Abstract
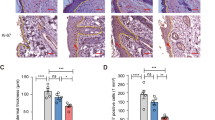
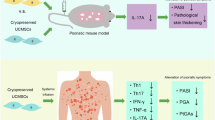
Psoriasis is a chronic, immune-mediated disease that affects 2–3% of the global population. Recently, mesenchymal stem cells (MSCs) have been used to alleviate psoriasis. However, the therapeutic mechanisms of MSCs remain unclear. Matrix metalloproteinase-13 (MMP13), a member of the MMPs family, is the key enzyme in the cleavage of type II collagen and plays a pivotal role in extracellular matrix (ECM) remodeling. Here, it was found that Mmp13 was upregulated in the skin lesions of an imiquimod-induced mouse model, which was downregulated after intravenous infusion of human umbilical cord MSCs (hUC-MSCs). Knockdown of MMP13 inhibited the proliferation of keratinocytes and arrested the cell cycle in G1 stage. In addition, hUC-MSCs were co-cultured with THP-1 or PMA-stimulated THP-1 directly in vitro to simulate the fate of systematically infused hUC-MSCs. The level of TNF-α was decreased in the supernatant of co-cultured hUC-MSCs and THP-1 or PMA-stimulated THP-1. Moreover, it was identified that TNF-α upregulated MMP13 through the NF-κB pathway in keratinocytes. In conclusion, we propose that systematically infused hUC-MSCs exert a therapeutic effect on psoriasis through the TNF-α/NF-κB/MMP13 pathway.






Similar content being viewed by others
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Greb, Jacqueline E., Ari M. Goldminz, James T. Elder, Mark G. Lebwohl, Dafna D. Gladman, Jashin J. Wu, Nehal N. Mehta, Andrew Y. Finlay, and Alice B. Gottlieb. 2016. Psoriasis. Nature Reviews Disease Primers 2. https://doi.org/10.1038/nrdp.2016.82.
Griffiths, Christopher E.M., April W. Armstrong, Johann E. Gudjonsson, and Jonathan N.W.N. Barker. 2021. Psoriasis. The Lancet. Berlin, Verlag: Elsevier. https://doi.org/10.1016/S0140-6736(20)32549-6.
Kaushik, Shivani B., and Mark G. Lebwohl. 2019. Psoriasis: Which therapy for which patient: Psoriasis comorbidities and preferred systemic agents. Journal of the American Academy of Dermatology. Mosby Inc. https://doi.org/10.1016/j.jaad.2018.06.057.
Nestle, Frank O, Daniel H Kaplan, and Jonathan Barker. 2009. Mechanism of disease: Psoriasis. The New England Journal of Medicine 361.
Alwan, Wisam, and Frank O. Nestle. 2015. Pathogenesis and treatment of psoriasis: Exploiting pathophysiological pathways for precision medicine. Clinical and Experimental Rheumatology 33.
Quesada, Jorge R., and Jordan U. Gutterman. 1986. Psoriasis and Alpha-Interferon. The Lancet 327. https://doi.org/10.1016/S0140-6736(86)91502-3.
Kamata, Masahiro, and Yayoi Tada. 2020. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: A literature review. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms21051690.
Guinard, E., C. Bulai Livideanu, H. Barthélémy, M. Viguier, Z. Reguiai, M. A. Richard, D. Jullien, et al. 2016. Active tuberculosis in psoriasis patients treated with TNF antagonists: a French nationwide retrospective study. Journal of the European Academy of Dermatology and Venereology 30. https://doi.org/10.1111/jdv.13633.
Fowler, E., R.I. Ghamrawi, N. Ghiam, W. Liao, and J.J. Wu. 2020. Risk of tuberculosis reactivation during interleukin-17 inhibitor therapy for psoriasis: A systematic review. Journal of the European Academy of Dermatology and Venereology. https://doi.org/10.1111/jdv.16254.
Nogueira, M., R.B. Warren, and T. Torres. 2021. Risk of tuberculosis reactivation with interleukin (IL)-17 and IL-23 inhibitors in psoriasis – time for a paradigm change. Journal of the European Academy of Dermatology and Venereology. https://doi.org/10.1111/jdv.16866.
Tropel, Philippe, Nadine Platet, Jean-Claude Platel, Danièle Noël, Mireille Albrieux, Alim-Louis Benabid, and François Berger. 2006. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells 24. https://doi.org/10.1634/stemcells.2005-0636.
Hu, Lifang, Chong Yin, Fan Zhao, Arshad Ali, Jianhua Ma, and Airong Qian. 2018. Mesenchymal stem cells: Cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms19020360.
Mackay, Alastair M., Stephen C. Beck, J. Mary Murphy, Frank P. Barry, Clinton O. Chichester, and Mark F. Pittenger. 1998. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Engineering 4. https://doi.org/10.1089/ten.1998.4.415.
Ferrari, Giuliana, Gabriella Cusella-De Angelis, Marcello Coletta, Egle Paolucci, Anna Stornaiuolo, Giulio Cossu, and Fulvio Mavilio. 1998. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279. https://doi.org/10.1126/science.279.5356.1528.
Romanov, Yuri A., Veronika A. Svintsitskaya, and Vladimir N. Smirnov. 2003. Searching for alternative sources of postnatal human mesenchymal stem cells: Candidate MSC-like cells from umbilical cord. Stem Cells 21. https://doi.org/10.1634/stemcells.21-1-105.
Steigman, Shaun A., and Dario O. Fauza. 2007. Isolation of mesenchymal stem cells from amniotic fluid and placenta. Current Protocols in Stem Cell Biology Chapter 1. https://doi.org/10.1002/9780470151808.sc01e02s1.
Ma, S., N. Xie, W. Li, B. Yuan, Y. Shi, and Y. Wang. 2014. Immunobiology of mesenchymal stem cells. Cell Death and Differentiation 21: 216–225. https://doi.org/10.1038/cdd.2013.158.
Wagoner, Zachary W., and Weian Zhao. 2021. Therapeutic implications of transplanted-cell death. Nature Biomedical Engineering 5: 379–384. US: Springer. https://doi.org/10.1038/s41551-021-00729-6.
Németh, Krisztián, Asada Leelahavanichkul, Peter S.T.. Yuen, Balázs Mayer, Alissa Parmelee, Kent Doi, Pamela G. Robey, et al. 2009. Bone marrow stromal cells attenuate sepsis via prostaglandin E 2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine 15: 42–49. https://doi.org/10.1038/nm.1905.
Chen, Maosheng, Jing Peng, Qi Xie, Na Xiao, Xian Su, Hua Mei, Yeping Lu, et al. 2019. Mesenchymal stem cells alleviate moderate-to-severe psoriasis by reducing the production of type i interferon (IFN-I) by plasmacytoid dendritic cells (pDCs). Stem Cells International 2019. https://doi.org/10.1155/2019/6961052.
Yao, Danni, Shuyan Ye, Ziyang He, Yu Huang, Jingwen Deng, Zehuai Wen, Xinsheng Chen, et al. 2021. Adipose-derived mesenchymal stem cells (AD-MSCs) in the treatment for psoriasis: results of a single-arm pilot trial. Annals of Translational Medicine 9:1653–1653. AME: Publishing Company. https://doi.org/10.21037/atm-21-5028.
Lee, Yun Sang, Shyam Kishor Sah, Ji Hyun Lee, Kwang Won Seo, Kyung Sun Kang, and Tae Yoon Kim. 2017. Human umbilical cord blood-derived mesenchymal stem cells ameliorate psoriasis-like skin inflammation in mice. Biochemistry and Biophysics Reports 9: 281–288. B.V.: Elsevier. https://doi.org/10.1016/j.bbrep.2016.10.002.
Lwin, S.M., J.A. Snowden, and C.E.M. Griffiths. 2021. The promise and challenges of cell therapy for psoriasis. British Journal of Dermatology. John Wiley and Sons Inc. https://doi.org/10.1111/bjd.20517.
de Witte, Samantha F.H.., Franka Luk, Jesus M. Sierra, Madhu Gargesha Parraga, Ana Merino, Sander S. Korevaar, Anusha S. Shankar, et al. 2018. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells 36: 602–615. https://doi.org/10.1002/stem.2779.
Vasandan, Anoop Babu, Sowmya Jahnavi, Chandanala Shashank, Priya Prasad, Anujith Kumar, and S. Jyothi Prasanna. 2016. Human mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE 2 -dependent mechanism. Scientific Reports 6. https://doi.org/10.1038/srep38308.
Howes, Joanna Marie, Dominique Bihan, David A. Slatter, Samir W. Hamaia, Len C. Packman, Vera Knauper, Robert Visse, and Richard W. Farndale. 2014. The recognition of collagen and triple-helical toolkit peptides by MMP-13: Sequence specificity for binding and cleavage. Journal of Biological Chemistry 289. https://doi.org/10.1074/jbc.M114.583443.
Hattori, Noriko, Satsuki Mochizuki, Kazuo Kishi, Tatsuo Nakajima, Hironari Takaishi, Jeanine D’Armiento, and Yasunori Okada. 2009. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. American Journal of Pathology 175. https://doi.org/10.2353/ajpath.2009.081080.
He, Yu Jie, Xu Liang, Xin Xin Zhang, Shan Shan Li, Yue Sun, and Tian Fang Li. 2021. PTH1–34 inhibited TNF-α expression and antagonized TNF-α-induced MMP13 expression in MIO mice. International Immunopharmacology 91. https://doi.org/10.1016/j.intimp.2020.107191.
Little, Christopher B., A. Barai, D. Burkhardt, S. M. Smith, A. J. Fosang, Z. Werb, M. Shah, and E. W. Thompson. 2009. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis and Rheumatism 60. https://doi.org/10.1002/art.25002.
Zhang, Bin, Xuchen Cao, Yanxue Liu, Wenfeng Cao, Fei Zhang, Shiwu Zhang, Hongtao Li, et al. 2008. Tumor-derived Matrix Metalloproteinase-13 (MMP-13) correlates with poor prognoses of invasive breast cancer. BMC Cancer 8. https://doi.org/10.1186/1471-2407-8-83.
Diani, Marco, Silvia Perego, Veronica Sansoni, Lucrezia Bertino, Marta Gomarasca, Martina Faraldi, Paolo Daniele Maria Pigatto, et al. 2019. Differences in osteoimmunological biomarkers predictive of psoriatic arthritis among a large Italian cohort of psoriatic patients. International Journal of Molecular Sciences 20. https://doi.org/10.3390/ijms20225617.
Xi, Chan, Chuanxi Xiong, Huiping Wang, Yuanjun Liu, and Suju Luo. 2021. Combination of retinoids and narrow-band ultraviolet B inhibits matrix metalloproteinase 13 expression in HaCaT keratinocytes and a mouse model of psoriasis. Scientific Reports 11. https://doi.org/10.1038/s41598-021-92599-w.
Kim, Ji Yon, Yu. Minhwa Park, Hee Kim, Kyung Ha Ryu, Kyung Ho Lee, Kyung Ah Cho, and So Youn Woo. 2018. Tonsil-derived mesenchymal stem cells (T-MSCs) prevent Th17-mediated autoimmune response via regulation of the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway. Journal of Tissue Engineering and Regenerative Medicine 12: e1022–e1033. https://doi.org/10.1002/term.2423.
Imai, Yasutomo, Kenichi Yamahara, Akiko Hamada, Yoshihiro Fujimori, and Kiyofumi Yamanishi. 2019. Human amnion-derived mesenchymal stem cells ameliorate imiquimod-induced psoriasiform dermatitis in mice. Journal of Dermatology 46: 276–278. https://doi.org/10.1111/1346-8138.14768.
Haider, Asifa S., Jules Cohen, Ji Fei, Lisa C. Zaba, Irma Cardinale, Kikuchi Toyoko, Jurg Ott, and James G. Krueger. 2008. Insights into gene modulation by therapeutic TNF and IFNγ antibodies: TNF regulates IFNγ production by T cells and TNF-regulated genes linked to psoriasis transcriptome. Journal of Investigative Dermatology 128: 655–666. Nature Publishing Group. https://doi.org/10.1038/sj.jid.5701064.
Saxton, Robert A., Naotaka Tsutsumi, Leon L. Su, Gita C. Abhiraman, Kritika Mohan, Lukas T. Henneberg, Nanda G. Aduri, Cornelius Gati, and K. Christopher Garcia. 2021. Structure-based decoupling of the pro- And anti-inflammatory functions of interleukin-10. Science 371. https://doi.org/10.1126/science.abc8433.
Wikan, Nitwara, Phateep Hankittichai, Phatarawat Thaklaewphan, Saranyapin Potikanond, and Wutigri Nimlamool. 2022. Oxyresveratrol inhibits TNF-α-stimulated cell proliferation in human immortalized keratinocytes (HaCaT) by suppressing AKT activation. Pharmaceutics 14. https://doi.org/10.3390/pharmaceutics14010063.
Patel, Arti B., Irene Tsilioni, Zuyi Weng, and Theoharis C. Theoharides. 2018. TNF stimulates IL-6, CXCL8 and VEGF secretion from human keratinocytes via activation of mTOR, inhibited by tetramethoxyluteolin. Experimental Dermatology 27. https://doi.org/10.1111/exd.13461.
Hawkes, Jason E., Tom C. Chan, and James G. Krueger. 2017. Psoriasis pathogenesis and the development of novel targeted immune therapies. Journal of Allergy and Clinical Immunology. https://doi.org/10.1016/j.jaci.2017.07.004.
Yan, Sha, Zhenyao Xu, Fangzhou Lou, Lingyun Zhang, Fang Ke, Jing Bai, Zhaoyuan Liu, et al. 2015. NF-κB-induced microRNA-31 promotes epidermal hyperplasia by repressing protein phosphatase 6 in psoriasis. Nature Communications 6. https://doi.org/10.1038/ncomms8652.
Rebholz, Bernd, Ingo Haase, Birgit Eckelt, Stephan Paxian, Michael J. Flaig, Kamran Ghoreschi, Sergei A. Nedospasov, et al. 2007. Crosstalk between keratinocytes and adaptive immune cells in an IκBα protein-mediated inflammatory disease of the skin. Immunity 27. https://doi.org/10.1016/j.immuni.2007.05.024.
Kessenbrock, Kai, Vicki Plaks, and Zena Werb. 2010. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. https://doi.org/10.1016/j.cell.2010.03.015.
Mezentsev, Alexandre, Alexander Nikolaev, and Sergey Bruskin. 2014. Matrix metalloproteinases and their role in psoriasis. Gene. https://doi.org/10.1016/j.gene.2014.01.068.
Suomela, S., A.L. Kariniemi, E. Snellman, and Ulpu Saarialho-Kere. 2001. Metalloelastase (MMP-12) and 92-kDa gelatinase (MMP-9) as well as their inhibitors, TIMP-1 and -3, are expressed in psoriatic lesions. Experimental Dermatology 10. https://doi.org/10.1034/j.1600-0625.2001.010003175.x.
Shi, Guang Hui, Yi Fei Cheng, Yang Zhang, Rui Guo, Shenglei Li, and Xin Hong. 2021. Long non-coding RNA LINC00511/miR-150/MMP13 axis promotes breast cancer proliferation, migration and invasion. Biochimica et Biophysica Acta - Molecular Basis of Disease 1867. https://doi.org/10.1016/j.bbadis.2020.165957
ACKNOWLEDGEMENTS
We would like to thank the Biomedical Research Institute of Shenzhen Peking University—The Hong Kong University of Science and Technology Medical Center for providing platform support and technical assistance. We would also like to thank Wingor company for donating hUC-MSCs.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81874249 and 82103726), Guangdong Basic and Applied Basic Research Foundation (2021A1515011558), Shenzhen Science and Technology Program (JCYJ20210324110008023 and JCYJ20180223181224405), Shenzhen Sanming Project (SZSM201812059), Shenzhen Key Medical Discipline Construction Fund (SZXK040), and Scientific Research Foundation of Peking University Shenzhen Hospital (KYQD2021038 and KYQD2021049).
Author information
Authors and Affiliations
Contributions
Xuanyao Ren contributed to the experimental design, performance, data collection/analysis, and manuscript preparation. Weilong Zhong contributed to the experimental design. Wenting Li and Mindan Tang helped with data analysis. Kaoyuan Zhang, Fenli Zhou, Xin Shi, Jun Wu, and Bo Yu helped to perform in vitro experiments. Cong Huang contributed to the data analysis and manuscript preparation. Xiaofan Chen contributed to the results’ discussion and manuscript preparation. Wei Zhang supervised the studies and contributed to the manuscript preparation.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, X., Zhong, W., Li, W. et al. Human Umbilical Cord-Derived Mesenchymal Stem Cells Alleviate Psoriasis Through TNF-α/NF-κB/MMP13 Pathway. Inflammation 46, 987–1001 (2023). https://doi.org/10.1007/s10753-023-01785-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01785-7