Abstract
Sepsis caused by a dysregulated host response to infection is a life-threatening disease that can lead to organ dysfunction. Due to its unclear and complex mechanism, effective medicine for the treatment of sepsis is urgently required. The extensive release of cytokines and other mediators like TNF-α and interleukin-6 (IL-6) play critical roles in the development of sepsis. The present study aims to evaluate the potential protective effects of an anti-TNF-α/HSA/IL-6R triple-specific fusion protein (TAL-6) under septic experimental conditions. The anti-TNF-α/HSA/IL-6R triple-specific fusion protein (TAL-6), which links three published single domain antibodies, was designed and constructed in our lab. High purity fusion proteins were obtained with high binding affinity for TNF-α (94.75 pM), human serum albumin (1.83 nM) and IL-6R (2.29 nM). TAL-6 protected mouse fibroblast fibrosarcoma cells (L929) from apoptosis induced by TNF-α, establishing that the expressed fusion proteins can selectively interact with TNF-α in vitro. In vivo, the survival rate of cecal ligation and puncture (CLP) was notably increased in the group with TAL-6 treatment and significantly higher compared with the single-targeted IL-6R and TNF-α fusion protein at the same dose. After treatment with TAL-6, the serum levels of TNF-α, IL-1β, and IL-6 were significantly decreased, and sepsis-induced pathological injuries in the kidney were remarkably attenuated. TAL-6 is therefore a potential candidate for the development of new drugs against sepsis in human.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sepsis constitutes a major healthcare problem and is expected to increase due to an aging population. In 2017, WHO recognized that sepsis was a global health priority [1]. Sepsis is one of the most severe diseases caused by a dysregulated host response to infection [2, 3]. Recent studies have demonstrated that the extensive release of cytokines and other mediators play critical roles in the development of sepsis [4]. During the pathogenesis of sepsis, inflammatory cells are stimulated to produce various inflammatory mediators, leading to the damage, dysfunction, and even exhaustion of multiple organs, and about 50% of sepsis cases exhibit myocardial dysfunction [4].
The cytokine network is activated to produce a large number of cytokines closely related to the development of inflammation in sepsis patients [4, 5]. Among the inflammatory mediators involved, tumor necrosis factor alpha (TNF-α) is the closest mediator of the cytokine cascade. Serum TNF-α levels increase within 1–2 h after injection of endotoxin that may be involved in sepsis [1, 5, 6]. Secreted TNF-α is an essential mediator of inflammation and induces marked vasodilatation leading to tissue hypoperfusion, shock, and organ failure. Based on this, TNF-α may be an important target that the pharmaceutical industry could develop antibodies or molecules against to treat sepsis. However, clinical trial results to date have not been ideal.
Interleukin-6 (IL-6) is produced as a prominent activator of the acute phase response with extensive biological activities [7, 8]. The plasma half-life of IL-6 is longer than other inflammatory factors such as TNF-α or IL-1β [9]. When the level of TNF-α in the plasma of patients with sepsis significantly decreases, the concentration of IL-6 significantly increases [10]. A link between high IL-6 levels and mortality in septic patients [6] has been found in the previous studies. Therefore, IL-6, widely accepted as a valuable biomarker for evaluating therapeutic response and the prognosis of sepsis [5], can be employed as a potential therapeutic target in the clinical treatment of sepsis.
Here, we constructed a triple-specific fusion protein (TAL-6) composed of published single domain anti-TNF-α, anti-human serum albumin (HSA) and anti-IL-6R antibodies. Different from other conventional single-chain Fv (scFv) antibodies from human IgGs, the single domain antibody derived from the natural camel heavy-chain only antibodies (HCAbs) [11, 12] presents superior features, including lower molecular weight, increased stability, high affinity, easy recombinant expression preparation, less immunogenicity, and better solubility [12, 13]. Additionally, the CDR3 of the single domain antibody is longer, which makes it more flexible in binding to the antigen. This structural feature allows the single domain antibody to bind to the recessed hidden epitopes of the antigen, such as the hidden epitopes of viruses and enzyme activation sites [14], or receptor protein molecule sites [15]. These characteristics allow it to play an important role in microscopy, structural biology, medical diagnosis, drug delivery, and disease treatment. Furthermore, recombinant fusion proteins can be easily constructed by different single domain antibodies allowing the resultant fusion protein to target one or two antigens at the same time. The anti-TNF-α/ HSA/IL-6R fusion protein (TAL-6) binds to the TNF-α and IL-6 receptors and to human serum albumin (HSA), leading to significantly increased half-life of fusion protein in vivo and blockage of cytokines at anatomical sites [16]. The present study aimed to explore the potential associations of the functions of TNF-α and IL-6 being blocked simultaneously by TAL-6 under experimental septic condition and to investigate the therapeutic effects of TAL-6 in vitro and vivo.
MATERIALS AND METHODS
Preparation of Anti-TNF-α/HSA/IL-6R Triple-Specific Fusion Protein
The gene synthesis of the anti-TNF-α/HSA/IL-6R triple-specific fusion protein (TAL-6) based on the sequence information of patents US2010/0172894 and US2012/0093839 was completed by Shanghai Gengray Biotech Co., Ltd. The synthetic gene fragment was inserted into the corresponding position of pET-28a/NcoI/XhoI and named pET28a-TAL-6 fusion protein. One hundred nanograms of the pET28a-TAL-6 plasmid was transformed into competent E. coli.BL21(DE3) cells (Beijing Quanshijin Biotechnology Company) by heat shock. Recombinant cells from 50 mL of culture were harvested by centrifugation at 10,000 g for 10 min, and the pellet was resuspended in a 5-mL TGE buffer (50 mM Tris pH 7.9, 0.5 mM EDTA, 50 mM NaCl, 5% glycerin), then ultrasonic crushed on ice for 30 min. The fusion proteins fused with His-tag were expressed and purified using BeaverBeads™ IDA-Nickel (Suzhou Beaver Bioengineering Company) and desalted with Äkta (GE Healthcare, USA). The purified fusion protein was analyzed by 10% SDS-PAGE.
Anti-TNF-α/HSA/IL-6R Triple-Specific Fusion Protein Specificity Analysis
Plates were first coated with IL-6R (1 μg·mL−1), TNF-α (1 μg·mL−1), and HSA (human serum albumin, 1 μg·mL−1) at 4 °C overnight (diluted in 0.1 M Na2CO3-NaHCO3 buffer, pH 9.6). The next day, the plates were washed 3 times with PBST (0.05% Tween-20 in PBS) and blocked for 2 h with 100 μL 1% BSA-PBS. The fusion protein diluent was added at various final concentrations after 3 washes and incubated for 1 h at 37 °C. Fifty microliters rabbit IgG anti-His-tag (CST, USA) was added and incubated for another hour. The plates were washed 3 times, and then 50 μL goat anti-rabbit IgG-HRP antibody (CST) was added and incubated for another hour. After washing, a total of 100 μL of reaction buffer (5 mg O-phenylenediamine, 10 μL H2O2, 5 mL citric acid-Na2HPO4) was added into the wells. The reaction was stopped by 2 M H2SO4 after 10 min. Absorbance was measured at 492 nm on an ELISA plate reader (Bio Tek, USA).
Cell-Based Functional Assays of Anti-TNF-α/HAS/IL-6R Triple-Specific Fusion Proteins In Vitro
Murine L929 fibroblast cells (Shanghai Institute of Cell Biology, Chinese Academy of Sciences) were cultured in 1640 medium (Gibco, USA) supplemented with 10% FBS (Gibco, USA). Approximately 12,000 cells were seeded in each well of a 96-well plate and incubated for 24 h at 37 °C under a humidified atmosphere and 5% CO2. The reaction mixtures were prepared using TNF-α (0.15 nM) and various concentrations of purified TAL-6 (ranging from 0.032 to 32 nM) in the presence of actinomycin (1 mg·mL−1). The mixtures were incubated on ice for 10 min and then added onto the previously L929 seeded wells for 12-h treatment. Twenty microliters of MTT (5 mg/mL) was added to each well and the plates were kept in the incubator for an additional 4 h. The supernatant was discarded, and 150 μL of dimethyl sulfoxide was added onto wells with incubation for 40 min at room temperature with agitation. The absorbance was measured at 570 nm, and the achieved results were fit by a dose–response inhibition curve in Prism (version 5.01, GraphPad Software Inc.).
For apoptotic cells analysis, L929 cells were treated with the same methods mentioned above. After incubation for 12 h, the supernatant was discarded. The plates were washed with PBS. One hundred microliters of PBS and 5 μL of PI (propidium iodide) were added to each well and incubated for 45 min at 37 °C. The L929 cells were then fixed in 4% paraformaldehyde for 15 min. The plates were washed, and 100 μL of PBS and 5 μL of DAPI (4′,6-diamidino- 2-phenylindol) were added to each well at room temperature for 30 min. After staining, the cells were imaged and analyzed using an Operetta™ High Content Screening instrument with Harmony™ software version 3.5.2 (PerkinElmer, Waltham, MA, USA) and different excitation/emission channels.
Therapeutic Effect of Anti-TNF-α/HSA/IL-6R Triple-Specific Fusion Protein on CLP Sepsis Model Rats
Specific pathogen free (SPF) male Sprague–Dawley rats, weighing 180–200 g, were supplied by Fujian Medical University Animal Center (Fuzhou, China). The rats were housed in Fujian Medical University Animal Center, with free access to food and water, under a light/dark (12 h/12 h cycle) at a temperature of 20–23 °C. The rats were acclimatized for more than 7 days before the experiments.
Rats were anesthetized with 2% pentobarbital sodium (40 mg·kg−1, i.p.) and received a laparotomy in the middle of the abdomen. The cecum was isolated and ligated using a 3–0 silk, then punctured twice with an 18-gauge needle. The fecal material was expelled by gently squeezing. In sham-operated rats, the cecum was isolated, but neither ligated nor punctured. The cecum was returned, and the wound was then closed. After surgery, 3 mL/100 g prewarmed saline was given intraperitoneally for fluid resuscitation. Rats were divided into 7 groups (n = 8): (1) sham group, (2) CLP model group, (3) low dose of TAL-6 group (CLP + TAL-6 1 mg·kg−1), (4) middle dose of TAL-6 group (CLP + TAL-6 5 mg·kg−1), (5) high dose of TAL-6 group (CLP + TAL-6 10 mg·kg−1), (6) anti-TNF-α fusion protein 5 mg·kg−1 group, and (7) anti-IL-6R fusion protein 5 mg·kg−1 group. The drugs were intravenously injected half an hour later after operation. Meanwhile, the sham group and the CLP model group were given the same saline intravenously. Seventy-two hours after surgery, rats were scarified, and the blood was collected. Left kidneys were harvested and fixed in 4% paraformaldehyde overnight. Right kidneys were harvested and frozen in liquid nitrogen for molecular biological examinations.
Survival Analysis and Blood Urea Nitrogen Examinations
For survival analysis, after surgery, the survival status of each group of rats was observed every 12 h and recorded. The levels of creatinine and BUN were examined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer’s instructions.
Hematoxylin–Eosin Staining
Kidney tissues were rinsed with ice-cold saline solution and fixed with paraformaldehyde (4%), embedded in paraffin, and cut into 5-mm sections. Then, all sections were stained with HE and photographed under an optical microscope with 20 × magnification.
Enzyme-Linked Immunosorbent Assays
The levels of TNF-α, IL-6, and IL-1β were detected with commercial kits following the manufacturer’s instructions (ELISA kits of TNF-α, IL-6, and IL-1β from Shanghai enzyme linked Biotechnology Co., Ltd, Shanghai, China).
Quantification of Inflammatory Mediator mRNA Levels by Real-Time PCR
Total mRNA was isolated using an RNA simple Total RNA Kit (Tianmo Biotech, Beijing, China). The concentration of mRNA was determined using a NanoDropND-2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Single-stranded cDNA was synthesized using oligonucleotide primer and super M-MLV (Takara, Japan) in a 20-μL reaction system. Quantitative real-time PCR was performed on 1-μL cDNA using the 2 × TB Green Premix Ex Taq II (TliRNaseH Plus) (Takara) in a 20-μL reaction system in a PCR System (Exicycle 96, Bioneer, Taejon, Korea). The synthesis and purchase of primers were completed by Biosune Biotech Co., Ltd. Relative gene expression was normalized to GAPDH and calculated using the 2ΔΔCt method. The following primers were used:
IL-1β primer sequences were forward 5′-TGGAGAAGCTGTGGCAGCTACCT-3′ and reverse 5′-GAACGTCACACACCAGCAGGTT-3′
IL-6 primer sequences were forward 5′-GCCAGAGTCCTTCAGAGAGA-3′ and reverse 5′-GGTCTTGGTCCTTAGCCACT-3′
TNF-α primer sequences were forward 5′-AGTGACAAGCCTGTAGCCCACGT-3′ and reverse 5′-CCATCGGCTGGCACCACTAGTT-3′
GAPDH primer sequences were forward 5′-GTGAAGGTCGGTGTGAACG-3′ and reverse 5′-CTCGCTCCTGGAAGATGGTG-3′
Statistical Analysis
All data are expressed as means ± SD. One-way ANOVA followed by Bonferroni’s multiple comparison test using GraphPad software was used for comparisons among experimental groups. A p-value of less than 0.05 was considered statistically significant.
RESULTS
The Preparation of Anti-TNF-α/HSA/IL-6R Triple-Specific Fusion Protein
The cDNA encoding the recombinant anti-TNF-α/HSA/IL-6R triple-specific fusion protein (TAL-6) was made synthetically based on the sequence information of patents US2010/0172894 and US2012/0093839. The 3D structure of TAL-6 was mimicked by SWISS-MODEL (Fig. 1A). The inserts were ligated into the multiple cloning sites region downstream of the pET28a + vector using EcoRI/NotI restriction sites. Colony PCR results showed that the size of the amplified gene fragment was 1600 bp (Fig. 1B) when the plasmid of TAL-6 was successfully transformed into competent cells BL21(DE3). The fusion protein was expressed and analyzed by 10% SDS-PAGE (Fig. 1C), indicating that TAL-6 is expressed in E. coli inclusion bodies [17]. The inclusion bodies were purified by BeaverBeads™ IDA-Nickel (Fig. 1D) and desalted with Äkta (Fig. 1E). The purity of TAL-6 reached 95%.
The preparation of anti-TNF-α/HSA/IL-6R triple-specific fusion protein TAL-6. (A) 3D structure of an anti-TNF-α/HSA/IL-6R triple-specific fusion protein TAL-6 simulated by SWISS-MODEL. (B) Colony PCR of TAL-6. (C) The expression product of TAL-6. Lane M, marker; lane 1: BL21(DE3); lane 2: BL21(DE3)/TAL-6, lane 3: supernatant; lane 4: sediment. (D) The process of purification. Lane M, marker; lane 1: BL21(DE3); lane 2: BL21(DE3)/TAL-6, lane 3: supernatant; lane 4: sediment, lanes 5–6: washed IBs, lane 7: renaturation protein, lane 8: further purified protein by one-step BeaverBeadsTM IDA-Nickel, lane 9: desalting. (E) Desalting with Äkta. (F) Binding capacity analysis of TAL-6.
Binding Capacity Analysis of Anti-TNF-α/HSA/IL-6R Triple-Specific Fusion Protein
As shown in Fig. 1F, TAL-6 was able to bind TNF-α, HSA, and IL-6R in a dose-dependent manner. The results indicate that the EC50 values of TAL-6 were 94.75 pM for TNF-α, 1.83 nM for has, and 2.29 nM for IL-6R.
Inhibition of TNF-α by Anti-TNF-α/HSA/IL-6R Triple-Specific Fusion Proteinin vitro
The MTT results (Fig. 2A) showed that TAL-6 inhibits TNF-α cytotoxicity in L929 cells in a dose-dependent manner with an IC50 value of 19.6 pM. DAPI/PI staining was employed to observe the effects of TAL-6 on apoptosis of L929 cells induced by TNF-α. Under a fluorescence microscope, the negative control group showed uniform blue fluorescence, and the nuclei treated with TNF-α were evenly colored red by PI (Fig. 2B). The results of cell death by high-content analysis showed that the control group had an apoptotic rate of 3.40 ± 0.77%, which was significantly different from the rate of 79.45 ± 5.89% of the TNF-α (2 ng/mL) group (P < 0.001). The apoptotic rates of the TAL-6-treated groups (32 nM, 3.2 nM, 0.32 nM) were 8.66 ± 4.93%, 8.93 ± 2.16%, and 64.89 ± 4.69%, respectively. These results indicate that TNF-α can induce apoptosis of L929 cells and that TAL-6 treatment effectively reduces the rate of TNF-α-induced apoptosis of L929 cells in a dose-dependent manner (Fig. 2C)
Inhibition of TNF-α cytotoxicity using TAL-6. (A) MTT assay for the abilities of TAL-6 to inhibit TNF-α activity. (B) Cells were cultured with 2 ng/mL TNF-α and 10 µg/mL actinomycin D in the presence of TAL-6 (32 nM, 3.2 nM, 0.32 nM, 0.032 nM), and then the cells were processed for DAPI/PI double stain assay. DAPI was used to stain the nuclei. PI was used to stain apoptotic cells. Images were taken under a fluorescent microscope (DAPI/PI at × 10). a. control, b. TNFα, c. TNF-α + 32 nM TAL-6, d. TNF-α + 3.2 nM TAL-6, e. TNF-α + 0.32 nM TAL-6, f. TNF-α + 0.032 nM TAL-6. (C) Apoptosis rate analysis. All data are represented as means ± SD, *P < 0.05, **P < 0.01, ***P < 0.001 vs. TNF-α group, ###P < 0.001 vs. the control group.
.
TAL-6 Significantly Alleviates CLP-Induced Sepsis
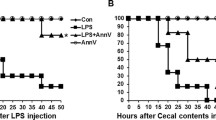
As demonstrated in Fig. 3A, the survival rate in the sham group was 100%, while the survival rate of the CLP group decreased to 10% within 72 h. Different doses of TAL-6 in the intervention groups improved the survival rate of rats within 72 h. The survival rate was 60% in the 5 mg·kg−1 group and 30% in the 1 mg·kg−1 group. The 10 mg·kg−1 group showed the most significant difference with a 70% survival rate. Moreover, compared with the single-targeted IL-6R or TNF-α fusion proteins constructed in-house, TAL-6 displayed the higher survival rate at the same dose.
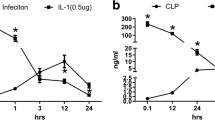
TAL-6 significantly alleviates CLP-induced sepsis. (A) Survival proportions of septic rats. (B) The expression levels of key inflammatory-related mediators in the serum of septic rats after treatment. Serum was collected after the sacrifice of rats and analyzed by commercial ELISA kits. The concentration is represented by bar graphs. (C) IL-6, (D) TNF-α, (E) IL-1β. All data are represented as means ± SD, *P < 0.05, **P < 0.01, ***P < 0.001 vs. CLP group, ###P < 0.001 vs. sham group.
As shown in Fig. 3B, compared with the sham group, the expression level of serum urea nitrogen (BUN) in the CLP group increased significantly (P < 0.001). The expression levels of BUN were significantly decreased in the single-targeted IL-6R or TNF-α fusion protein-treated group and the TAL-6 treated groups (P < 0.05, P < 0.01, and P < 0.001, respectively) compared with the CLP group. The same dose of TAL-6 downregulated BUN expression more prominently.
TAL-6 Inhibits Inflammatory Mediators in the Serum of Septic Rats
Cytokines, including IL-1β, IL-6, and TNF-α, have been identified as key mediators of sepsis. In our experiment, these biomarkers were selected to demonstrate the therapeutic effects of TAL-6 against sepsis (Fig. 3C–E). Satisfactorily, TAL-6 treatment significantly reduced these inflammatory mediators in a dose-dependent manner. The single-targeted IL-6R or TNF-α fusion protein also had downregulated these cytokines, although the effects were significantly weaker than those of TAL-6 at the same dose.
TAL-6 Alleviates Histopathological Lesions in the Kidneys of CLP Rats
Acute kidney injury (AKI) is a serious complication of sepsis which occurs frequently, particularly in the elderly. The kidneys of different groups of rats were collected for histopathological analysis. In the sham operation group, the structure of the renal tubules and glomeruli was normal, with no damage (Fig. 4A). The histopathological changes of the CLP group, including marked interstitial hemorrhage, renal proximal tubular congestion, edema, necrosis, and exfoliated cells were visible (Fig. 4B). In contrast, these lesions were obviously attenuated in a dose-dependent manner in the groups treated with TAL-6 (Fig. 4C–E), and minimal lesions were seen in CLP rats treated with 10 mg/kg of TAL-6 (Fig. 4E). The histologic features were less severe and extensive in the rats treated with single-targeted TNF-α (Fig. 4F) or IL-6R (Fig. 4G) fusion proteins, through the effects were significantly weaker than those of TAL-6 at the same dose. The bar graph shows that all the treatment groups had a therapeutic effect compared to CLP group.
TAL-6 alleviates histopathological lesions in septic rats. (A) Sham, (B) CLP, (C) CLP + TAL-6 1 mg·kg−1 (D) CLP + TAL-6 5 mg·kg−1, (E) CLP + TAL-6 10 mg·kg−1, (F) CLP + anti-TNF-α 5 mg·kg−1, (G) CLP + anti-IL-6R 5 mg·kg−1. TAL-6 downregulates the expression of inflammatory cytokines in the kidney of CLP group. (H) IL-6, TNF-α and IL-1β expression, **P < 0.01, ***P < 0.01 vs. CLP group, ###P < 0.001 vs. sham group. (I) TAL-6 decreases mRNA levels of inflammatory mediators in the kidney: relative mRNA of IL-6, relative mRNA of TNF-α and relative mRNA of IL-1β, *P < 0.05, **P < 0.01, ***P < 0.01 vs. CLP, ###P < 0.001 vs. sham group. Scale bars, 50 μm (a–g).
Pro-inflammatory cytokine levels were also detected in the kidney tissue. Compared with the sham operation group (P < 0.01), the levels of TNF-α, IL-1β, and IL-6 in the CLP group were significantly increased, while the fusion protein group was significantly decreased (Fig. 4H). TAL-6 (10 mg·kg−1 and 5 mg·kg−1) administration markedly decreased all three cytokines (P < 0.01, P < 0.01 vs. CLP). As shown in Fig. 4I, mRNA levels of IL-1β, TNF-α, and IL-6 in the CLP group were significantly elevated compared with the sham group. All fusion protein-treated groups exhibited decreased expression levels of these inflammatory mediators, and TAL-6 remarkably inhibited the expression of these genes in a dose-dependent manner.
DISCUSSION
Sepsis caused by the host response to infection is a life-threatening condition that leads to organ dysfunction and that can significantly extend the length of hospitalization of patients [18, 19]. However, the underlying mechanisms of sepsis are complicated and still unclear, and thus the clinical treatment of sepsis lacks effective tools. It is widely accepted that the extensive release of cytokines and other mediators like TNF-α and IL-6 are valuable biomarkers in septic condition, which can be employed not only to evaluate the prognosis of sepsis [6], but also as therapeutic targets for the development of medicine to treat sepsis.
Serum TNF-α concentration in human sepsis is elevated significantly at the beginning of the disease, though with a short half-life [20]. The TNF-α response is likely the result of predominantly local production and the presence of circulating inhibitors. When the level of TNF-α decreased in patients, other mediators like IL-6 change notably. IL-6 considered a pro- and an anti-inflammatory cytokine that is important for sepsis diagnosis and monitoring [9] — physiologically normal levels of IL-6 are in the range of 5–25 pg/mL, but these levels increase to 1000 pg/mL in patients with sepsis [21]. IL-6 enhances mitochondrial biogenesis and survival in astrocytes in septic conditions and engages in pro-survival activities through the IL-6/AMPK signaling pathway [22].
In this study, we first constructed a triple-specific fusion protein (TAL-6) by linking 3 single domain antibodies, anti-TNF-α/HSA/IL-6R, which simultaneously block the functions of TNF-α and IL-6 under the septic experimental conditions. The anti-serum albumin (HSA) moiety single-domain antibody in TAL-6 extends the half-life of the fusion protein in vivo to improve its pharmacokinetic behavior [16]. Based on this new prokaryotic expression system developed in our lab, the triple-specific fusion protein allows for efficient expression of a soluble and stable molecule in E. coli in large quantities with purity over 95% and without compromising its properties. The TAL-6 fusion protein produced with this method displayed high binding affinity with TNF-α (94.75 pM), HSA (1.83 nM), and IL-6R (2.29 nM), analyzed by ELISA binding assay. In vitro, TAL-6 protects L929 cells from apoptosis induced by TNF-α with high efficiency.
In our CLP septic model rats, the serum levels of sepsis biomarkers TNF-α, IL-1β, and IL-6 were significantly increased, indicating that sepsis had been initiated. After intervention treatment with TAL-6 alone, the survival rate of CLP rats was significantly increased compared with the 10% survival rate of CLP rats within 72 h. Compared with the single-targeted IL-6R or TNF-α fusion proteins, the same dose of TAL-6 increased the survival rate of CLP more significantly. Meanwhile, serum urea nitrogen (BUN), TNF-α, IL-1β, and IL-6 collected from different CLP groups showed that TAL-6 decreases the levels of cytokine mediators more than monospecific fusion proteins at the same concentration, supplying more evidence to support that TAL-6 has more potential as an effective treatment for sepsis.
Sepsis is a life-threatening condition caused by a dysregulated host response to infection where several organs, specifically the heart, lungs, and kidney, can be seriously damaged [2]. In our experiments, kidneys collected from the CLP group displayed histopathological changes including marked interstitial hemorrhage, renal proximal tubular congestion, edema, necrosis, and exfoliated cells. With TAL-6 treatment, the lesions were significantly attenuated based on histopathological analysis compared to the untreated CLP group. Levels of TNF-α, IL-1β, and IL-6 detected in the kidney tissue by ELISA assays and by RT-PCR were significantly decreased in the TAL-6-treated CLP groups.
In conclusion, we showed that TAL-6 presented potent therapeutic effects in an animal model of sepsis, indicating that simultaneously blocking the bioactivities of important inflammatory mediators, such as TNF-α and IL-6, is a potential additional treatment method that can be deployed along with antibiotic drugs during clinical treatment of sepsis. Continued studies in this direction with animal experiments and clinical trials have the potential to shed more light on possible avenues for the treatment for sepsis.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Skirecki, T., and J.M. Cavaillon. 2019. Inner sensors of endotoxin-implications for sepsis research and therapy. FEMS Microbiology Reviews 43: 239–256.
Finfer, S., and F.R. Machado. 2016. The global epidemiology of sepsis. does it matter that we know so little?. American journal of respiratory and critical care medicine 193: 228–230.
Singer, M., C.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane, M. Bauer, R. Bellomo, G.R. Bernard, J.D. Chiche, C.M. Coopersmith, R.S. Hotchkiss, M.M. Levy, J.C. Marshall, G.S. Martin, S.M. Opal, G.D. Rubenfeld, T. van der Poll, J.L. Vincent, and D.C. Angus. 2016. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810.
Hotchkiss, R.S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. The New England journal of medicine 348: 138–150.
Pantović-Stefanović, M., N. Petronijević, B. Dunjić-Kostić, M. Velimirović, T. Nikolić, V. Jurišić, M. Lačković, A. Damjanović, S. Totić-Poznanović, A.A. Jovanović, and M. Ivković. 2018. sVCAM-1, sICAM-1, TNF-α and IL-6 levels in bipolar disorder type I: Acute, longitudinal and therapeutic implications. The world journal of biological psychiatry: The official journal of the World Federation of Societies of Biological Psychiatry 19: S41–S51.
Song, R., J. Kim, D. Yu, C. Park, and J. Park. 2012. Kinetics of IL-6 and TNF-α changes in a canine model of sepsis induced by endotoxin. Veterinary immunology and immunopathology 146: 143–149.
Alicja, K.P., C.T. Aleksandra, S.K. Agnieszka, K.B. Maciej, and B. Alina. 2020. Biochemical parameters in cognitive functions. Neuropsychiatric Disease and Treatment. 16: 2479–2489.
Kang, S., T. Tanaka, and T. Kishimoto. 2015. Therapeutic uses of anti-interleukin-6 receptor antibody. International immunology 27: 21–29.
Rau, S., B. Kohn, C. Richter, N. Fenske, H. Küchenhoff, K. Hartmann, S. Härtle, B. Kaspers, and J. Hirschberger. 2007. Plasma interleukin-6 response is predictive for severity and mortality in canine systemic inflammatory response syndrome and sepsis. Veterinary clinical pathology 36: 253–260.
Watanabe, E., Hirasawa, H., Oda, S., Matsuda, K., Hatano, M., and T. Tokuhisa. 2005. Extremely high interleukin-6 blood levels and outcome in the critically ill are associated with tumor necrosis factor- and interleukin-1-related gene polymorphisms. Critical care medicine 33: 89–97; discussion 242–243.
Hamers-Casterman, C., T. Atarhouch, S. Muyldermans, G. Robinson, C. Hamers, E.B. Songa, N. Bendahman, and R. Hamers. 1993. Naturally occurring antibodies devoid of light antibodies. Nature 363: 446–448.
Arbabi-Ghahroudi, M. 2017. Camelid single-domain antibodies: Historical perspective and future outlook. Frontiers in immunology 8: 1589.
Muyldermans, S. 2013. Nanobodies: Natural single-domain antibodies. Annual review of biochemistry 82: 775–797.
Detalle, L., T. Stohr, C. Palomo, P.A. Piedra, B.E. Gilbert, V. Mas, A. Millar, U.F. Power, C. Stortelers, K. Allosery, J.A. Melero, and E. Depla. 2016. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrobial agents and chemotherapy 60: 6–13.
Crasson, O., N. Rhazi, O. Jacquin, A. Freichels, C. Jérôme, N. Ruth, M. Galleni, P. Filée, and M. Vandevenne. 2015. Enzymatic functionalization of a nanobody using protein insertion technology. Protein engineering, design & selection: PEDS 28: 451–460.
Kontermann, R.E. 2012. Dual targeting strategies with bispecific antibodies. mAbs 4: 182–197.
Jäger, V.D., R. Lamm, K. Küsters, G. Ölçücü, M. Oldiges, K.E. Jaeger, J. Büchs, and U. Krauss. 2020. Catalytically-active inclusion bodies for biotechnology-general concepts, optimization, and application. Applied microbiology and biotechnology 104: 7313–7329.
Fleischmann, C., A. Scherag, N.K. Adhikari, C.S. Hartog, T. Tsaganos, P. Schlattmann, D.C. Angus, and K. Reinhart. 2016. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. American journal of respiratory and critical care medicine 193: 259–272.
Hotchkiss, R.S., G. Monneret, and D. Payen. 2013. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. The Lancet. Infectious diseases 13: 260–268.
Pantovi-Stefanovi, M., N. Petronijevi, and B. Dunji-Kosti et al. 2016. sVCAM-1, sICAM-1, TNF-α, and IL-6 levels in bipolar disorder type I: acute, longitudinal, and therapeutic implications [J]. The World Journal of Biological Psychiatry 2016: 1–34.
Miguel-Bayarri, V., E.B. Casanoves-Laparra, L. Pallás-Beneyto, et al. 2012. Prognostic value of the biomarkers procalcitonin, interleukin-6 and C-reactive protein in severe sepsis [J]. Medicina Intensiva (English Edition) 36 (8): 556–562.
Chen, X.L., Y. Wang, W.W. Peng, et al. 2018. Effects of interleukin-6 and IL-6/AMPK signaling pathway on mitochondrial biogenesis and astrocytes viability under experimental septic condition[J]. International Immunopharmacology 59: 287–294.
Acknowledgements
The authors would like to thank Fujian Medical University for providing their equipment to conduct the experiment.
Funding
This work was supported by the National Natural Science Foundation, China (Grant No. 81402842), The Outstanding Talents Training Project of the Educational Office of Fujian Province, China (Grant No. 2017052), Fujian Provincial Health Technology Project, China (Grant Nos. 2018-ZQN-61, 2018-ZQN-64), Natural Science Foundation of Fujian Province, China (Grant Nos. 2019J01306, 2020J01634), The Emergency Project on New Coronavirus Pneumonia Prevention Research of Fujian Medical University (Grant No. 2020YJ004).
Author information
Authors and Affiliations
Contributions
Conceptualization: Juhua Yang and Xiaole Chen; methodology: Xiaole Chen and Nanwen Zhang; software: Rui Liu and Kaimei Nie; validation: Qingmei Zheng, Yaduan Wang, and Menru Yan; writing-original draft preparation: Xiaole Chen and Shuangyu Tang; writing-review and editing: He Wang and Nanwen Zhang; supervision: He Wang and Juhua Yang; project administration: Kaimei Nie; funding acquisition: He Wang, Xiaole Chen, and Nanwen Zhang.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All the procedures were performed in accordance with protocols approved by the Ethics Review Committee for Animal Experimentation of Fujian Medical University (No. 2018–104). All the animals were raised in the Laboratory Animal Center of Fujian Medical University (Certificate No. SCXK (Fujian) 2016–0006), where the animal work has taken place, and animal handling procedures were performed in strict accordance with the care of laboratory animals, according to the Fujian Province Zoological Society.
Consent for Publication
The manuscript is approved by all the authors for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Tan, S., Yan, M. et al. The Therapeutic Effect of an Anti-TNF-α/HSA/IL-6R Triple-Specific Fusion Protein Under Experimental Septic Conditions. Inflammation 45, 919–928 (2022). https://doi.org/10.1007/s10753-021-01595-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01595-9