Abstract
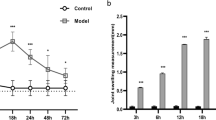
The aim of the present study was to observe the changes of TTX-R, Nav1.8, and Nav1.9 Na+ currents in MSU-induced gouty arthritis mice, and to explore the possibility of Nav1.8 and Nav1.9 channels as potential targets for gout pain treatment. Acute gouty arthritis was induced by monosodium urate (MSU) in mice. Swelling degree was evaluated by measuring the circumference of the ankle joint. Mechanical allodynia was assessed by applying the electronic von Frey. Na+ currents were recorded by patch-clamp techniques in acute isolated dorsal root ganglion (DRG) neurons. MSU treatment significantly increased the swelling degree of ankle joint and decreased the mechanical pain threshold. The amplitude of TTX-R Na+ current was significantly increased and reached its peak on the 4th day after injection of MSU. For TTX-R Na+ channel subunits, Nav1.8 current density was significantly increased, but Nav1.9 current density was markedly decreased after MSU treatment. MSU treatment shifted the steady-state activation curves of TTX-R Na+ channel, Nav1.8 and Nav1.9 channels, and the inactivation curves of TTX-R Na+ channel and Nav1.8 channels to the depolarizing direction, but did not affect the inactivation curve of Nav1.9 channel. Compared with the normal group, the recovery of Nav1.8 channel was faster, while that of Nav1.9 channel was slower. The recovery of TTX-R Na+ channel remained unchanged after MSU treatment. Additionally, MSU treatment increased DRG neurons excitability by reducing action potential threshold. Nav1.8 channel, not Nav1.9 channel, may be involved in MSU-induced gout pain by increasing nerve excitability.





Similar content being viewed by others
References
Zhu, Y., B.J. Pandya, and H.K. Choi. 2011. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis and Rheumatism 63 (10): 3136–3141.
Chia, E.W., R. Grainger, and J.L. Harper. 2008. Colchicine suppresses neutrophil superoxide production in a murine model of gouty arthritis: a rationale for use of low-dose colchicine. British Journal of Pharmacology 153 (6): 1288–1295.
Dalbeth, N., T.R. Merriman, and L.K. Stamp. 2016. Gout. Lancet 388 (10055): 2039–2052.
Lemos Lima Rde, C., F.C. Ferrari, M.R. de Souza, B.M. de Sa Pereira, C.A. de Paula, and D.A. Saude-Guimaraes. 2015. Effects of extracts of leaves from Sparattosperma leucanthum on hyperuricemia and gouty arthritis. Journal of Ethnopharmacology 161: 194–199.
Terkeltaub, R. 2010. Update on gout: new therapeutic strategies and options. Nature reviews. Rheumatology 6 (1): 30–38.
Chamaa, F., M. Chebaro, B. Safieh-Garabedian, R. Saadeh, S.J. Jabbur, and N.E. Saade. 2016. Transcriptional expression of inflammatory mediators in various somatosensory relay centers in the brain of rat models of peripheral mononeuropathy and local inflammation. Journal of Neuroimmunology 297: 81–91.
McCleskey, E.W., and M.S. Gold. 1999. Ion channels of nociception. Annual Review of Physiology 61: 835–856.
Waxman, S.G., T.R. Cummins, S. Dib-Hajj, J. Fjell, and J.A. Black. 1999. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle & Nerve 22 (9): 1177–1187.
Leo, S., R. D’Hooge, and T. Meert. 2010. Exploring the role of nociceptor-specific sodium channels in pain transmission using Nav1.8 and Nav1.9 knockout mice. Behavioural Brain Research 208 (1): 149–157.
Yu, Y.Q., Z.Y. Zhao, X.F. Chen, F. Xie, Y. Yang, and J. Chen. 2013. Activation of tetrodotoxin-resistant sodium channel Nav1.9 in rat primary sensory neurons contributes to melittin-induced pain behavior. Neuromolecular Medicine 15 (1): 209–217.
Goldin, A.L. 2001. Resurgence of sodium channel research. Annual Review of Physiology 63: 871–894.
John, V.H., M.J. Main, A.J. Powell, Z.M. Gladwell, C. Hick, H.S. Sidhu, J.J. Clare, S. Tate, and D.J. Trezise. 2004. Heterologous expression and functional analysis of rat Nav1.8 (SNS) voltage-gated sodium channels in the dorsal root ganglion neuroblastoma cell line ND7-23. Neuropharmacology 46 (3): 425–438.
Zhang, F., Y. Wang, Y. Liu, H. Han, D. Zhang, X. Fan, X. Du, N. Gamper, and H. Zhang. 2019. Transcriptional regulation of voltage-gated sodium channels contributes to GM-CSF-induced pain. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience 39 (26): 5222–5233.
Coste, B., M. Crest, and P. Delmas. 2007. Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. The Journal of General Physiology 129 (1): 57–77.
Cummins, T.R., S.D. Dib-Hajj, J.A. Black, A.N. Akopian, J.N. Wood, and S.G. Waxman. 1999. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience 19 (24): RC43.
Huang, J., C. Han, M. Estacion, D. Vasylyev, J.G. Hoeijmakers, M.M. Gerrits, L. Tyrrell, et al. 2014. Gain-of-function mutations in sodium channel Nav1.9 in painful neuropathy. Brain : A Journal of Neurology 137 (Pt 6): 1627–1642.
Huang, C. P., H. N. Chen, H. L. Su, C. L. Hsieh, W. H. Chen, Z. R. Lai, and Y. W. Lin. 2013. Electroacupuncture Reduces Carrageenan- and CFA-Induced Inflammatory Pain Accompanied by Changing the Expression of Nav1.7 and Nav1.8, rather than Nav1.9, in Mice Dorsal Root Ganglia. Evidence-based Complementary and Alternative Medicine : eCAM 2013:312184.
Gould, H.J., 3rd, J.D. England, R.D. Soignier, P. Nolan, L.D. Minor, Z.P. Liu, S.R. Levinson, and D. Paul. 2004. Ibuprofen blocks changes in Nav 1.7 and 1.8 sodium channels associated with complete Freund’s adjuvant-induced inflammation in rat. The Journal of Pain : Official Journal of the American Pain Society 5 (5): 270–280.
Strickland, I.T., J.C. Martindale, P.L. Woodhams, A.J. Reeve, I.P. Chessell, and D.S. McQueen. 2008. Changes in the expression of Nav1.7, Nav1.8 and Nav1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. European Journal of Pain 12 (5): 564–572.
Yu, Y. Q., F. Zhao, S. M. Guan, and J. Chen. 2011. Antisense-mediated knockdown of Nav1.8, but not Nav1.9, generates inhibitory effects on complete Freund’s adjuvant-induced inflammatory pain in rat. PloS One 6 (5):e19865.
Amir, R., C.E. Argoff, G.J. Bennett, T.R. Cummins, M.E. Durieux, P. Gerner, M.S. Gold, F. Porreca, and G.R. Strichartz. 2006. The role of sodium channels in chronic inflammatory and neuropathic pain. The Journal of Pain : Official Journal of the American Pain Society 7 (5 Suppl 3): S1–S29.
Hong, J., J. Qiu, X. Wang, and G. Zhang. 2020. Characteristics of voltage-gated potassium currents in monosodium urate induced gouty arthritis in mice. Inflammation Research 69 (6): 589–598.
Berta, T., O. Poirot, M. Pertin, R.R. Ji, S. Kellenberger, and I. Decosterd. 2008. Transcriptional and functional profiles of voltage-gated Na+ channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Molecular and Cellular Neuroscience 37 (2): 196–208.
Bonin, R.P., C. Bories, and Y. De Koninck. 2014. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Molecular Pain 10: 26.
Tena, B., B. Escobar, M.J. Arguis, C. Cantero, J. Rios, and C. Gomar. 2012. Reproducibility of Electronic von Frey and von Frey monofilaments testing. The Clinical Journal of Pain 28 (4): 318–323.
Villarreal, C.F., D. Sachs, F.Q. Cunha, C.A. Parada, and S.H. Ferreira. 2005. The role of Nav1.8 sodium channel in the maintenance of chronic inflammatory hypernociception. Neuroscience Letters 386 (2): 72–77.
Black, J.A., S. Liu, M. Tanaka, T.R. Cummins, and S.G. Waxman. 2004. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 108 (3): 237–247.
Tate, S., S. Benn, C. Hick, D. Trezise, V. John, R.J. Mannion, M. Costigan, C. Plumpton, D. Grose, Z. Gladwell, G. Kendall, K. Dale, C. Bountra, and C.J. Woolf. 1998. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nature Neuroscience 1 (8): 653–655.
Kim, H.I., T.H. Kim, and J.H. Song. 2005. Resveratrol inhibits Na+ currents in rat dorsal root ganglion neurons. Brain Research 1045 (1-2): 134–141.
Bennett, D.L., A.J. Clark, J. Huang, S.G. Waxman, and S.D. Dib-Hajj. 2019. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiological Reviews 99 (2): 1079–1151.
Dib-Hajj, S.D., J.A. Black, and S.G. Waxman. 2009. Voltage-gated sodium channels: therapeutic targets for pain. Pain Medicine 10 (7): 1260–1269.
Ekberg, J., and D.J. Adams. 2006. Neuronal voltage-gated sodium channel subtypes: key roles in inflammatory and neuropathic pain. The International Journal of Biochemistry & Cell Biology 38 (12): 2005–2010.
Jarvis, M.F., P. Honore, C.C. Shieh, M. Chapman, S. Joshi, X.F. Zhang, M. Kort, W. Carroll, B. Marron, R. Atkinson, J. Thomas, D. Liu, M. Krambis, Y. Liu, S. McGaraughty, K. Chu, R. Roeloffs, C. Zhong, J.P. Mikusa, G. Hernandez, D. Gauvin, C. Wade, C. Zhu, M. Pai, M. Scanio, L. Shi, I. Drizin, R. Gregg, M. Matulenko, A. Hakeem, M. Gross, M. Johnson, K. Marsh, P.K. Wagoner, J.P. Sullivan, C.R. Faltynek, and D.S. Krafte. 2007. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proceedings of the National Academy of Sciences of the United States of America 104 (20): 8520–8525.
Bulaj, G., M.M. Zhang, B.R. Green, B. Fiedler, R.T. Layer, S. Wei, J.S. Nielsen, S.J. Low, B.D. Klein, J.D. Wagstaff, L. Chicoine, T.P. Harty, H. Terlau, D. Yoshikami, and B.M. Olivera. 2006. Synthetic muO-conotoxin MrVIB blocks TTX-resistant sodium channel Nav1.8 and has a long-lasting analgesic activity. Biochemistry 45 (23): 7404–7414.
Ekberg, J., A. Jayamanne, C.W. Vaughan, S. Aslan, L. Thomas, J. Mould, R. Drinkwater, M.D. Baker, B. Abrahamsen, J.N. Wood, D.J. Adams, M.J. Christie, and R.J. Lewis. 2006. muO-conotoxin MrVIB selectively blocks Nav1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proceedings of the National Academy of Sciences of the United States of America 103 (45): 17030–17035.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
Open Fund of Key Laboratory of Medical Electrophysiology, Ministry of Education, Southwest Medical University (No. KeyME-2018-08).
Author information
Authors and Affiliations
Contributions
Jie Qiu performed research and analyzed the data. Xiuqi Xu and Shijia Zhang performed material preparation and data collection. Guang Li designed the study. Guangqin Zhang designed the study and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The experimental protocol was approved by the Animal Experimentation Ethics Committee of China Pharmaceutical University.
Consent for Publication
The manuscript is approved by all authors for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qiu, J., Xu, X., Zhang, S. et al. Modulations of Nav1.8 and Nav1.9 Channels in Monosodium Urate–Induced Gouty Arthritis in Mice. Inflammation 44, 1405–1415 (2021). https://doi.org/10.1007/s10753-021-01425-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01425-y