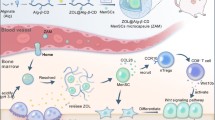
Abstract
Psoriasis, an inflammatory autoimmune skin disease, is characterized by scaly white or erythematous plaques, which severely influence patients’ quality of life and social activities. Mesenchymal stem cells derived from the human umbilical cord (UCMSCs) represent a promising therapeutic approach for psoriasis because of its unique superiority in ethical agreeableness, abundant source, high proliferation capacity, and immunosuppression. Although cryopreservation provided multiple benefits to the cell therapy, it also greatly compromised clinical benefits of MSCs due to impaired cell functions. The current study aims to evaluate the therapeutic efficacy of cryopreserved UCMSCs in a mouse model of psoriasis as well as in patients with psoriasis. Our results showed that cryopreserved and fresh UCMSCs have comparable effects on the suppression of psoriasis-like symptoms such as thickening, erythema, and scaling, and serum IL-17 A secretion in mice model of psoriasis. Moreover, psoriatic patients injected with cryopreserved UCMSCs had a significant improvement in the Psoriasis Area and Severity Index (PASI), Physician Global Assessment (PGA), and Patient Global Assessments (PtGAs) scores compared to baseline values. Mechanically, cryopreserved UCMSCs markedly inhibit the proliferation of PHA-activated PBMCs, type 1 T helper (Th1) and type 17 T helper (Th17) cell differentiation and secretion of inflammatory cytokines including IFN-γ, TNF-a and IL-17 A in PBMCs stimulated by anti-CD3/CD28 beads. Taken together, these data indicated that cryopreserved UCMSCs exhibited great beneficial effect on psoriasis. Thus, cryopreserved UCMSCs can be systemically administered as ‘‘off-the-shelf’’ cell product for psoriasis therapy. Trial Registration ChiCTR1800019509. Registered on November 15, 2018—Retrospectively registered, http://www.chictr.org.cn/.
Graphical Abstract







Similar content being viewed by others
Data Availability
All data are available in the main manuscript or the supplemental information or provided upon availability and reasonable request.
Abbreviations
- UCMSCs :
-
Umbilical cord-derived mesenchymal stem cells
- IMQ :
-
Imiquimod
- PHA :
-
Phytohemagglutinin
- PASI :
-
Psoriasis Area Severity Index
- H&E :
-
Hematoxylin and eosin
- PGA :
-
Physician Global Assessment
- PtGAs :
-
Patient Global Assessments
- PBMC :
-
Peripheral blood mononuclear cell
- Th1 :
-
Type 1 T helper
- Th17 :
-
Type 17 T helper
- UC :
-
Umbilical cord
- UCMSCs (FL) :
-
Low dose of fresh UCMSCs
- UCMSCs (FH) :
-
High dose of fresh UCMSCs
- UCMSCs (CL) :
-
Low dose of cryopreserved UCMSCs
- UCMSCs (CH) :
-
High dose of cryopreserved UCMSCs
- SEM :
-
Standard error of the mean
- hBMMSCs :
-
Human bone marrow derived MSCs
- M :
-
Million
References
Griffiths, C. E. M., van der Walt, J. M., Ashcroft, D. M., Flohr, C., Naldi, L., Nijsten, T., & Augustin, M. (2017). The global state of psoriasis disease epidemiology: A workshop report. The British Journal of Dermatology, 177, e4–e7.
Naik, P. P. (2022). Stem cell therapy as a potential treatment option for psoriasis. Anais Brasileiros de Dermatologia, 97, 471–4773.
Zhou, X., Chen, Y., Cui, L., Shi, Y., & Guo, C. (2022). Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death & Disease, 13, 81.
Armstrong, A. W., & Read, C. (2020). Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA, 323, 1945–1960.
Chiricozzi, A., Guttman-Yassky, E., Suárez-Fariñas, M., Nograles, K. E., Tian, S., Cardinale, I., Chimenti, S., & Krueger, J. G. (2011). Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. The Journal of Investigative Dermatology, 131, 677–687.
Monteleone, G., Pallone, F., MacDonald, T. T., Chimenti, S., & Costanzo, A. (2011). Psoriasis: From pathogenesis to novel therapeutic approaches. Clinical Science (London England: 1979), 120, 1–11.
Lowes, M. A., Kikuchi, T., Fuentes-Duculan, J., Cardinale, I., Zaba, L. C., Haider, A. S., Bowman, E. P., & Krueger, J. G. (2008). Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. The Journal of Investigative Dermatology, 128, 1207–1211.
Ariza, M. E., Williams, M. V., & Wong, H. K. (2013). Targeting IL-17 in psoriasis: From cutaneous immunobiology to clinical application. Clinical Immunology (Orlando Fla), 146, 131–139.
Leonardi, C., Matheson, R., Zachariae, C., Cameron, G., Li, L., Edson-Heredia, E., Braun, D., & Banerjee, S. (2012). Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. The New England Journal of Medicine, 366, 1190–1199.
Papp, K. A., Langley, R. G., Sigurgeirsson, B., Abe, M., Baker, D. R., Konno, P., Haemmerle, S., Thurston, H. J., Papavassilis, C., & Richards, H. B. (2013). Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled phase II dose-ranging study. The British Journal of Dermatology, 168, 412–421.
Coates, L. C., Merola, J. F., Grieb, S. M., Mease, P. J., & Duffin, C. (2020). Methotrexate in psoriasis and psoriatic arthritis. The Journal of Rheumatology Supplement, 96, 31–35.
Mozzanica, N., Pigatto, P. D., & Finzi, A. F. (1993). Cyclosporin in psoriasis: Pathophysiology and experimental data. Dermatology (Basel Switzerland), 187(Suppl 1), 3–7.
Gooderham, M., Posso-De Los Rios, C. J., Rubio-Gomez, G. A., & Papp, K. (2015). Interleukin-17 (IL-17) inhibitors in the treatment of plaque psoriasis: A review. Skin Therapy Letter, 20, 1–5.
Al-Janabi, A., & Yiu, Z. Z. N. (2022). Biologics in psoriasis: Updated perspectives on long-term safety and risk management. Psoriasis (Auckland N Z), 12, 1–14.
Chen, M., Peng, J., Xie, Q., Xiao, N., Su, X., Mei, H., Lu, Y., Zhou, J., Dai, Y., Wang, S., Li, C., Lin, G., & Cheng, L. (2019). Mesenchymal stem cells alleviate moderate-to-severe psoriasis by reducing the production of type I interferon (IFN-I) by plasmacytoid dendritic cells (pDCs). Stem Cells International, 2019, 6961052.
Lin, Y., Wang, H., Jiang, C., Chen, C., Shen, D., Xie, F., Zhang, H., Yang, J., & Wang, H. (2022). Effects of different concentrations of human umbilical cord mesenchymal stem cells to ameliorate psoriasis-like skin lesions in BALB/c mice. Annals of Translational Medicine, 10, 86.
Ahn, H., Lee, S. Y., Jung, W. J., Pi, J., & Lee, K. H. (2021). Psoriasis treatment using minimally manipulated umbilical cord-derived mesenchymal stem cells: A case report. World Journal of Clinical Cases, 9, 6798–6803.
Chen, Y., Hu, Y., Zhou, X., Zhao, Z., Yu, Q., Chen, Z., Wang, Y., Xu, P., Yu, Z., Guo, C., Zhang, X., & Shi, Y. (2022). Human umbilical cord-derived mesenchymal stem cells ameliorate psoriasis-like dermatitis by suppressing IL-17-producing γδ T cells. Cell and Tissue Research, 388, 549–563.
Woods, E. J., Thirumala, S., Badhe-Buchanan, S. S., Clarke, D., & Mathew, A. J. (2016). Off the shelf cellular therapeutics: Factors to consider during cryopreservation and storage of human cells for clinical use. Cytotherapy, 18, 697–71120.
Marquez-Curtis, L. A., Janowska-Wieczorek, A., McGann, L. E., & Elliott, J. A. (2015). Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology, 71, 181–197.
Müllers, Y., Meiser, I., Stracke, F., Riemann, I., Lautenschläger, F., Neubauer, J. C., & Zimmermann, H. (2019). Quantitative analysis of F-actin alterations in adherent human mesenchymal stem cells: Influence of slow-freezing and vitrification-based cryopreservation. PLoS One, 14, e0211382.
Bahsoun, S., Coopman, K., & Akam, E. C. (2020). Quantitative assessment of the impact of cryopreservation on human bone marrow-derived mesenchymal stem cells: Up to 24 h post-thaw and beyond. Stem Cell Research & Therapy, 11, 540.
Fu, X., Xu, B., Jiang, J., Du, X., Yu, X., Yan, Y., Li, S., Inglis, B. M., Ma, H., Wang, H., Pei, X., & Si, W. (2020). Effects of cryopreservation and long-term culture on biological characteristics and proteomic profiles of human umbilical cord-derived mesenchymal stem cells. Clinical Proteomics, 17, 15.
Wang, Z., Li, H., Fang, J., Wang, X., Dai, S., Cao, W., Guo, Y., Li, Z., & Zhu, H. (2022). Comparative analysis of the therapeutic effects of amniotic membrane and umbilical cord derived mesenchymal stem cells for the treatment of type 2 diabetes. Stem Cell Reviews and Reports, 18, 1193–1206.
Cai, Y. H., Lu, Z. Y., Shi, R. F., Xue, F., Chen, X. Y., Pan, M., Yuan, W. R., Xu, H., Li, W. P., & Zheng, J. (2009). Enhanced proliferation and activation of peripheral blood mononuclear cells in patients with psoriasis vulgaris mediated by streptococcal antigen with bacterial DNA. The Journal of Investigative Dermatology, 129, 2653–2660.
Kaushik, S. B., & Lebwohl, M. G. (2019). Psoriasis: Which therapy for which patient: Psoriasis comorbidities and preferred systemic agents. Journal of the American Academy of Dermatology, 80, 27–40.
Chinnadurai, R., Garcia, M. A., Sakurai, Y., Lam, W. A., Kirk, A. D., Galipeau, J., & Copland, I. B. (2014). Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Reports, 3, 60–72.
Shaik, S., Wu, X., Gimble, J., & Devireddy, R. (2018). Effects of decade long freezing storage on adipose derived stem cells functionality. Scientific Reports, 8, 8162.
Zhang, M., Zhao, Y., Wang, L., Zheng, Y., Yu, H., Dong, X., He, W., Yin, Z., & Wang, Z. (2022). Study of the biological characteristics of human umbilical cord mesenchymal stem cells after long-time cryopreservation. Cell and Tissue Banking, 23, 739–752.
Horie, S., Gonzalez, H., Brady, J., Devaney, J., Scully, M., O’Toole, D., & Laffey, J. G. (2021). Fresh and cryopreserved human umbilical-cord-derived mesenchymal stromal cells attenuate injury and enhance resolution and repair following ventilation-induced lung injury. International Journal of Molecular Sciences, 22, 12842.
Tan, Y., Salkhordeh, M., Wang, J. P., McRae, A., Souza-Moreira, L., McIntyre, L., Stewart, D. J., & Mei, S. H. J. (2019). Thawed mesenchymal stem cell product shows comparable immunomodulatory potency to cultured cells in vitro and in polymicrobial septic animals. Scientific Reports, 9, 18078.
González, M. A., Gonzalez-Rey, E., Rico, L., Büscher, D., & Delgado, M. (2009). Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology, 136, 978–989.
Maria, A. T., Toupet, K., Bony, C., Pirot, N., Vozenin, M. C., Petit, B., Roger, P., Batteux, F., Le Quellec, A., Jorgensen, C., Noël, D., & Guilpain, P. (2016). Antifibrotic, antioxidant, and immunomodulatory effects of mesenchymal stem cells in hocl-induced systemic sclerosis. Arthritis & Rhematology, 68, 1013–1025.
Robinson, A. M., Rahman, A. A., Miller, S., Stavely, R., Sakkal, S., & Nurgali, K. (2017). The neuroprotective effects of human bone marrow mesenchymal stem cells are dose-dependent in TNBS colitis. Stem Cell Research & Therapy, 8, 87.
Blauvelt, A., & Chiricozzi, A. (2018). The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clinical Reviews in Allergy & Immunology, 55, 379–390.
Furue, K., Ito, T., & Furue, M. (2018). Differential efficacy of biologic treatments targeting the TNF-α/IL-23/IL-17 axis in psoriasis and psoriatic arthritis. Cytokine, 111, 182–188.
Shi, Y., Wang, Y., Li, Q., Liu, K., Hou, J., Shao, C., & Wang, Y. (2018). Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nature Reviews, 14, 493–507.
Schrepfer, S., Deuse, T., Reichenspurner, H., Fischbein, M. P., Robbins, R. C., & Pelletier, M. P. (2006). Stem cell transplantation: the lung barrier. Transplantation Proceedings, 2, 573–576.
Fan, J., Tang, X., Wang, Q., Zhang, Z., Wu, S., Li, W., Liu, S., Yao, G., Chen, H., & Sun, L. (2018). Mesenchymal stem cells alleviate experimental autoimmune cholangitis through immunosuppression and cytoprotective function mediated by galectin-9. Stem Cell Research & Therapy, 1, 237.
Acknowledgements
The authors thank all of the MSC donors and the patients for their willingness to participate in this study. The authors also appreciate Yu Zhang, Chenxi Ge, Ziying Lin, Zhiqian Zhou, and Ke Liu at the Sinoneural Cell Engineering Group Holdings Co., Ltd. for their help with preparation of MSCs.
Funding
This work was sponsored by grants from National Natural Science Foundation of China (No. 81872522, 82073429, 82273510, 82203908), Innovation Program of Shanghai Municipal Education Commission (No.2019-01-07-00-07-E00046), Clinical Research Plan of SHDC (No. SHDC2020CR1014B, SHDC2020CR6022, SHDC12018X06) and Program of Shanghai Academic Research Leader (No. 20XD1403300).
Author information
Authors and Affiliations
Contributions
The study was designed by Z.W., D.F.W., H.J., H.Z., and Y.S.; The experiments were carried out by Z.W., Y.H., X.W., Y.C., C.Y., J.F., C.P., L.W., Y.G., Y.L., D.W., and F.R.; Z.W., X.W., C.Y., J.F., and S.W. wrote the paper; all authors analyzed the data; H.Z., and Y.S. supervised the project. All authors approved the final submitted version.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The number of animals used in the research as well as experimental procedures had been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC No. ACU20-1719). Mouse care and experiments were performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The clinical study was approved by the Stem Cell Research Ethics Committee of Shanghai Tenth People’s Hospital of Tongji University, China (approval No. SC-2020-01).
Consent for Publication
Consent for publication was obtained from every participant.
Competing Interests
The authors declare no competing interests. The authors from Sinoneural Cell Engineering Group Holdings Co., Ltd. helped in the preparation of the MSCs.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(PDF 632 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Hu, Y., Wang, X. et al. Comparative Analysis of the Therapeutic Effects of Fresh and Cryopreserved Human Umbilical Cord Derived Mesenchymal Stem Cells in the Treatment of Psoriasis. Stem Cell Rev and Rep 19, 1922–1936 (2023). https://doi.org/10.1007/s12015-023-10556-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10556-8