Abstract
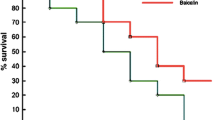
(-)-α-Bisabolol (BISA) is an unsaturated monocyclic sesquiterpenes compound, mainly found in the essential oil of chamomile (Matricaria chamomilla). It has been reported that this compound has several biological activities, but there are few studies evaluating the activity of this compound in the systemic inflammatory response in infectious processes. The aim of this study was to evaluate the effect of BISA on the inflammatory response and survival rate in a systemic infection model, and in vitro neutrophils phagocytic activity. BISA at concentration of 3, 10, 30, and 90 μg/ml did not presented in vitro cytotoxicity in MTT assay, and at concentrations of 1 and 3 μg/ml the BISA treatment increased in vitro phagocytic neutrophil activity. For the inflammatory response study, we verified the BISA treatment effect in a cecal ligation and puncture (CLP)-induced systemic infection model in mice; in this model, we demonstrate that BISA at dose of 100 mg/kg reduced the leukocyte recruitment in peritoneal cavity; at dose of 200 mg/kg, the NO concentration was increased in the peritoneal cavity. The bacteria CFU number was reduced in mice blood in the BISA treatment, at doses of 100 and 200 mg/kg. The BISA treatment at doses of 50 and 100 mg/kg increased the myeloperoxidase activity and reduction NO production in lung tissue of mice in CLP model. At dose of 100 mg/kg, the BISA treatment was able to reduce the mortality rate of mice submitted to CLP-induced sepsis and observed for 7 days. The results suggest an effect of BISA on inflammatory response, with activity on leukocyte chemotactic and NO production, in addition to increasing the survival rate of animals submitted to CLP model.





Similar content being viewed by others
References
Andrade, S.F., L.G.V. Cardoso, J.C.T. Carvalho, and J.K. Bastos. 2007. Anti-inflammatory and antinociceptive activities of extract, fractions and populnoic acid from bark wood of Austroplenckia populnea. Journal of Ethnopharmacology 109: 464–471. https://doi.org/10.1016/j.jep.2006.08.023.
Aderem, A., and R.J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406: 782–787. https://doi.org/10.1038/35021228.
Buckley, Christopher D., D.W. Gilroy, and C.N. Serhan. 2014. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40: 315–327. https://doi.org/10.1016/j.immuni.2014.02.009.
Franceschi, C., and J. Campisi. 2014. Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 69: S4–S9. https://doi.org/10.1093/gerona/glu057.
Araújo, C.V., V. Estato, E. Tibiriçá, P.T. Bozza, H.C. Castro-Faria-Neto, and A.R. Silva. 2012. PPAR gamma activation protects the brain against microvascular dysfunction in sepsis. Microvascular Research 84: 218–221. https://doi.org/10.1016/j.mvr.2012.05.006.
Akdis, M., A. Aab, C. Altunbulakli, K. Azkur, R.A. Costa, R. Crameri, S. Duan, T. Eiwegger, A. Eljaszewicz, R. Ferstl, R. Frei, M. Garbani, A. Globinska, L. Hess, C. Huitema, T. Kubo, Z. Komlosi, P. Konieczna, N. Kovacs, U.C. Kucuksezer, N. Meyer, H. Morita, J. Olzhausen, L. O'Mahony, M. Pezer, M. Prati, A. Rebane, C. Rhyner, A. Rinaldi, M. Sokolowska, B. Stanic, K. Sugita, A. Treis, W. van de Veen, K. Wanke, M. Wawrzyniak, P. Wawrzyniak, O.F. Wirz, J.S. Zakzuk, and C.A. Akdis. 2016. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. Journal of Allergy and Clinical Immunology 138: 984–1010. https://doi.org/10.1016/j.jaci.2016.06.033.
Rocha, N.F.M., E.T. Venâncio, B.A. Moura, M.I.G. Silva, M.R.A. Neto, E.R.V. Rios, D.P. Sousa, S.M.M. Vasconcelos, M.M.F. Fonteles, and F.C.F. Sousa. 2010. Gastroprotection of (-)-alpha-bisabolol on acute gastric mucosal lesions in mice: the possible involved pharmacological mechanisms. Fundamental & Clinical Pharmacology 24: 63–71. https://doi.org/10.1111/j.1472-8206.2009.00726.x.
McAndrew, B.A. 1992. Sesquiterpenoids: the lost dimension of perfumery. Perfumer Flavorist 17: 1–12.
Forrer, M., E.M. Kulik, A. Filippi, and T. Waltimo. 2013. The antimicrobial activity of alpha-bisabolol and tea tree oil against Solobacterium moorei, a Gram-positive bacterium associated with halitosis. Archives of Oral Biology 58: 10–16. https://doi.org/10.1016/j.archoralbio.2012.08.001.
Braga, P.C., M. Dal Sasso, E. Fonti, and M. Culici. 2009. Antioxidant activity of bisabolol: inhibitory effects on chemiluminescence of human neutrophil bursts and cell-free systems. Pharmacology 83: 110–115. https://doi.org/10.1159/000186049.
Gomes-Carneiro, M.R., D.M.M. Dias, A.C.A.X. De-Oliveira, and F.J.R. Paumgartten. 2005. Evaluation of mutagenic and antimutagenic activities of α-bisabolol in the Salmonella/microsome assay. Mutation Research, Genetic Toxicology and Environmental Mutagenesis 585: 105–112. https://doi.org/10.1016/j.mrgentox.2005.04.007.
Vila, R., A.I. Santana, R. Pérez-Rosés, A. Valderrama, M.V. Castelli, S. Mendonca, S. Zacchino, M.P. Gupta, and S. Cañigueral. 2010. Composition and biological activity of the essential oil from leaves of Plinia cerrocampanensis, a new source of alpha-bisabolol. Bioresource Technology 101: 2510–2514. https://doi.org/10.1016/j.biortech.2009.11.021.
Rocha, N.F.M., E.R.V. Rios, A.M.R. Carvalho, G.S. Cerqueira, A.A. Lopes, L.K.A.M. Leal, M.L. Dias, D.P. Sousa, and F.C.F. Sousa. 2011. Anti-nociceptive and anti-inflammatory activities of (−)-α-bisabolol in rodents. Naunyn-Schmiedeberg's Archives of Pharmacology 384: 525–533. https://doi.org/10.1007/s00210-011-0679-x.
Bezerra, S.B., L.K.A.M. Leal, N.A.P. Nogueira, N.A.N. Pinto, and A.R. Campos. 2009. Bisabolol-induced gastroprotection against acute gastric lesions: role of prostaglandins, nitric oxide, and KATP+ channels. Journal of Medicinal Food 12: 1403–1406. https://doi.org/10.1089/jmf.2008.0290.
Maurya, A.K., M. Singh, V. Dubey, S. Srivastava, S. Luqman, and D.U. Bawankule. 2014. α-(-)-bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Current Pharmaceutical Biotechnology 15: 173–181.
Sun, L., H. Zhang, L. Zhi, Q. Wen, Z. Qi, S. Yan, W. Li, and G. Zhang. 2017. Bisabolol attenuates sepsis-induced acute lung injury through inhibiting NF-κB-mediated inflammatory response. International Journal of Clinical and Experimental Pathology 10: 1052–1062.
Budavari, S. 1996. The Merck index : an encyclopedia of chemicals, drugs, and biologicals. 12th ed. Whitehouse Station.
Silva-Filho, S.E., L.A.M. Wiirzler, H.A.O. Cavalcante, N.S. Uchida, F.M.S. Silva-Comar, G.F.E. Cardia, E.L. da Silva, R.P. Aguiar, C.A. Bersani-Amado, and R.K.N. Cuman. 2016. Effect of patchouli (Pogostemon cablin) essential oil on in vitro and in vivo leukocytes behavior in acute inflammatory response. Biomedicine and Pharmacotherapy 84. https://doi.org/10.1016/j.biopha.2016.10.084.
Rittirsch, D., M.S. Huber-Lang, M.A. Flierl, and P.A. Ward. 2008. Immunodesign of experimental sepsis by cecal ligation and puncture. Nature Protocols 4: 31–36. https://doi.org/10.1038/nprot.2008.214.
Freitas, J.C.O.C., A.C. Medeiros, and V.S.F. Sales. 2004. Proteção pela glucana em modelo experimental de sepse. Acta Cirúrgica Brasileira 19: 296–307. https://doi.org/10.1590/S0102-86502004000300012.
Maciel, M.C.G., J.C. Farias, M.J. Maluf, E.A. Gomes, P.V.S. Pereira, W.C. Aragão-Filho, J.B. Frazão, C.G. Costa, S.M. Sousa, L.A. Silva, Flávia M.M. Amaral, M. Russo, R.N.M. Guerra, and F.R.F. Nascimento. 2008. Syzygium jambolanum treatment improves survival in lethal sepsis induced in mice. BMC Complementary and Alternative Medicine 8: 57–57. https://doi.org/10.1186/1472-6882-8-57.
Benjamim, C.F. 2001. Atualização sobre mediadores e modelos experimentais de sepse. Medicina 34: 18–26.
Newham, P., D. Ross, P. Ceuppens, S. Das, J.W.T. Yates, C. Betts, J. Reens, K.J. Randall, R. Knight, and J.S. McKay. 2014. Determination of the safety and efficacy of therapeutic neutralization of tumor necrosis factor-α (TNF-α) using AZD9773, an anti-TNF-α immune Fab, in murine CLP sepsis. Inflammation Research 63: 149–160. https://doi.org/10.1007/s00011-013-0683-3.
Kuo, M.C., C.Y. Chang, T.S. Cheng, and M.J. Wu. 2008. Immunomodulatory effect of Antrodia camphorata mycelia and culture filtrate. Journal of Ethnopharmacology 120: 196–203. https://doi.org/10.1016/j.jep.2008.08.011.
Amulic, B., C. Cazalet, G.L. Hayes, K.D. Metzler, and A. Zychlinsky. 2012. Neutrophil Function: From Mechanisms to Disease. Annual Review of Immunology 30: 459–489. https://doi.org/10.1146/annurev-immunol-020711-074942.
Ritter, C., M. Andrades, A. Reinke, J.C.F. Moreira, and F. Dal-Pizzol. 2004. Drug intervention trials in sepsis. The Lancet 364: 498. https://doi.org/10.1016/S0140-6736(04)16799-8.
Ziaja, M. 2012. Sepsis and septic encephalopathy: characteristics and experimental models. Folia Neuropathologica 50: 231–239.
Michels, M., A.S. Vieira, F. Vuolo, H.G. Zapelini, B. Mendonça, F. Mina, D. Dominguini, A. Steckert, P.F. Schuck, J. Quevedo, F. Petronilho, and F. Dal-Pizzol. 2015. The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain, Behavior, and Immunity 43: 54–59. https://doi.org/10.1016/j.bbi.2014.07.002.
Mina, F., C.M. Comim, D. Dominguini, O.J. Cassol-Jr, D.M. Dall’Igna, G.K. Ferreira, M.C. Silva, L.S. Galant, E.L. Streck, J. Quevedo, and F. Dal-Pizzol. 2014. Il1-β Involvement in Cognitive Impairment after Sepsis. Molecular Neurobiology 49: 1069–1076. https://doi.org/10.1007/s12035-013-8581-9.
Blackwell, T.S., and J.W. Christman. 1996. Sepsis and cytokines: current status. British Journal of Anaesthesia 77: 110–117.
Kim, S., E. Jung, J.H. Kim, Y.H. Park, J. Lee, and D. Park. 2011. Inhibitory effects of (−)-α-bisabolol on LPS-induced inflammatory response in RAW264.7 macrophages. Food and Chemical Toxicology 49: 2580–2585. https://doi.org/10.1016/j.fct.2011.06.076.
Korhonen, L., I. Hansson, J.P. Kukkonen, K. Brännvall, M. Kobayashi, K. Takamatsu, and D. Lindholm. 2005. Hippocalcin protects against caspase-12-induced and age-dependent neuronal degeneration. Molecular and Cellular Neurosciences 28: 85–95. https://doi.org/10.1016/j.mcn.2004.08.015.
Clancy, R.M., A.R. Amin, and S.B. Abramson. 1998. The role of nitric oxide in inflammation and immunity. Arthritis and Rheumatism 41: 1141–1151. https://doi.org/10.1002/1529-0131(199807)41:7<1141::AID-ART2>3.0.CO;2-S.
Atzler, D., E. Schwedhelm, and C.U. Choe. 2015. L-Homoarginine and cardiovascular disease. Current Opinion in Clinical Nutrition and Metabolic Care 18: 83–88. https://doi.org/10.1097/MCO.0000000000000123.
Kirkeboen, K.A., and O.A. Strand. 1999. The role of nitric oxide in sepsis—an overview. Acta Anaesthesiologica Scandinavica 43: 275–288. https://doi.org/10.1034/j.1399-6576.1999.430307.x.
Taysi, S., Z. Umudum, R.A. Sari, S. Kuskay, and N. Bakan. 2003. Nitric oxide level and superoxide dismutase activity in serum of patients with rheumatoid arthritis. The Pain Clinic 15: 429–434. https://doi.org/10.1163/156856903770196818.
Van Dervort, A.L., L. Yan, P.J. Madara, J.P. Cobb, R.A. Wesley, C.C. Corriveau, M.M. Tropea, and R.L. Danner. 1994. Nitric oxide regulates endotoxin-induced TNF-alpha production by human neutrophils. Journal of Immunology 152: 4102–4109.
Fukatsu, K., H. Saito, I. Han, S. Furukawa, M.T. Lin, T. Matsuda, S. Ikeda, T. Inoue, H. Yasuhara, and T. Muto. 1998. Nitric oxide donor decreases neutrophil adhesion in both lung and peritoneum during peritonitis. The Journal of Surgical Research 74: 119–124. https://doi.org/10.1006/jsre.1997.5234.
Xie, C.Y., W. Yang, J. Ying, Q.C. Ni, X.D. Pan, J.H. Dong, K. Li, and X.S. Wang. 2011. B-cell lymphoma-2 over-expression protects δ-elemene-induced apoptosis in human lung carcinoma mucoepidermoid cells via a nuclear factor kappa B-related pathway. Biological and Pharmaceutical Bulletin 34: 1279–1286.
Manuele, M.G., M.L.B. Arcos, R. Davicino, G. Ferraro, G. Cremaschi, and C. Anesini. 2009. Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: relationship with oxidative stress. Cancer Investigation 28: 135–145. https://doi.org/10.3109/07357900903179583.
Qi, J., N. Li, J.H. Zhou, B.Y. Yu, and S.X. Qiu. 2010. Isolation and anti-inflammatory activity evaluation of triterpenoids and a monoterpenoid glycoside from Harpagophytum procumbens. Planta Medica 76: 1892–1896. https://doi.org/10.1055/s-0030-1250029.
Thimmulappa, R.K., R.J. Fuchs, D. Malhotra, C. Scollick, K. Traore, J.H. Bream, M.A. Trush, K.T. Liby, M.B. Sporn, T.W. Kensler, and S. Biswal. 2007. Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxidants & Redox Signaling 9: 1963–1970. https://doi.org/10.1089/ars.2007.1745.
Dulhunty, J.M., J.A. Roberts, J.S. Davis, S.A.R. Webb, R. Bellomo, C. Gomersall, C. Shirwadkar, G.M. Eastwood, J. Myburgh, D.L. Paterson, and J. Lipman. 2013. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clinical Infectious Diseases 56: 236–244. https://doi.org/10.1093/cid/cis856.
Pedro, T.C.S., A.M. Morcillo, and E.C.E. Baracat. 2015. Etiology and prognostic factors of sepsis among children and adolescents admitted to the intensive care unit. Revista Brasileira de Terapia Intensiva: 27. https://doi.org/10.5935/0103-507X.20150044.
Bradley, P.P., D.A. Priebat, R.D. Christensen, and G. Rothstein. 1982. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. The Journal of Investigative Dermatology 78: 206–209.
Huang, J., A. Milton, R.D. Arnold, H. Huang, F. Smith, J.R. Panizzi, and P. Panizzi. 2016. Methods for measuring myeloperoxidase activity toward assessing inhibitor efficacy in living systems. Journal of Leukocyte Biology 99: 541–548. https://doi.org/10.1189/jlb.3RU0615-256R.
Mathias, J.R., B.J. Perrin, T.X. Liu, J. Kanki, A.T. Look, and A. Huttenlocher. 2006. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. Journal of Leukocyte Biology 80: 1281–1288. https://doi.org/10.1189/jlb.0506346.
Osborn, L. 1990. Leukocyte adhesion to endothelium in inflammation. Cell 62: 3–6. https://doi.org/10.1016/0092-8674(90)90230-C.
Hogg, J.C., and C.M. Doerschuk. 1995. Leukocyte Traffic in the Lung. Annual Review of Physiology 57: 97–114. https://doi.org/10.1146/annurev.ph.57.030195.000525.
Lien, D.C., W.W. Wagner, R.L. Capen, C. Haslett, W.L. Hanson, S.E. Hofmeister, P.M. Henson, and G.S. Worthen. 1987. Physiological neutrophil sequestration in the lung: visual evidence for localization in capillaries. Journal of applied physiology (Bethesda, Md. : 1985) 62: 1236–1243.
Deshmane, S.L., S. Kremlev, S. Amini, and B.E. Sawaya. 2009. Monocyte chemoattractant protein-1 (MCP-1): an overview. Journal of Interferon & Cytokine Research 29: 313–326. https://doi.org/10.1089/jir.2008.0027.
Speyer, C.L., H. Gao, N.J. Rancilio, T.A. Neff, G.B. Huffnagle, J.V. Sarma, and P.A. Ward. 2004. Novel chemokine responsiveness and mobilization of neutrophils during sepsis. The American Journal of Pathology 165: 2187–2196. https://doi.org/10.1016/S0002-9440(10)63268-3.
Acknowledgments
The authors thank Jailson Araujo Dantas and Celia Regina Miranda for technical assistance. This study was supported by grants from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cavalcante, H.A.O., Silva-Filho, S.E., Wiirzler, L.A.M. et al. Effect of (-)-α-Bisabolol on the Inflammatory Response in Systemic Infection Experimental Model in C57BL/6 Mice. Inflammation 43, 193–203 (2020). https://doi.org/10.1007/s10753-019-01109-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01109-8