Abstract
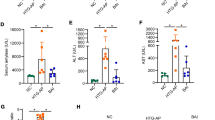
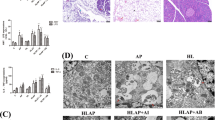
Hypertriglyceridemia (HTG) can aggravate acute pancreatitis (AP), but its pathogenesis remains unclear. As autophagic activity is closely related to lipid metabolism and AP, we investigated the autophagic response in models of AP aggravated by HTG and explored whether rapamycin has a protective effect against HTG-related pancreatitis. HTG-associated AP models were established in vivo in rats and in vitro. The degree of inflammation, pancreatic injury, the expression of endoplasmic reticulum (ER) stress, and autophagy markers (P62, LC3) were compared. Autophagic flux were assessed using immunostaining, electron microscopy, and immunoblotting. Compared with the normal diet group, the high-fat diet (HFD) AP group exhibited more severe pancreatic injury, apoptosis, and blocked autophagic flux. In addition, the three branches (PERK–eIF2α, ATF-6–GRP78, and IRE1–sXBP1) of the unfolded protein response and mTORC1/S6K1 pathway were activated in HFD AP models. Moreover, the same phenomena were confirmed in vitro in palmitic acid–stimulated pancreatic acinar cells. Preincubation with the mTOR inhibitor rapamycin restored the autophagic flux and markedly reduced the adverse effects of HTG. In conclusion, the autophagic flux is impaired in HFD-induced AP models and is strongly associated with ER stress. Rapamycin could prevent the aggravation of HTG-associated AP via inhibiting mTORC1/S6K1 pathway.







Similar content being viewed by others
Abbreviations
- AP:
-
acute pancreatitis
- ND:
-
normal diet
- HFD:
-
high-fat diet
- PA:
-
palmitic acid
- ERS:
-
endoplasmic reticulum stress
- HTG:
-
hypertriglyceridemia
- UPR:
-
unfolded protein response
- PACs:
-
pancreatic acinar cells
- TG:
-
triglycerides
- TC:
-
total cholesterol
- CQ:
-
chloroquine
References
Banks, P.A., T.L. Bollen, C. Dervenis, H.G. Gooszen, C.D. Johnson, M.G. Sarr, G.G. Tsiotos, and S.S. Vege. 2013. Classification of acute pancreatitis--2012: Revision of the Atlanta classification and definitions by international consensus. Gut 62 (1): 102–111. https://doi.org/10.1136/gutjnl-2012-302779.
Valdivielso, P., A. Ramirez-Bueno, and N. Ewald. 2014. Current knowledge of hypertriglyceridemic pancreatitis. European Journal of Internal Medicine 25 (8): 689–694. https://doi.org/10.1016/j.ejim.2014.08.008.
Bosques-Padilla, F.J., G. Vazquez-Elizondo, O. Gonzalez-Santiago, L. Del Follo-Martinez, O.P. Gonzalez, J.A. Gonzalez-Gonzalez, H.J. Maldonado-Garza, and E. Garza-Gonzalez. 2015. Hypertriglyceridemia-induced pancreatitis and risk of persistent systemic inflammatory response syndrome. The American Journal of the Medical Sciences 349 (3): 206–211. https://doi.org/10.1097/maj.0000000000000392.
Noel, P., K. Patel, C. Durgampudi, R.N. Trivedi, C. de Oliveira, M.D. Crowell, R. Pannala, K. Lee, R. Brand, J. Chennat, A. Slivka, G.I. Papachristou, A. Khalid, D.C. Whitcomb, J. DeLany, R.A. Cline, C. Acharya, D. Jaligama, F.M. Murad, D. Yadav, S. Navina, and V.P. Singh. 2016. Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut 65 (1): 100–111. https://doi.org/10.1136/gutjnl-2014-308043.
Gregor, M.F., and G.S. Hotamisligil. 2007. Thematic review series: Adipocyte biology. Adipocyte stress: The endoplasmic reticulum and metabolic disease. Journal of Lipid Research 48 (9): 1905–1914. https://doi.org/10.1194/jlr.R700007-JLR200.
Aoi, K., A. Nishio, T. Okazaki, M. Takeo, M. Masuda, T. Fukui, K. Uchida, and K. Okazaki. 2019. Inhibition of the dephosphorylation of eukaryotic initiation factor 2alpha ameliorates murine experimental pancreatitis. Pancreatology 19 (4): 548–556. https://doi.org/10.1016/j.pan.2019.04.005.
Wu, J., G. Hu, Y. Lu, J. Zheng, J. Chen, X. Wang, and Y. Zeng. 2016. Palmitic acid aggravates inflammation of pancreatic acinar cells by enhancing unfolded protein response induced CCAAT-enhancer-binding protein beta-CCAAT-enhancer-binding protein alpha activation. The International Journal of Biochemistry & Cell Biology 79: 181–193. https://doi.org/10.1016/j.biocel.2016.08.035.
Hotamisligil, G.S. 2010. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140 (6): 900–917. https://doi.org/10.1016/j.cell.2010.02.034.
Czaja, M.J. 2011. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology 140 (7): 1895–1908. https://doi.org/10.1053/j.gastro.2011.04.038.
Mizushima, N., and B. Levine. 2010. Autophagy in mammalian development and differentiation. Nature Cell Biology 12 (9): 823–830. https://doi.org/10.1038/ncb0910-823.
Takabatake, Y., T. Yamamoto, and Y. Isaka. 2017. Stagnation of autophagy: A novel mechanism of renal lipotoxicity. Autophagy 13 (4): 775–776. https://doi.org/10.1080/15548627.2017.1283084.
Xiao, Y., H. Liu, J. Yu, Z. Zhao, F. Xiao, T. Xia, C. Wang, K. Li, J. Deng, Y. Guo, S. Chen, Y. Chen, and F. Guo. 2016. MAPK1/3 regulate hepatic lipid metabolism via ATG7-dependent autophagy. Autophagy 12 (3): 592–593. https://doi.org/10.1080/15548627.2015.1135282.
Grootaert, M.O.J., L. Roth, D.M. Schrijvers, G.R.Y. De Meyer, and W. Martinet. 2018. Defective autophagy in atherosclerosis: To die or to senesce? Oxidative Medicine and Cellular Longevity 2018: 7687083. https://doi.org/10.1155/2018/7687083.
Koga, H., S. Kaushik, and A.M. Cuervo. 2010. Altered lipid content inhibits autophagic vesicular fusion. The FASEB Journal 24 (8): 3052–3065. https://doi.org/10.1096/fj.09-144519.
Komiya, K., T. Uchida, T. Ueno, M. Koike, H. Abe, T. Hirose, R. Kawamori, Y. Uchiyama, E. Kominami, Y. Fujitani, and H. Watada. 2010. Free fatty acids stimulate autophagy in pancreatic beta-cells via JNK pathway. Biochemical and Biophysical Research Communications 401 (4): 561–567. https://doi.org/10.1016/j.bbrc.2010.09.101.
Yang, L., P. Li, S. Fu, E.S. Calay, and G.S. Hotamisligil. 2010. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metabolism 11 (6): 467–478. https://doi.org/10.1016/j.cmet.2010.04.005.
Nguyen, T.B., and J.A. Olzmann. 2017. Lipid droplets and lipotoxicity during autophagy. Autophagy 13 (11): 2002–2003. https://doi.org/10.1080/15548627.2017.1359451.
Fortunato, F., H. Burgers, F. Bergmann, P. Rieger, M.W. Buchler, G. Kroemer, and J. Werner. 2009. Impaired autolysosome formation correlates with Lamp-2 depletion: Role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology 137 (1): 350–360. e351-355. https://doi.org/10.1053/j.gastro.2009.04.003.
Hashimoto, D., M. Ohmuraya, M. Hirota, A. Yamamoto, K. Suyama, S. Ida, Y. Okumura, E. Takahashi, H. Kido, K. Araki, H. Baba, N. Mizushima, and K. Yamamura. 2008. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. The Journal of Cell Biology 181 (7): 1065–1072. https://doi.org/10.1083/jcb.200712156.
Abdullah, A., and P. Ravanan. 2018. The unknown face of IRE1alpha - Beyond ER stress. European Journal of Cell Biology 97 (5): 359–368. https://doi.org/10.1016/j.ejcb.2018.05.002.
Miyagawa, K., S. Oe, Y. Honma, H. Izumi, R. Baba, and M. Harada. 2016. Lipid-induced endoplasmic reticulum stress impairs selective autophagy at the step of autophagosome-lysosome fusion in hepatocytes. The American Journal of Pathology 186 (7): 1861–1873. https://doi.org/10.1016/j.ajpath.2016.03.003.
Yamamoto, T., Y. Takabatake, A. Takahashi, T. Kimura, T. Namba, J. Matsuda, S. Minami, J.Y. Kaimori, I. Matsui, T. Matsusaka, F. Niimura, M. Yanagita, and Y. Isaka. 2017. High-fat diet-induced Lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J Am Soc Nephrol 28 (5): 1534–1551. https://doi.org/10.1681/asn.2016070731.
Bjorkoy, G., T. Lamark, A. Brech, H. Outzen, M. Perander, A. Overvatn, H. Stenmark, and T. Johansen. 2005. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. The Journal of Cell Biology 171 (4): 603–614. https://doi.org/10.1083/jcb.200507002.
Cheng, Y.C., J.M. Chang, C.A. Chen, and H.C. Chen. 2015. Autophagy modulates endoplasmic reticulum stress-induced cell death in podocytes: A protective role. Experimental Biology and Medicine (Maywood, N.J.) 240 (4): 467–476. https://doi.org/10.1177/1535370214553772.
Cybulsky, A.V. 2017. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nature Reviews. Nephrology 13 (11): 681–696. https://doi.org/10.1038/nrneph.2017.129.
Biczo, G., E.T. Vegh, N. Shalbueva, O.A. Mareninova, J. Elperin, E. Lotshaw, S. Gretler, A. Lugea, S.R. Malla, D. Dawson, P. Ruchala, J. Whitelegge, S.W. French, L. Wen, S.Z. Husain, F.S. Gorelick, P. Hegyi, Rakonczay Z Jr, I. Gukovsky, and A.S. Gukovskaya. 2018. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology 154 (3): 689–703. https://doi.org/10.1053/j.gastro.2017.10.012.
Alers, S., A.S. Loffler, S. Wesselborg, and B. Stork. 2012. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Molecular and Cellular Biology 32 (1): 2–11. https://doi.org/10.1128/mcb.06159-11.
Muoio, D.M., and C.B. Newgard. 2008. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nature Reviews. Molecular Cell Biology 9 (3): 193–205. https://doi.org/10.1038/nrm2327.
Gorman, A.M., S.J. Healy, R. Jager, and A. Samali. 2012. Stress management at the ER: Regulators of ER stress-induced apoptosis. Pharmacology & Therapeutics 134 (3): 306–316. https://doi.org/10.1016/j.pharmthera.2012.02.003.
Korolchuk, V.I., S. Saiki, M. Lichtenberg, F.H. Siddiqi, E.A. Roberts, S. Imarisio, L. Jahreiss, S. Sarkar, M. Futter, F.M. Menzies, C.J. O'Kane, V. Deretic, and D.C. Rubinsztein. 2011. Lysosomal positioning coordinates cellular nutrient responses. Nature Cell Biology 13 (4): 453–460. https://doi.org/10.1038/ncb2204.
Choi, S.E., S.M. Lee, Y.J. Lee, L.J. Li, S.J. Lee, J.H. Lee, Y. Kim, H.S. Jun, K.W. Lee, and Y. Kang. 2009. Protective role of autophagy in palmitate-induced INS-1 beta-cell death. Endocrinology 150 (1): 126–134. https://doi.org/10.1210/en.2008-0483.
Durgampudi, C., P. Noel, K. Patel, R. Cline, R.N. Trivedi, J.P. DeLany, D. Yadav, et al. 2014. Acute lipotoxicity regulates severity of biliary acute pancreatitis without affecting its initiation. The American Journal of Pathology 184 (6): 1773–1784. https://doi.org/10.1016/j.ajpath.2014.02.015.
Hu, G., J. Shen, L. Cheng, C. Guo, X. Xu, F. Wang, L. Huang, et al. 2011. Reg4 protects against acinar cell necrosis in experimental pancreatitis. Gut 60 (6): 820–828. https://doi.org/10.1136/gut.2010.215178.
Cousin, S.P., S.R. Hugl, C.E. Wrede, H. Kajio, M.G. Myers Jr., and C.J. Rhodes. 2001. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology 142 (1): 229–240. https://doi.org/10.1210/endo.142.1.7863.
Schmidt, J., D.W. Rattner, K. Lewandrowski, C.C. Compton, U. Mandavilli, W.T. Knoefel, and A.L. Warshaw. 1992. A better model of acute pancreatitis for evaluating therapy. Annals of Surgery 215 (1): 44–56.
Chao, K.C., K.F. Chao, C.C. Chuang, and S.H. Liu. 2006. Blockade of interleukin 6 accelerates acinar cell apoptosis and attenuates experimental acute pancreatitis in vivo. The British Journal of Surgery 93 (3): 332–338. https://doi.org/10.1002/bjs.5251.
Funding
This work was supported by grants from the Natural Science Foundation of China (No. 81970555),the Natural Science Foundation for Young Scholars of China (No. 8160030431), the Medical-engineering Cross Project of Shanghai JiaoTong University (YG2015MS29 and YG2014ZD10), and the Clinical Cultivation Research Project of Shanghai Shenkang Hospital Development Center (SHDC12017X09).
Author information
Authors and Affiliations
Contributions
Y.Z designed the research plan, and Q.X.M performed the research. C.L.H, J.Y.Z, X.Y.F, and Y.C.G assisted in the animal experiments. J.J.F and J.Y.Z interpreted the results. Q.X.M and Y.Y.L generated the draft of the manuscript. X.P.W and Y.Y.L edited and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Disclosure Statement
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qixiang Mei and Yue Zeng should be regarded as equivalent first authors
Electronic Supplementary Material
ESM 1.
Supplement 1 (a) Flow chart of pancreatitis modeling (b-c) Plasma levels of TG and TC in the normal diet and HFD groups. (d-f) Serum levels of inflammatory cytokines (IL-1β, TNFα and IL-6) in rats 3, 6, 9, and 12 h after the initial caerulein injection. The data are provided as the mean ± SEM (n = 6 per group).*p < 0.05 versus nor-AP group, #p < 0.05 versus normal-diet AP group. (PNG 2189 kb)
ESM 2.
Supplement 2 (a-b) Representative anti-LC3 immunostaining in pancreatic tissue obtained from rats 9 h after the AP induction. Scale bar = 50 μm. (c-d) Expression of P62, LC3 in the pancreatic tissues by Western Blot at 9 h after ANP induction with or without chloroquine(CQ) in both normal diet and HFD groups. The data are provided as the mean ± SEM (n = 6 per group).*p < 0.05 versus nor-AP or nor-CQ group, #p < 0.05 versus normal-diet AP group. (PNG 1961 kb)
Rights and permissions
About this article
Cite this article
Mei, Q., Zeng, Y., Huang, C. et al. Rapamycin Alleviates Hypertriglyceridemia-Related Acute Pancreatitis via Restoring Autophagy Flux and Inhibiting Endoplasmic Reticulum Stress. Inflammation 43, 1510–1523 (2020). https://doi.org/10.1007/s10753-020-01228-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01228-7