerol (c) and the percent of glycerol converted to glucose (d). In addition, BBR improved hepatic lipogenesis (e) and obviously promoted fatty acid β oxidation (f). The data are expressed as the means ± SEM. *P < 0.05, NCD vs NAFLD; #P < 0.05, NAFLD vs NAFLD + BBR
Berberine inhibited hepatic lipogenesis and promoted fatty acid β oxidation in skeletal muscle
The new synthesis of triglycerides in the liver displayed an increasing trend in the NAFLD group (3.47 ± 0.53 vs NCD: 3.05 ± 0.56, P > 0.05). Compared with the NAFLD group, BBR significantly reduced hepatic lipogenesis (NAFLD + BBR: 2.25 ± 0.44, P < 0.05) (Fig. 5e). The rates of fatty acid β oxidation in skeletal muscle decreased dramatically in the NAFLD group (3.07 ± 1.70%, P < 0.05), but no difference was observed between the NCD (6.01 ± 1.93%) and NAFLD + BBR (5.04 ± 0.98%) groups (Fig. 5f).
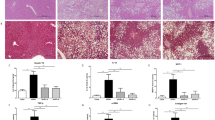
Changes in liver morphology
Under the light microscope, normal liver tissue structures and well-arranged hepatic lobules without liquid droplets were observed in the NCD group (Fig. 6a), whereas a disordered arrangement of the hepatic lobules and fatty degeneration of the hepatocytes was observed in the NAFLD group (Fig. 6b). However, the injury to the hepatic lobules and hepatocyte steatosis observed in the rats in the NAFLD + BBR group were all noticeably improved (Fig. 6c).
Histopathological changes in the liver (H&E staining, magnification × 200). Compared with the NCD group (a), the NAFLD group (b) exhibited a disordered arrangement of the hepatic lobules and fatty degeneration of hepatocytes. BBR noticeably improved the injury to the hepatic lobules and hepatocyte steatosis in the rats in the NAFLD + BBR group (c)
Discussion
NAFLD is already considered a critical hepatic manifestation of metabolic syndrome [4]. In addition, dietary habits and genetic background are thought to be responsible for the pathogenesis and development of hyperlipidemia with NAFLD; therefore, many mouse and rat models of NAFLD have been induced by feeding the animals a high-fat-diet in previous studies [4, 20, 21]. In our study, the rat model of NAFLD was also successfully established by providing nourishment with a high-fat diet for 16 weeks, at which time, the body weight was greatly increased. Meanwhile, increased fasting insulin concentrations, HOMA-IR and decreased ISI were observed in the rats with NAFLD. This result verified that obesity and insulin resistance are two main risk factors for the initiation or exacerbation of NAFLD [14]. After the BBR treatment, obvious weight loss was observed. The possible mechanism was associated with changes in the expression of multiple key genes controlling energy expenditure [22]. Previous studies found that at least a 5–7% of weight loss was required to improve hepatic steatosis [23]. Weight loss could improve hepatic insulin resistance and attenuate liver fat accumulation by reducing FFA flux to the liver for hepatic de novo lipogenesis [24]. Also, weight loss likely attenuates mitochondrial oxidative flux by alleviating the load of FFA and lipotoxicity to hepatic mitochondria [25]. Thus, weight loss is one of the cornerstones of treatment of NAFLD. Moreover, the fasting insulin levels and HOMA-IR decreased, and ISI obviously increased, although the differences were not significant. However, in some previous animal experiments, BBR significantly attenuated insulin resistance [4, 6]. This finding may be associated with the different species of animals and the different degree of obesity induced by the HFD. In addition, concomitant reductions in the total cholesterol, triglyceride, and low-density lipoprotein-cholesterol levels were observed in the BBR-treated rats, and the levels of the latter two were dramatically reduced. These findings may be attributed to the observation that berberine modulates the gut microbiota by up-regulating intestinal Bacteroidetes-to-Firmicutes ratio, which might decrease the animals’ capacity to harvest energy from the diet. Moreover, berberine increases the levels of serum glucagon-like peptide-1 and neuropeptide Y and decreases the levels of orexin-A, which may also modify the gut bacteria and regulate food intake, energy metabolism, circadian rhythm, etc. [26]. Therefore, based on these results, BBR indeed exerted a certain protective effect against hepatic steatosis.
According to the results of the isotope perfusion analysis, Ragly was much lower in the NAFLD + BBR group than that in the NAFLD group, although the differences between the three groups were not significant. Glycerol is one of the products of lipolysis. During fasting, the main source of glycerol is peripheral adipose tissue degradation. Moreover, glycerol released by lipolysis cannot be resynthesized into adipose tissue, since it lacks glycerokinase. Therefore, the Ragly in the blood can reflect the extent of lipolysis [16]. According to the results, BBR partially inhibited lipolysis in the rats with NAFLD. In fact, the obesity-associated dysfunction of adipose tissue plays an important role in the development of NAFLD, since adipose tissue not only delivers excess free fatty acids to the liver to facilitate hepatic steatosis but also secretes proinflammatory factors to trigger or exacerbate liver inflammation [4]. Although the levels of the associated inflammatory factors were not detected in the current study, BBR, a recognized anti-inflammatory drug, certainly had a powerful anti-inflammatory effect on both hepatocytes and adipocytes.
We also used 9,10-3H-palmitic acid to assess hepatic lipogenesis in vivo and its metabolic utilization in skeletal muscle in vitro. 9,10-3H-palmitic acid has many of the same features as normal fatty acids; it is able to be taken up by the liver for triglyceride synthesis and by skeletal muscle to produce 3H2O via β oxidation. The results showed that BBR significantly reduced the rates of hepatic lipogenesis, whereas the BBR treatment increased the rates of fatty acid β oxidation in skeletal muscle. In addition, liver histology revealed that BBR obviously attenuated ectopic fat accumulation in the liver, consistent with previous findings showing that oral administration of BBR alleviates TG deposition in the liver [6]. Based on these results, BBR comprehensively improved lipid metabolism in NAFLD by inhibiting hepatic lipogenesis and lipolysis in adipose tissue, as well as by promoting fatty acid β oxidation in skeletal muscle. Moreover, BBR exerted the strongest effects on hepatic lipogenesis and fat deposition, possibly because BBR was typically concentrated in the liver (at levels 50–70 times higher than the plasma levels) after oral administration [10, 13]. BBR may have improved lipolysis in adipose tissue and fatty acid β oxidation in skeletal muscle by adjusting energy metabolism pathways, such as PPAR signaling pathways; however, additional animal experiments are required to confirm this hypothesis.
In the basal state, hepatic glucose production (HGP) is equivalent to the glucose appearance rates (Raglu) after an overnight fast. In the current study, the highest Raglu was observed in NAFLD group and was apparently reduced in the BBR-treated rats. The FBG levels were also significantly decreased in the first 8 weeks of BBR treatment, although there were no differences between the three groups at the end of the experiment. Gluconeogenesis is one of the major mechanisms used to maintain normal FBG levels [16]. Gluconeogenesis was primarily increased in rats with NAFLD and significantly decreased (including glycerol converted to glucose (%) and GNG from glycerol) in the BBR-treated rats compared with the vehicle-treated rats in this study, indicating that BBR attenuated high-fat-diet-induced GNG from glycerol. As described above, insulin resistance, with an increased HOMA-IR and decreased ISI, was apparent in the NAFLD group, but the FBG levels remained within the normal range. Thus, the FBG levels alone cannot predict the metabolic risk in NAFLD. However, the increased Raglu and GNG in combination with the unaffected FBG levels indicate that the dynamics of glucose metabolism have been actively initiated in the early state. BBR may not only have a beneficial anti-hyperglycemic effect but may also begin to decrease hepatic glucose production in the early stage of glucose metabolic disorder, suggesting that BBR may be a more effective therapeutic strategy for patients with NAFLD and glucose metabolic disorder. Based on these findings, BBR might be an effective treatment to prevent the progression of prediabetes to diabetes, but further animal studies and human trials are required to confirm this hypothesis.
In our study, we comprehensively observed the effects of BBR on hepatic and extra hepatic glucose and lipid metabolism in rats with NAFLD using isotope tracer technology. Tracer techniques have been widely used to study the metabolism of glucose, lipid and other molecules. Previous tracer studies in rat models often involved invasive surgical placement of catheters in the carotid artery and jugular vein [27], which would obviously cause stress. This stress may considerably interfere with the metabolic flux of the substrate and thus affect the results. In the present study, we inserted catheters into the rats’ tail arteries and veins and maintained the rats in a conscious and relaxed state throughout the experiment to limit and reduce stress and to prevent disturbances in glucose and lipid metabolism (Fig. 1). However, our study has some limitations. For example, a single dose of BBR was used; therefore, we could not observe the effects of different doses of BBR on NAFLD. In future studies, we will make up for these deficiencies and further explore the molecular biological mechanism by which BBR regulates glucose and lipid metabolism.
Conclusions
BBR, a compound derived from herbal medicine, improved NAFLD and prevented its metabolic disorder-related complications by comprehensively regulating glucose and lipid metabolism. The improvement may be partly mediated by weight loss. The most important and beneficial effects of BBR on NAFLD were to inhibit hepatic lipogenesis and fat deposition. In clinical practice, BBR may have a better therapeutic effect on patients with NAFLD and glucose metabolic disorder. Further clinical trials need to be conducted to confirm these effects.
Abbreviations
- BBR:
-
Berberine
- FBG:
-
Fasting blood glucose
- FFA:
-
Free fatty acid
- FINS:
-
Fasting insulin
- GNG:
-
Gluconeogenesis
- HOMA-IR:
-
Insulin resistance index
- ISI:
-
Insulin sensitivity index
- LDL:
-
Low-density lipoprotein
- NAFLD:
-
Nonalcoholic fatty liver disease
- Raglu :
-
Rate of glucose appearance
- Ragly :
-
Rate of glycerol appearance
- TC:
-
Total cholesterol
- TG:
-
Total triglycerides
References
Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61(3):409–15.
Jamali R, Arj A, Razavizade M, Aarabi MH. Prediction of nonalcoholic fatty liver disease via a novel panel of serum adipokines. Medicine. 2016;95(5):e2630.
Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci. 2011;48(3):97–113.
Guo T, Woo SL, Guo X, Li H, Zheng J, Botchlett R, et al. Berberine ameliorates hepatic steatosis and suppresses liver and adipose tissue inflammation in mice with diet-induced obesity. Sci Rep. 2016;6:22612.
Cheng Y, Hou T, Ping J, Chen G, Chen J. Quantitative succinylome analysis in the liver of non-alcoholic fatty liver disease rat model. Proteome Sci. 2016;14:3.
Zhang Z, Li B, Meng X, Yao S, Jin L, Yang J, et al. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci Rep. 2016;6:20848.
Tillhon M, Guaman Ortiz LM, Lombardi P, Scovassi AI. Berberine: new perspectives for old remedies. Biochem Pharmacol. 2012;84(10):1260–7.
Chang X, Yan H, Fei J, Jiang M, Zhu H, Lu D, et al. Berberine reduces methylation of the MTTP promoter and alleviates fatty liver induced by a high-fat diet in rats. J Lipid Res. 2010;51(9):2504–15.
Zhang Z, Zhang H, Li B, Meng X, Wang J, Zhang Y, et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun. 2014;5:5493.
Yan HM, Xia MF, Wang Y, Chang XX, Yao XZ, Rao SX, et al. Efficacy of berberine in patients with Non-alcoholic fatty liver disease. PLoS One. 2015;10(8):e0134172.
Chen W, Miao YQ, Fan DJ, Yang SS, Lin X, Meng LK, et al. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech. 2011;12(2):705–11.
Hua W, Ding L, Chen Y, Gong B, He J, Xu G. Determination of berberine in human plasma by liquid chromatography-electrospray ionization-mass spectrometry. J Pharm Biomed Anal. 2007;44(4):931–7.
Liu YT, Hao HP, Xie HG, Lai L, Wang Q, Liu CX, et al. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab Dispos. 2010;38(10):1779–84.
Xie W, Gu D, Li J, Cui K, Zhang Y. Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS One. 2011;6(9):e24520.
Xing XY, Li YF, Fu ZD, Chen YY, Wang YF, Liu XL, et al. Antihypertensive effect of metformin in essential hypertensive patients with hyperinsulinemia. Zhonghua Nei Ke Za Zhi. 2010;49(1):14–8.
Wu H, Sui C, Xu H, Xia F, Zhai H, Zhang H, et al. The GLP-1 analogue exenatide improves hepatic and muscle insulin sensitivity in diabetic rats: tracer studies in the basal state and during hyperinsulinemic-euglycemic clamp. J Diabetes Res. 2014;2014:524517.
Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509.
Zhai HL, Wu H, Xu H, Weng P, Xia FZ, Chen Y, et al. Trace glucose and lipid metabolism in high androgen and high-fat diet induced polycystic ovary syndrome rats. Reprod Biol Endocrinol. 2012;10:5.
Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 2007;56(5):1369–75.
Woo SL, Xu H, Li H, Zhao Y, Hu X, Zhao J, et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS One. 2014;9(3):e91111.
Kucera O, Cervinkova Z. Experimental models of non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2014;20(26):8364–76.
Hu Y, Young AJ, Ehli EA, Nowotny D, Davies PS, Droke EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310.
Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification signnificantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–78.
Ratziu V. Non-pharmacological interventions in non-alcoholic fatty liver disease patients. Liver Int. 2017;37 Suppl 1:90–6.
Sunny NE, Bril F, Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver : novel mechanisms and treatment strategies. Trends Endocrinol Metab. 2016; doi: 10.1016/j.tem.2016.11.006.
Sun H, Wang N, Cang Z, Zhu C, Zhao L, Nie X, et al. Modulation of microbiota-gut-brain axis by berberine resulting in improved metabolic status in high-fat diet-fed rats. Obes Facts. 2016;9(6):365–78.
Guo Z, Jensen MD. Blood glycerol is an important precursor for intramuscular triacylglycerol synthesis. J Biol Chem. 1999;274(34):23702–6.
Acknowledgments
Not applicable
Funding
This work was supported by the National Key Basic Research Program of China (973 Program: 2012CB524900), the National Natural Science Foundation of China (81070677, 81270885, and 81300653), and the Science and Technology Commission of Shanghai Municipality (114119b2800, 11140903002, and 10JC1409002).
Availability of data and materials
We wish not to share the raw data as the authors are aiming for future publications from the data. However, the data are available from the corresponding author upon reasonable individual request.
Authors’ contributions
LZ and ZC contributed equally to this study and shared first co-authorship. YL designed this study; LZ, ZC, HS and XN performed the experiments; LZ and NW analyzed the data and interpreted the results of the experiments; LZ prepared the figures and wrote the manuscript; and YL edited and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethical approval and consent to participate
All animal procedures were conducted in accordance with the ethical principles in animal research adopted by the Department of Laboratory Animal Science and approved by the Animal Experimental Ethical Committee of Jiaotong University School of Medicine, Shanghai, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, L., Cang, Z., Sun, H. et al. Berberine improves glucogenesis and lipid metabolism in nonalcoholic fatty liver disease. BMC Endocr Disord 17, 13 (2017). https://doi.org/10.1186/s12902-017-0165-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-017-0165-7