Abstract
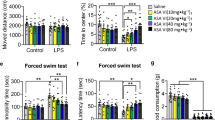
Neuroinflammation and oxidative stress play critical roles in pathogenesis of depression. Diallyl disulfide (DADS), an active compound in garlic oil, has been shown to exhibit obvious anti-inflammatory and anti-oxidative activities. Preliminary evidence indicates that depression is associated with high levels of pro-inflammatory cytokines and oxidative markers, suggesting that inhibition of neuroinflammatory response and oxidative stress may be beneficial for depression interruption. Here, we investigated the antidepressant effect of DADS as well as it mechanisms in a depression-like model induced by lipopolysaccharide (LPS). Similarly to imipramine (10 mg/kg), a clinical antidepressant, DADS (40 or 80 mg/kg), which was administered 1 h before LPS treatment (pre-LPS) or 1.5 h and 23.5 h after LPS treatment (post-LPS), prevented and reversed LPS (100 μg/kg)-induced increase in immobility time in the tail suspension test (TST) and forced swim test (FST) in mice. Mechanistic studies revealed that DADS pre-treatment or post-treatment at the dose of 40 and 80 mg/kg prevented and reversed (i) LPS-induced increases in interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and nitric oxide (NO) levels in the hippocampus and prefrontal cortex, (ii) LPS-induced increases in contents of malondialdehyde (MDA), a parameter reflecting high levels of oxidative stress, and (iii) LPS-induced decreases in contents of GSH, a marker reflecting weakened anti-oxidative ability, in the hippocampus and prefrontal cortex in mice. These results indicate that DADS is comparable to imipramine in effectively ameliorating LPS-induced depression-like behaviors in mice, providing a potential value for DADS in prevention and/or therapy of depression.







Similar content being viewed by others
References
Trivedi, M.H., and E.J. Daly. 2008. Treatment strategies to improve and sustain remission in major depressive disorder. Dialogues Clin Neurosci 10 (4): 377–384.
Terracciano, A., T. Tanaka, A.R. Sutin, S. Sanna, B. Deiana, S. Lai, M. Uda, D. Schlessinger, G.R. Abecasis, L. Ferrucci, and P.T. Costa Jr. 2010. Genome-wide association scan of trait depression. Biol Psychiatry 68 (9): 811–817.
Uher, R., O. Mors, M. Rietschel, A. Rajewska-Rager, A. Petrovic, A. Zobel, N. Henigsberg, J. Mendlewicz, K.J. Aitchison, A. Farmer, and P. McGuffin. 2011. Early and delayed onset of response to antidepressants in individual trajectories of change during treatment of major depression: a secondary analysis of data from the Genome-Based Therapeutic Drugs for Depression (GENDEP) study. J Clin Psychiatry 72 (11): 1478–1484.
Schwartz, J., J.W. Murrough, and D.V. Iosifescu. 2016. Ketamine for treatment-resistant depression: recent developments and clinical applications. Evid Based Ment Health 19 (2): 35–38.
Kapur, S., T. Mieczkowski, and J.J. Mann. 1992. Antidepressant medications and the relative risk of suicide attempt and suicide. JAMA 268 (24): 3441–3445.
Lee, M.M., A. Reif, and A.G. Schmitt. 2013. Major depression: a role for hippocampal neurogenesis? Curr Top Behav Neurosci 14: 153–179.
Klimes-Dougan, B., M. Westlund Schreiner, M. Thai, M. Gunlicks-Stoessel, K. Reigstad, and K.R. Cullen. 2018. Neural and neuroendocrine predictors of pharmacological treatment response in adolescents with depression: a preliminary study. Prog Neuropharmacol Biol Psychiatry 81: 194–202.
Levy, M.J.F., F. Boulle, H.W. Steinbusch, D.L.A. van den Hove, G. Kenis, and L. Lanfumey. 2018. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology (Berl) 235 (8): 2195–2220.
Raison, C.L., and A.H. Miller. 2011. Is depression an inflammatory disorder? Curr Psychiatry Rep 13 (6): 467–475.
Miller, A.H., V. Maletic, and C.L. Raison. 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65 (9): 732–741.
Lindqvist, D., F.S. Dhabhar, S.J. James, C.M. Hough, F.A. Jain, F.S. Bersani, V.I. Reus, J.E. Verhoeven, E.S. Epel, L. Mahan, R. Rosser, O.M. Wolkowitz, and S.H. Mellon. 2017. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 76: 197–205.
Huang, C., J. Wang, X. Lu, W. Hu, F. Wu, B. Jiang, Y. Ling, R. Yang, and W. Zhang. Z-guggulsterone negatively controls microglia-mediated neuroinflammation via blocking IkappaB-alpha-NF-kappaB signals. Neurosci Lett 619: 34–42.
Yirmiya, R. 1996. Endotoxin produces a depressive-like episode in rats. Brain Res 711 (1-2): 163–174.
Zou, W., R. Feng, and Y. Yang. 2018. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naive patients with major depression. PLoS One 13 (6): e0197267.
Bajpai, A., A.K. Verma, M. Srivastava, and R. Srivastava. 2014. Oxidative stress and major depression. J Clin Diagn Res 8 (12): CC04–CC07.
Yang, R., P. Wang, Z. Chen, W. Hu, Y. Gong, W. Zhang, and C. Huang. 2017. WY-14643, a selective agonist of peroxisome proliferator-activated receptor-alpha, ameliorates lipopolysaccharide-induced depressive-like behaviors by preventing neuroinflammation and oxido-nitrosative stress in mice. Pharmacol Biochem Behav 153: 97–104.
Sulakhiya, K., G.P. Keshavlal, B.B. Bezbaruah, S. Dwivedi, S.S. Gurjar, N. Munde, A. Jangra, M. Lahkar, and R. Gogoi. Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci Lett 611: 106–111.
Aalling, N., I. Hageman, K. Miskowiak, D. Orlowski, G. Wegener, and G. Wortwein. 2018. Erythropoietin prevents the effect of chronic restraint stress on the number of hippocampal CA3c dendritic terminals-relation to expression of gene involved in synaptic plasticity, angiogenesis, inflammation, and oxidative stress in male rats. J Neurosci Res 96 (1): 103–116.
Lu, X., R.R. Yang, J.L. Zhang, P. Wang, Y. Gong, W.F. Hu, Y. Wu, M.H. Gao, and C. Huang. 2018. Tauroursodeoxycholic acid produces antidepressant-like effects in a chronic unpredictable stress model of depression via attenuation of neuroinflammation, oxido-nitrosative stress, and endoplasmic reticulum stress. Fundam Clin Pharmacol 32 (4): 363–377.
Baune, B.T. 2017. Are non-steroidal anti-inflammatory drugs clinically suitable for the treatment of symptoms in depression-associated inflammation? Curr Top Behav Neurosci 31: 303–319.
Husain, M.I., I.B. Chaudhry, N. Husain, A.B. Khoso, R.R. Rahman, M.M. Hamirani, J. Hodsoll, I. Qurashi, J.F. Deakin, and A.H. Young. Minocycline as an adjunct for treatment-resistant depressive symptoms: a pilot randomised placebo-controlled trial. J Psychopharmacol 31 (9): 1166–1175.
Durairaj, H., M.D. Steury, and N. Parameswaran. 2015. Paroxetine differentially modulates LPS-induced TNFalpha and IL-6 production in mouse macrophages. Int Immunopharmacol 25 (2): 485–492.
Liu, F.Y., J. Cai, C. Wang, W. Ruan, G.P. Guan, H.Z. Pan, J.R. Li, C. Qian, J.S. Chen, L. Wang, and G. Chen. 2018. Fluoxetine attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage: a possible role for the regulation of TLR4/MyD88/NF-κB signaling pathway. J Neuroinflammation 15 (1): 347.
Chavant, F., J. Deguil, S. Pain, I. Ingrand, S. Milin, B. Fauconneau, M.C. Pérault-Pochat, and C. Lafay-Chebassier. 2010. Imipramine, in part through tumor necrosis factor alpha inhibition, prevents cognitive decline and beta-amyloid accumulation in a mouse model of Alzheimer’s disease. J Pharmacol Exp Ther 332 (2): 505–514.
Domingues, M., A.M. Casaril, P.T. Birmann, D.A. Lourenço, B. Vieira, K. Begnini, E.J. Lenardão, T. Collares, F.K. Seixas, and L. Savegnago. 2018. Selanylimidazopyridine prevents lipopolysaccharide-induced depressive-like behavior in mice by targeting neurotrophins and inflammatory/oxidative mediators. Front Neurosci 12: 486.
Reiter, J., N. Levina, M. van der Linden, M. Gruhlke, C. Martin, and A.J. Slusarenko. 2017. Diallylthiosulfinate (Allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules 22 (10).
Mathai, K., S. Anand, A. Aravind, P. Dinatius, A.V. Krishnan, and M. Mathai. Antimicrobial effect of ginger, garlic, honey, and lemon extracts on Streptococcus mutans. J Contemp Dent Pract 18 (11): 1004–1008.
Keusgen, M., R.M. Fritsch, H. Hisoriev, P.A. Kurbonova, and F.O. Khassanov. 2006. Wild Allium species (Alliaceae) used in folk medicine of Tajikistan and Uzbekistan. J Ethnobiol Ethnomed 2: 18.
Rojas, P., N. Serrano-García, O.N. Medina-Campos, J. Pedraza-Chaverri, P.D. Maldonado, and E. Ruiz-Sánchez. 2011. S-Allylcysteine, a garlic compound, protects against oxidative stress in 1-methyl-4-phenylpyridinium-induced parkinsonism in mice. J Nutr Biochem 22 (10): 937–944.
Chauhan, N.B., and J. Sandoval. 2007. Amelioration of early cognitive deficits by aged garlic extract in Alzheimer’s transgenic mice. Phytother Res 21 (7): 629–640.
Rahmani, G., F. Farajdokht, G. Mohaddes, S. Babri, V. Ebrahimi, and H. Ebrahimi. 2018. Garlic (Allium sativum) improves anxiety- and depressive-related behaviors and brain oxidative stress in diabetic rats. Arch Physiol Biochem 31: 1–6.
Song, H., Y. Lu, Z. Qu, V.V. Mossine, M.B. Martin, J. Hou, J. Cui, B.A. Peculis, T.P. Mawhinney, J. Cheng, C.M. Greenlief, K. Fritsche, F.J. Schmidt, R.B. Walter, D.B. Lubahn, G.Y. Sun, and Z. Gu. Effects of aged garlic extract and FruArg on gene expression and signaling pathways in lipopolysaccharide-activated microglial cells. Sci Rep 6: 35323.
Lee, S.H., Y.T. Liu, K.M. Chen, C.K. Lii, and C.T. Liu. 2012. Effect of garlic sulfur compounds on neutrophil infiltration and damage to the intestinal mucosa by endotoxin in rats. Food Chem Toxicol 50 (3-4): 567–574.
Feng, C., Y. Luo, Y. Nian, D. Liu, X. Yin, J. Wu, J. Di, R. Zhang, and J. Zhang. 2017. Diallyl disulfide suppresses the inflammation and apoptosis resistance induced by DCA through ROS and the NF-kappaB signaling pathway in human Barrett’s epithelial cells. Inflammation 40 (3): 818–831.
Shin, I.S., J. Hong, C.M. Jeon, N.R. Shin, O.K. Kwon, H.S. Kim, J.C. Kim, S.R. SR, and K.S. Ahn. 2013. Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression. Food Chem Toxicol 62: 506–513.
Yang, J., R. Tang, J. Yi, Y. Chen, X. Li, T. Yu, and J. Fei. 2019. Diallyl disulfide alleviates inflammatory osteolysis by suppressing osteoclastogenesis via NF-kappaB-NFATc1 signal pathway. FASEB J 33 (6): 7261–7273.
Paylor, R., C.M. Spencer, L.A. Yuva-Paylor, and S. Pieke-Dahl. 2006. The use of behavioral test batteries, II: effect of test interval. Physiol Behav 87 (1): 95–102.
Wang, P., Y. Zhang, Y. Gong, R. Yang, Z. Chen, W. Hu, Y. Wu, M. Gao, X. Xu, Y. Qin, and C. Huang. 2018. Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol Dis 111: 12–25.
Liu, C.T., P.L. Wong, C.K. Lii, H. Hse, and L.Y. Sheen. Antidiabetic effect of garlic oil but not diallyl disulfide in rats with streptozotocin-induced diabetes. Food Chem Toxicol 44 (8): 1377–1384.
Mello, B.S., A.S. Monte, R.S. McIntyre, J.K. Soczynska, C.S. Custódio, R.C. Cordeiro, J.H. Chaves, S.M. Vasconcelos, H.V. Nobre Jr., F.C. Florenço de Sousa, T.N. Hyphantis, A.F. Carvalho, and D.S. Macêdo. 2013. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res 47 (10): 1521–1529.
Steru, L., R. Chermat, B. Thierry, and P. Simon. 1985. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85 (3): 367–370.
You, Z., Q. Yao, J. Shen, Z. Gu, H. Xu, Z. Wu, C. Chen, and L. Li. 2017. Antidepressant-like effects of ginsenoside Rg3 in mice via activation of the hippocampal BDNF signaling cascade. J Nat Med 71 (2): 367–379.
Porsolt, R.D., A. Bertin, and M. Jalfre. 1977. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229 (2): 327–336.
Jangra, A., A.K. Datusalia, S. Khandwe, and S.S. Sharma. 2013. Amelioration of diabetes-induced neurobehavioral and neurochemical changes by melatonin and nicotinamide: implication of oxidative stress-PARP pathway. Pharmacol Biochem Behav 114-115: 43–51.
Beutler, E., O. Duron, and B.M. Kelly. 1963. Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882–888.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193 (1): 265–275.
Barbaccia, M.L. 2011. Much excitement about antidepressants, DBI and c-FOS. Pharmacol Res 64 (4): 333–335.
Arias, H.R., E. Vázquez-Gómez, A. Hernández-Abrego, S. Gallino, D. Feuerbach, M.O. Ortells, A.B. Elgoyhen, and J. García-Colunga. 2018. Tricyclic antidepressants inhibit hippocampal α7* and α9α10 nicotinic acetylcholine receptors by different mechanisms. Int J Biochem Cell Biol 100: 1–10.
Peng, C.H., S.H. Chiou, S.J. Chen, Y.C. Chou, H.H. Ku, C.K. Cheng, C.J. Yen, T.H. Tsai, Y.L. Chang, and C.L. Kao. 2008. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol 18 (2): 128–140.
Hutchinson, M.R., Y. Zhang, M. Shridhar, J.H. Evans, M.M. Buchanan, T.X. Zhao, P.F. Slivka, B.D. Coats, N. Rezvani, J. Wieseler, T.S. Hughes, K.E. Landgraf, S. Chan, S. Fong, S. Phipps, J.J. Falke, L.A. Leinwand, S.F. Maier, H. Yin, K.C. Rice, and L.R. Watkins. 2010. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun 24 (1): 83–95.
Li, J., A. Csakai, J. Jin, F. Zhang, and H. Yin. 2016. Therapeutic developments targeting toll-like receptor-4-mediated neuroinflammation. Chem Med Chem 11 (2): 154–165.
Bhattacharyya, D., G.M. Mohite, J. Krishnamoorthy, N. Gayen, S. Mehra, A. Navalkar, S.A. Kotler, B.N. Ratha, A. Ghosh, R. Kumar, K. Garai, A.K. Mandal, S.K. Maji, and A. Bhunia. 2019. Lipopolysaccharide from gut microbiota modulates α-synuclein aggregation and alters its biological function. ACS Chem Neurosci 10 (5): 2229–2236.
Sahu, P., J. Mudgal, D. Arora, M. Kinra, S.B. Mallik, C.M. Rao, K.S.R. Pai, and M. Nampoothiri. 2019. Cannabinoid receptor 2 activation mitigates lipopolysaccharide-induced neuroinflammation and sickness behavior in mice. Psychopharmacology (Berl) 236 (6): 1829–1838.
Draper, A., R.M. Koch, J.W. van der Meer, M. Aj Apps, P. Pickkers, M. Husain, and M.E. van der Schaaf. 2018. Effort but not reward sensitivity is altered by acute sickness induced by experimental endotoxemia in humans. Neuropsychopharmacology 43 (5): 1107–1118.
de La Serre, C.B., G. de Lartigue, and H.E. Raybould. 2015. Chronic exposure to low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol Behav 139: 188–194.
Cui, B., D. Su, W. Li, X. She, M. Zhang, R. Wang, and Q. Zhai. 2018. Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: implications for Alzheimer’s disease. J Neuroinflammation 15 (1): 190.
Anisman, H., J. Gibb, and S. Hayley. 2008. Influence of continuous infusion of interleukin-1beta on depression-related processes in mice: corticosterone, circulating cytokines, brain monoamines, and cytokine mRNA expression. Psychopharmacology (Berl) 199 (2): 231–244.
Brüning, C.A., F. Martini, S.M. Soares, L. Savegnago, T.B. Sampaio, and C.W. Nogueira. 2015. Depressive-like behavior induced by tumor necrosis factor-α is attenuated by m-trifluoromethyl-diphenyl diselenide in mice. J Psychiatr Res 66-67: 75–83.
Thomas, A.J., S. Davis, C. Morris, E. Jackson, R. Harrison, and J.T. O’Brien. 2005. Increase in interleukin-1beta in late-life depression. Am J Psychiatry 162 (1): 175–177.
Nguyen, K.T., T. Deak, S.M. Owens, T. Kohno, M. Fleshner, L.R. Watkins, and S.F. Maier. 1998. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci 18 (6): 2239–2246.
Koo, J.W., and R.S. Duman. 2008. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA 105 (2): 751–756.
Han, Y., L. Zhang, Q. Wang, D. Zhang, Q. Zhao, J. Zhang, L. Xie, G. Liu, and Z. You. 2019. Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology 107: 37–45.
Moylan, S., M. Berk, O.M. Dean, Y. Samuni, L.J. Williams, A. O’Neil, A.C. Hayley, J.A. Pasco, G. Anderson, F.N. Jacka, and M. Maes. 2014. Oxidative & nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev 45: 46–62.
Siwek, M., M. Sowa-Kućma, D. Dudek, K. Styczeń, B. Szewczyk, K. Kotarska, P. Misztakk, A. Pilc, M. Wolak, and G. Nowak. 2013. Oxidative stress markers in affective disorders. Pharmacol Rep 65 (6): 1558–1571.
Madrigal, J.L., O. Hurtado, M.A. Moro, I. Lizasoain, P. Lorenzo, A. Castrillo, L. Boscá, and J.C. Leza. 2002. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology 26 (2): 155–163.
Kasala, R.R., L.N. Bodduluru, Y. Maneti, and R. Thipparaboina. 2014. Effect of meditation on neurophysiological changes in stress mediated depression. Complement Ther Clin Pract 20 (1): 74–80.
Valles-Colomer, M., G. Falony, Y. Darzi, E.F. Tigchelaar, J. Wang, R.Y. Tito, C. Schiweck, A. Kurilshikov, M. Joossens, C. Wijmenga, S. Claes, L. Van Oudenhove, A. Zhernakova, S. Vieira-Silva, and J. Raes. 2019. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 4 (4): 623–632.
Qu, Y., C. Yang, Q. Ren, M. Ma, C. Dong, and K. Hashimoto. 2017. Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci Rep 7: 15725.
Zhang, J.C., W. Yao, C. Dong, C. Yang, Q. Ren, M. Ma, and K. Hashimoto. 2017. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl Psychiatry 7 (5): e1138.
Ramirez, K., D.T. Shea, D.B. McKim, B.F. Reader, and J.F. Sheridan. 2015. Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain Behav Immun 46: 212–220.
Ramirez, K., and J.F. Sheridan. 2016. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive-like behaviors. Brain Behav Immun 57: 293–303.
Réus, G.Z., R.B. Stringari, B. de Souza, F. Petronilho, F. Dal-Pizzol, J.E. Hallak, A.W. Zuardi, J.A. Crippa, and J. Quevedo. 2010. Harmine and imipramine promote antioxidant activities in prefrontal cortex and hippocampus. Oxid Med Cell Longev 3 (5): 325–331.
Acknowledgements
The authors would like to thank all the members for the helpful discussions on the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (81771467, 81571323, 81701286, 81974216, and 31771172), the Natural Science Foundation of Jiangsu Province (BK20180267 and BK20191207), and the Six Talent Peaks Project in Jiangsu Province (SWYY-071).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: J. Chen, X. Yuan, and Y. Ma. Performed the experiments: X. Wei, F. Li, H. He, H. Huang, C. Huang, Z. Chen, and D. Chen. Analyzed the data: X. Wei, F. Li, H. He, H. Huang, C. Huang, and Z. Chen. Wrote the paper: X. Wei, F. Li, X. Yuan, and Y. Ma.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All animal experiments in the present study were approved by the University Animal Ethics Committee of Nantong University (Permit Number: 2110836) and were carried out according to internationally accepted guidelines for the use of animals in toxicology as adopted by the Society of Toxicology in 1999.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, X., Ma, Y., Li, F. et al. Acute Diallyl Disulfide Administration Prevents and Reveres Lipopolysaccharide-Induced Depression-Like Behaviors in Mice via Regulating Neuroinflammation and Oxido-Nitrosative Stress. Inflammation 44, 1381–1395 (2021). https://doi.org/10.1007/s10753-021-01423-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01423-0