Abstract—
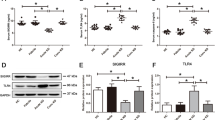
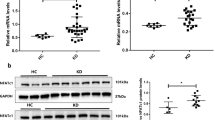
Kawasaki disease (KD) is an acute, self-limiting, febrile systemic vasculitis of unknown cause associated with the development of coronary artery lesions (CALs) during childhood. Damage-associated molecular patterns (DAMPs) from cell death and oxidative stress have been shown to be involved in the development of KD vasculitis. Interleukin (IL)-33 is released from damaged endothelial cells and acts as a DAMP. We studied whether IL-33 and its receptor (ST2) might be involved in KD pathogenesis. Serum levels of soluble ST2 (sST2) in KD patients were measured before their first therapy. Furthermore, we investigated the impact of IL-33 on human coronary artery endothelial cells (HCAECs). Serum levels of sST2 were significantly higher in KD patients with CALs than in those with normal coronary arteries. In vitro, IL-33 upregulated the expression of ST2L and increased production of sST2, IL-6, IL-8, and monocyte chemoattractant protein−1 in HCAECs in a time- and concentration-dependent manner. Moreover, IL-33 induced significantly greater production of IL-6 and IL-8 in HCAECs compared to the condition stimulated with isoconcentration of tumor necrosis factor-α. The results of the present study suggest that the IL-33/ST2 axis might be involved in the development of KD vasculitis. The IL-33/ST2 axis may be a therapeutic target for the treatment of KD.
Graphical abstract






Similar content being viewed by others
Availability of Data and Materials
All data will be available upon publication. Reagents and material will be shared upon request.
References
Hara, T., K. Yamamura, and Y. Sakai. 2021. The up-to-date pathophysiology of Kawasaki disease. Clinical & Translational Immunology 10: e1284. https://doi.org/10.1002/cti2.1284.
Fukazawa, R., J. Kobayashi, M. Ayusawa, H. Hamada, M. Miura, Y. Mitani, E. Tsuda, H. Nakajima, H. Matsuura, K. Ikeda, K. Nishigaki, H. Suzuki, K. Takahashi, K. Suda, H. Kamiyama, Y. Onouchi, T. Kobayashi, H. Yokoi, K. Sakamoto, M. Ochi, S. Kitamura, K. Hamaoka, H. Senzaki, T. Kimura, and Japanese Circulation Society Joint Working Group. 2020. JCS/JSCS 2020 guideline on diagnosis and management of cardiovascular sequelae in Kawasaki disease. Circulation Journal 84: 1348–1407. https://doi.org/10.1253/circj.CJ-19-1094.
McCrindle, B.W., A.H. Rowley, J.W. Newburger, J.C. Burns, A.F. Bolger, M. Gewitz, A.L. Baker, M.A. Jackson, M. Takahashi, P.B. Shah, T. Kobayashi, M.H. Wu, T.T. Saji, E. Pahl, American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Council on Epidemiology and Prevention. 2017. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 135: 927–999. https://doi.org/10.1161/CIR.0000000000000484.
Hara, T., Y. Nakashima, Y. Sakai, H. Nishio, Y. Motomura, and S. Yamasaki. 2016. Kawasaki disease: A matter of innate immunity. Clinical and Experimental Immunology 186: 134–143. https://doi.org/10.1111/cei.12832.
Kusuda, T., Y. Nakashima, K. Murata, S. Kanno, H. Nishio, M. Saito, T. Tanaka, K. Yamamura, Y. Sakai, H. Takada, T. Miyamoto, Y. Mizuno, K. Ouchi, K. Waki, and T. Hara. 2014. Kawasaki disease-specific molecules in the sera are linked to microbe-associated molecular patterns in the biofilms. PLoS ONE 9: e113054. https://doi.org/10.1371/journal.pone.0113054.
Hoshina, T., K. Kusuhara, K. Ikeda, Y. Mizuno, M. Saito, and T. Hara. 2008. High mobility group box 1 (HMGB1) and macrophage migration inhibitory factor (MIF) in Kawasaki disease. Scandinavian Journal of Rheumatology 37: 445–449. https://doi.org/10.1080/03009740802144143.
Foell, D., F. Ichida, T. Vogl, X. Yu, R. Chen, T. Miyawaki, C. Sorg, and J. Roth. 2003. S100A12 (EN-RAGE) in monitoring Kawasaki disease. The Lancet 361: 1270–1272. https://doi.org/10.1016/S0140-6736(03)12986-8.
Nakashima, Y., Y. Sakai, Y. Mizuno, K. Furuno, K. Hirono, S. Takatsuki, H. Suzuki, Y. Onouchi, T. Kobayashi, K. Tanabe, K. Hamase, T. Miyamoto, R. Aoyagi, M. Arita, K. Yamamura, T. Tanaka, H. Nishio, H. Takada, S. Ohga, and T. Hara. 2021. Lipidomics links oxidized phosphatidylcholines and coronary arteritis in Kawasaki disease. Cardiovascular Research 117: 96–108. https://doi.org/10.1093/cvr/cvz305.
Takeshita, S., H. Kawase, M. Yamamoto, T. Fujisawa, I. Sekine, and S. Yoshioka. 1994. Increased expression of human 63-kD heat shock protein gene in Kawasaki disease determined by quantitative reverse transcription-polymerase chain reaction. Pediatric Research 35: 179–183. https://doi.org/10.1203/00006450-199402000-00010.
Abe, J., T. Jibiki, S. Noma, T. Nakajima, H. Saito, and M. Terai. 2005. Gene expression profiling of the effect of high-dose intravenous Ig in patients with Kawasaki disease. The Journal of Immunology 174: 5837–5845. https://doi.org/10.4049/jimmunol.174.9.5837.
Jia, C., J. Zhang, H. Chen, Y. Zhuge, H. Chen, F. Qian, K. Zhou, C. Niu, F. Wang, H. Qiu, Z. Wang, J. Xiao, X. Rong, and M. Chu. 2019. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death & Disease 10: 778. https://doi.org/10.1038/s41419-019-2021-3.
Ikeda, K., K. Yamaguchi, T. Tanaka, Y. Mizuno, A. Hijikata, O. Ohara, H. Takada, K. Kusuhara, and T. Hara. 2010. Unique activation status of peripheral blood mononuclear cells at acute phase of Kawasaki disease. Clinical and Experimental Immunology 160: 246–255. https://doi.org/10.1111/j.1365-2249.2009.04073.x.
Ueno, K., Y. Nomura, Y. Morita, and Y. Kawano. 2021. Prednisolone suppresses the extracellular release of HMGB-1 and associated inflammatory pathways in Kawasaki disease. Frontiers in Immunology 12: 640315. https://doi.org/10.3389/fimmu.2021.640315.
Inoue, T., S. Murakami, K. Matsumoto, and A. Matsuda. 2020. Functional benefits of corticosteroid and IVIG combination therapy in a coronary artery endothelial cell model of Kawasaki disease. Pediatric Rheumatology Online Journal 18: 76. https://doi.org/10.1186/s12969-020-00461-6.
Armaroli, G., E. Verweyen, C. Pretzer, K. Kessel, K. Hirono, F. Ichida, M. Okabe, D.A. Cabral, D. Foell, K.L. Brown, and C. Kessel. 2019. Monocyte-derived interleukin-1β as the driver of S100A12-induced sterile inflammatory activation of human coronary artery endothelial cells: Implications for the pathogenesis of Kawasaki disease. Arthritis & Rhematology 71: 792–804. https://doi.org/10.1002/art.40784.
Silvis, M.J.M., S.E. Kaffka Genaamd Dengler, C.A. Odille, M. Mishra, N.P. van der Kaaij, P.A. Doevendans, J.P.G. Sluijter, D.P.V. de Kleijn, S.C.A. de Jager, L. Bosch, and G.P.J. van Hout. 2020. Damage-associated molecular patterns in myocardial infarction and heart transplantation: the road to translational success. Frontiers in Immunology 11: 599511. https://doi.org/10.3389/fimmu.2020.599511.
Huang, J., S. Wu, S. Cao, X. Zhu, and S. Zhang. 2020. Neutrophil-derived semaphorin 4D induces inflammatory cytokine production of endothelial cells via different plexin receptors in Kawasaki disease. BioMed Research International 2020: 6663291. https://doi.org/10.1155/2020/6663291.
Su, Y., S. Feng, L. Luo, R. Liu, and Q. Yi. 2019. Association between IL-35 and coronary arterial lesions in children with Kawasaki disease. Clinical and Experimental Medicine 19: 87–92. https://doi.org/10.1007/s10238-018-0513-6.
Demyanets, S., C. Kaun, R. Pentz, K.A. Krychtiuk, S. Rauscher, S. Pfaffenberger, A. Zuckermann, A. Aliabadi, M. Gröger, G. Maurer, K. Huber, and J. Wojta. 2013. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. Journal of Molecular and Cellular Cardiology 60: 16–26. https://doi.org/10.1016/j.yjmcc.2013.03.020.
Liew, F.Y., J.P. Girard, and H.R. Turnquist. 2016. Interleukin-33 in health and disease. Nature Reviews Immunology 16: 676–689. https://doi.org/10.1038/nri.2016.95.
De la Fuente, M., T.T. MacDonald, and M.A. Hermoso. 2015. The IL-33/ST2 axis: Role in health and disease. Cytokine & Growth Factor Reviews 26: 615–623. https://doi.org/10.1016/j.cytogfr.2015.07.017.
Günther, S., D. Deredge, A.L. Bowers, A. Luchini, D.A. Bonsor, R. Beadenkopf, L. Liotta, P.L. Wintrode, and E.J. Sundberg. 2017. IL-1 family cytokines use distinct molecular mechanisms to signal through their shared co-receptor. Immunity 47: 510–523. https://doi.org/10.1016/j.immuni.2017.08.004.
Coronado, M.J., K.A. Bruno, L.A. Blauwet, C. Tschöpe, M.W. Cunningham, S. Pankuweit, S. van Linthout, E.S. Jeon, D.M. McNamara, J. Krejčí, J. Bienertová-Vašků, E.J. Douglass, E.D. Abston, A. Bucek, J.A. Frisancho, M.S. Greenaway, A.R. Hill, H.P. Schultheiss, L.T. Cooper Jr., and D. Fairweather. 2019. Elevated sera sST2 is associated with heart failure in men ≤ 50 years old with myocarditis. Journal of the American Heart Association 8: e008968. https://doi.org/10.1161/JAHA.118.008968.
Sato, Y.Z., D.P. Molkara, L.B. Daniels, A.H. Tremoulet, C. Shimizu, J.T. Kanegaye, B.M. Best, J.V. Snider, J.R. Frazer, A. Maisel, and J.C. Burns. 2013. Cardiovascular biomarkers in acute Kawasaki disease. International Journal of Cardiology 164: 58–63. https://doi.org/10.1016/j.ijcard.2011.06.065.
Ko, T.M., H.C. Kuo, J.S. Chang, S.P. Chen, Y.M. Liu, H.W. Chen, F.J. Tsai, Y.C. Lee, C.H. Chen, J.Y. Wu, and Y.T. Chen. 2015. CXCL10/IP-10 is a biomarker and mediator for Kawasaki disease. Circulation Research 116: 876–883. https://doi.org/10.1161/CIRCRESAHA.116.305834.
Hoshino, S., S. Jain, C. Shimizu, S. Roberts, F. He, L.B. Daniels, A.M. Kahn, A.H. Tremoulet, J.B. Gordon, and J.C. Burns. 2021. Biomarkers of inflammation and fibrosis in young adults with history of Kawasaki disease. International Journal of Cardiology Heart & Vasculature 36: 100863. https://doi.org/10.1016/j.ijcha.2021.100863.
Ayusawa, M., T. Sonobe, S. Uemura, S. Ogawa, Y. Nakamura, N. Kiyosawa, M. Ishii, K. Haradam, and Kawasaki Disease Research Committee. 2005. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatrics International 47: 232–234. https://doi.org/10.1111/j.1442-200x.2005.02033.x.
Kobayashi, T., S. Fuse, N. Sakamoto, M. Mikami, S. Ogawa, K. Hamaoka, Y. Arakaki, T. Nakamura, H. Nagasawa, T. Kato, T. Jibiki, S. Iwashima, M. Yamakawa, T. Ohkubo, S. Shimoyama, K. Aso, S. Sato, T. Saji, and Z Score Project Investigators. 2016. A new Z score curve of the coronary arterial internal diameter using the lambda-mu-sigma method in a pediatric population. Journal of the American Society of Echocardiography 29: 794–801. https://doi.org/10.1016/j.echo.2016.03.017.
Hasegawa, S., T. Ichiyama, I. Sonaka, A. Ohsaki, S. Okada, H. Wakiguchi, K. Kudo, S. Kittaka, M. Hara, and S. Furukawa. 2012. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clinical and Experimental Immunology 167: 269–274. https://doi.org/10.1111/j.1365-2249.2011.04519.x.
Matsubara, T. 2018. Infliximab for the treatment of Kawasaki disease. Pediatrics International 60: 775. https://doi.org/10.1111/ped.13663.
Kawasaki, T., and S. Naoe. 2014. History of Kawasaki disease. Clinical and Experimental Nephrology 18: 301–304. https://doi.org/10.1007/s10157-013-0877-6.
Tremoulet, A.H. 2018. Adjunctive therapies in Kawasaki disease. International Journal of Rheumatic Diseases 21: 76–79. https://doi.org/10.1111/1756-185X.13208.
Zhu, F., and J.Y. Ang. 2021. 2021 Update on the clinical management and diagnosis of Kawasaki disease. Current Infectious Disease Reports 23: 3. https://doi.org/10.1007/s11908-021-00746-1.
Masuda, H., T. Kobayashi, A. Hachiya, Y. Nakashima, H. Shimizu, T. Nozawa, Y. Ogihara, S. Ito, S. Takatsuki, N. Katsumata, Y. Suzuki, S. Takenaka, K. Hirono, T. Kobayashi, H. Suzuki, E. Suganuma, K. Takahashi, T. Saji, and Committee of Survey on Infliximab use for Kawasaki disease. 2018. Infliximab for the treatment of refractory Kawasaki disease: a nationwide survey in Japan. The Journal of Pediatrics 195: 115–120. https://doi.org/10.1016/j.jpeds.2017.10.013.
Ohnishi, Y., S. Okada, A. Kawakami-Miyake, T. Furuta, R. Fukano, H. Yasudo, M. Shimokawa, and S. Hasegawa. 2022. Safety and feasibility of infliximab therapy in children with Kawasaki disease who received live vaccinations. The Pediatric Infectious Disease Journal 41: e388–e392. https://doi.org/10.1097/INF.0000000000003611.
Furuta, T., H. Yasudo, S. Okada, Y. Ohnishi, A. Kawakami-Miyake, Y. Suzuki, S. Ohga, and S. Hasegawa. 2022. Third-line therapies in patients with Kawasaki disease refractory to first- and second-line intravenous immunoglobulin therapy. World Journal of Pediatrics. https://doi.org/10.1007/s12519-022-00602-9 Online ahead of print.
Takahashi, K., T. Oharaseki, Y. Yokouchi, N. Hiruta, and S. Naoe. 2010. Kawasaki disease as a systemic vasculitis in childhood. Annals of Vascular Diseases 3: 173–181. https://doi.org/10.3400/avd.sasvp01003.
Demyanets, S., V. Konya, S.P. Kastl, C. Kaun, S. Rauscher, A. Niessner, R. Pentz, S. Pfaffenberger, K. Rychli, C.E. Lemberger, R. de Martin, A. Heinemann, I. Huk, M. Gröger, G. Maurer, K. Huber, and J. Wojta. 2011. Interleukin-33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human atherosclerotic plaques. Arteriosclerosis, Thrombosis, and Vascular Biology 31: 2080–2089. https://doi.org/10.1161/ATVBAHA.111.231431.
Wang, Y., W. Wang, F. Gong, S. Fu, Q. Zhang, J. Hu, Y. Qi, C. Xie, and Y. Zhang. 2013. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis and Rheumatism 65: 805–814. https://doi.org/10.1002/art.37815.
Kim, H.J., E.H. Choi, and H.R. Kil. 2014. Association between adipokines and coronary artery lesions in children with Kawasaki Disease. Journal of Korean Medical Science 29: 1385–1390. https://doi.org/10.3346/jkms.2014.29.10.1385.
Song, H.B., Y.D. Zhang, Q.W. Dong, L.P. Han, R.F. Qi, B.B. Bi, L. Ma, L. Ma, and H. Zhang. 2020. Significance of serum NT-proBNP and endogenous H2S for predicting coronary artery lesions in pediatric Kawasaki disease. Journal of the College of Physicians and Surgeons Pakistan 30: 37–40. https://doi.org/10.29271/jcpsp.2020.01.37.
Matsubara, T., T. Ichiyama, and S. Furukawa. 2005. Immunological profile of peripheral blood lymphocytes and monocytes/macrophages in Kawasaki disease. Clinical and Experimental Immunology 141: 381–387. https://doi.org/10.1111/j.1365-2249.2005.02821.x.
Saunders, P.T.K., and A.W. Horne. 2021. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 184: 2807–2824. https://doi.org/10.1016/j.cell.2021.04.041.
Qi, Y., J. Xu, Z. Lin, Y. Tao, F. Zheng, Y. Wang, Y. Sun, S. Fu, W. Wang, C. Xie, Y. Zhang, and F. Gong. 2021. The network of pro-inflammatory factors CD147, DcR3, and IL33 in the development of Kawasaki disease. Journal of Inflammation Research 14: 6043–6053. https://doi.org/10.2147/JIR.S338763.
Ferrara, G., T. Giani, M.C. Caparello, C. Farella, L. Gamalero, and R. Cimaz. 2020. Anakinra for treatment-resistant kawasaki disease: Evidence from a literature review. Paediatric Drugs 22: 645–652. https://doi.org/10.1007/s40272-020-00421-3.
Hicar, M.D. 2020. Antibodies and immunity during kawasaki disease. Frontiers in Cardiovascular Medicine 7: 94. https://doi.org/10.3389/fcvm.2020.00094.
Funding
This work was supported by JSPS KAKENHI (Grant number, JP21K15906) (S.O.), AMED (Grant number, JP22ek0109606) (S.O.), and a grant from Japan Blood Products Organization and partially supported by Inokuma Prize.
Author information
Authors and Affiliations
Contributions
Seigo Okada contributed to conception, design, acquisition, analysis, and interpretation of data, and drafting the article. Hiroki Yasudo contributed to conception, design, and interpretation of data. Yuji Ohnishi contributed to design, acquisition, analysis, and interpretation of data. Chie Matsuguma, Takahiro Motonaga, and Takako Waniishi contributed to acquisition and analysis of data. Reiji Fukano contributed to analysis and interpretation of data. Shunji Hasegawa contributed to revising it critically for important intellectual content and final approval of the version to be published. The first draft of the manuscript was written by Seigo Okada and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Yamaguchi University Hospital Institutional Review Board (Date 27/06/2016/No. H28-047).
Consent to Participate
Written informed consent was obtained from the parents.
Consent to Publish
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1 and 2.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hiroki Yasudo and Yuji Ohnishi contributed equally to this paper.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Okada, S., Yasudo, H., Ohnishi, Y. et al. Interleukin-33/ST2 Axis as Potential Biomarker and Therapeutic Target in Kawasaki Disease. Inflammation 46, 480–490 (2023). https://doi.org/10.1007/s10753-022-01753-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01753-7