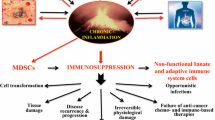
Abstract
Recent data have demonstrated that chronic inflammation is a crucial component of tumor initiation and progression. We previously reported that immature myeloid-derived suppressor cells (MDSCs) with immunosuppressive activity toward effector T cells were expanded in experimental chronic inflammation. We hypothesized that elevated levels of MDSCs, induced by chronic inflammation, may contribute to the progression of tumor growth. Using the Ehrlich carcinoma animal model, we found increased tumor growth in mice with chronic adjuvant arthritis, which was accompanied by a persistent increase in the proportion of splenic monocytic and granulocytic MDSCs expressing CD62L (l-selectin), when compared to tumor mice without adjuvant arthritis. Depletion of inflammation-induced MDSCs resulted in decreased tumor growth. In vitro studies demonstrated that increased expression of CD62L by MDSCs was mediated by TNFα, elevated concentrations of which were found in tumor mice subjected to chronic inflammation. Moreover, the addition of exogenous TNFα markedly enhanced the suppressive activity of bone marrow-derived MDSCs, as revealed by the ability to impair the proliferation of CD8+ T cells in vitro. This study provides evidence that chronic inflammation may promote tumor growth via induction of CD62L expression by MDSCs that can facilitate their migration to tumor and lymph nodes and modulation of their suppressor activity.







Similar content being viewed by others
Abbreviations
- AA:
-
Arthritic animals
- Ab:
-
Antibody
- APC:
-
Allophycocyanin
- Arg-1:
-
Arginase-1
- ATA:
-
Arthritic animals with tumor
- BM:
-
Bone marrow
- CA:
-
Control animals
- CFA:
-
Complete Freund’s adjuvant
- CFSE:
-
5(6)-Carboxyfluorescein diacetate N-succinimidyl ester
- ConA:
-
Concanavalin A
- CXCR4:
-
C-X-C chemokine receptor type 4
- DCFDA:
-
2′-7′-dichlorofluorescein diacetate
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- GHVD:
-
Graft-versus-host disease
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- HIV:
-
Human immunodeficiency virus
- IFNγ:
-
Interferon gamma
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- LBS:
-
Lysing buffer solution
- MCP-1:
-
Monocyte chemoattractant protein-1
- MDSC:
-
Myeloid-derived suppressor cells
- MIP-1α:
-
Macrophage inflammatory protein 1 alpha
- NF-κB:
-
Nuclear factor kappa B
- NO:
-
Nitric oxide
- PE:
-
Phycoerythrin
- PerCP:
-
Peridinin chlorophyll protein complex
- PGE2:
-
Prostaglandin E2
- RAGE:
-
Receptor for advanced glycation end products
- ROS:
-
Reactive oxygen species
- SDF-1α:
-
Stromal cell-derived factor one alpha
- TA:
-
Tumor animals
- TGFβ:
-
Transforming growth factor beta
- TLR:
-
Toll-like receptors
- TNFα:
-
Tumor necrosis factor alpha
- VEGF:
-
Vascular endothelial growth factor
References
Multhoff, G., M. Molls, and J. Radons. 2012. Chronic inflammation in cancer development. Frontiers in Immunology 2: 98. https://doi.org/10.3389/fimmu.2011.00098.
Wang, Dingzhi, and Raymond N. DuBois. 2015. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 36: 1085–1093. https://doi.org/10.1093/carcin/bgv123.
Bronte, Vincenzo, Sven Brandau, Shu Hsia Chen, Mario P. Colombo, Alan B. Frey, Tim F. Greten, Susanna Mandruzzato, Peter J. Murray, Augusto Ochoa, Suzanne Ostrand-Rosenberg, Paulo C. Rodriguez, Antonio Sica, Viktor Umansky, Robert H. Vonderheide, and Dmitry I. Gabrilovich. 2016. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nature Communications 7: 12150. https://doi.org/10.1038/ncomms12150.
Xia, Sheng, Haibo Sha, Liu Yang, Yewei Ji, Suzanne Ostrand-Rosenberg, and Ling Qi. 2011. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. Journal of Biological Chemistry 286: 23591–23599. https://doi.org/10.1074/jbc.M111.237123.
Höchst, Bastian, Julita Mikulec, Tania Baccega, Christina Metzger, Meike Welz, Julia Peusquens, Frank Tacke, Percy Knolle, Christian Kurts, Linda Diehl, and Isis Ludwig-Portugall. 2015. Differential induction of Ly6G and Ly6C positive myeloid derived suppressor cells in chronic kidney and liver inflammation and fibrosis. PLoS One 10: e0119662. https://doi.org/10.1371/journal.pone.0119662.
Singh, Udai P., Narendra P. Singh, Balwan Singh, Lorne J. Hofseth, Dennis D. Taub, Robert L. Price, Mitzi Nagarkatti, and Prakash S. Nagarkatti. 2012. Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(-/-) mice. Brain, Behavior, and Immunity 26: 72–82. https://doi.org/10.1016/j.bbi.2011.07.236.
Obregón-Henao, Andrés, Marcela Henao-Tamayo, Ian M. Orme, and Diane J. Ordway. 2013. Gr1intCD11b+ myeloid-derived suppressor cells in mycobacterium tuberculosis infection. PLoS One 8: e80669. https://doi.org/10.1371/journal.pone.0080669.
Vollbrecht, Thomas, Renate Stirner, Amanda Tufman, Julia Roider, Rudolf M. Huber, Johannes R. Bogner, Andreas Lechner, Carole Bourquin, and Rika Draenert. 2012. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS 26. https://doi.org/10.1097/QAD.0b013e328354b43f.
Dorhoi, Anca, and Nelita Du Plessis. 2017. Monocytic myeloid-derived suppressor cells in chronic infections. Front Immunol 8:1895. doi:https://doi.org/10.3389/fimmu.2017.01895.
Verschoor, C.P., J. Johnstone, J. Millar, M.G. Dorrington, M. Habibagahi, A. Lelic, M. Loeb, J.L. Bramson, and D.M.E. Bowdish. 2013. Blood CD33(+)HLA-DR(-) myeloid-derived suppressor cells are increased with age and a history of cancer. Journal of Leukocyte Biology 93: 633–637. https://doi.org/10.1189/jlb.0912461.
Flores, Rafael R., Cheryl L. Clauson, Joonseok Cho, Byeong Chel Lee, Sara J. McGowan, Darren J. Baker, Laura J. Niedernhofer, and Paul D. Robbins. 2017. Expansion of myeloid-derived suppressor cells with aging in the bone marrow of mice through a NF-κB-dependent mechanism. Aging Cell 16: 480–487. https://doi.org/10.1111/acel.12571.
Perfilyeva, Yuliya V., Nurshat Abdolla, Yekaterina O. Ostapchuk, Raikhan Tleulieva, Vladimir C. Krasnoshtanov, and Nikolai N. Belyaev. 2017. Expansion of CD11b(+)Ly6G(high) and CD11b(+)CD49d(+) myeloid cells with suppressive potential in mice with chronic inflammation and light-at-night-induced circadian disruption. Inflammation Research 66 (8): 711–724. https://doi.org/10.1007/s00011-017-1052-4.
Zhang, Hui, Shuang Wang, Yuefang Huang, Hongyue Wang, Jijun Zhao, Felicia Gaskin, Niansheng Yang, and Shu Man Fu. 2015. Myeloid-derived suppressor cells are proinflammatory and regulate collagen-induced arthritis through manipulating Th17 cell differentiation. Clinical Immunology 157: 175–186. https://doi.org/10.1016/j.clim.2015.02.001.
Guo, Chunqing, Hu Fanlei, Huanfa Yi, Zhitao Feng, Changzheng Li, Lianjie Shi, Yingni Li, et al. 2014. Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. Annals of the Rheumatic Diseases 75 (1): 278–285. https://doi.org/10.1136/annrheumdis-2014-205508.
Zhan, Xiaoxia, Yimin Fang, Shengfeng Hu, Yongjian Wu, Kun Yang, Chunxin Liao, Yuanqing Zhang, Xi Huang, and Minhao Wu. 2015. IFN-γ differentially regulates subsets of Gr-1+CD11b+ myeloid cells in chronic inflammation. Molecular Immunology 66: 451–462. https://doi.org/10.1016/j.molimm.2015.05.011.
Poh, Tze Wei, Cathy S. Madsen, Jessica E. Gorman, Ronald J. Marler, Jonathan A. Leighton, Peter A. Cohen, and Sandra J. Gendler. 2013. Downregulation of hematopoietic MUC1 during experimental colitis increases tumor-promoting myeloid-derived suppressor cells. Clinical Cancer Research 19: 5039–5052. https://doi.org/10.1158/1078-0432.CCR-13-0278.
Katoh, Hiroshi, Dingzhi Wang, Takiko Daikoku, Haiyan Sun, Sudhansu K. Dey, and Raymond N. DuBois. 2013. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 24: 631–644. https://doi.org/10.1016/j.ccr.2013.10.009.
Wang, Zibing, Jing Jiang, Zhiguang Li, Jinhua Zhang, Hui Wang, and Zhihai Qin. 2010. A myeloid cell population induced by Freund adjuvant suppresses T-cell-mediated antitumor immunity. Journal of Immunotherapy 33: 167–177. https://doi.org/10.1097/CJI.0b013e3181bed2ba.
Ostrand-Rosenberg, Suzanne, and Pratima Sinha. 2009. Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of Immunology 182: 4499–4506. https://doi.org/10.4049/jimmunol.0802740.
Zhao, Xueqiang, Lijie Rong, Xiaopu Zhao, Xiao Li, Xiaoman Liu, Jingjing Deng, Wu Hao, et al. 2012. TNF signaling drives myeloid-derived suppressor cell accumulation. Journal of Clinical Investigation 122: 4094–4104. https://doi.org/10.1172/JCI64115.
Sade-Feldman, Moshe, Julia Kanterman, Eliran Ish-Shalom, Mazal Elnekave, Elad Horwitz, and Michal Baniyash. 2013. Tumor necrosis factor-alpha blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity 38: 541–554. https://doi.org/10.1016/j.immuni.2013.02.007.
Marigo, Ilaria, Erika Bosio, Samantha Solito, Circe Mesa, Audry Fernandez, Luigi Dolcetti, Stefano Ugel, Nada Sonda, Silvio Bicciato, Erika Falisi, Fiorella Calabrese, Giuseppe Basso, Paola Zanovello, Emanuele Cozzi, Susanna Mandruzzato, and Vincenzo Bronte. 2010. Tumor-induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity 32: 790–802. https://doi.org/10.1016/j.immuni.2010.05.010.
Le, Hanh K., Laura Graham, Esther Cha, Johanna K. Morales, Masoud H. Manjili, and Harry D. Bear. 2009. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Intl Immunopharmacol 9 (7–8): 900–909. https://doi.org/10.1016/j.intimp.2009.03.015.
Kumar, Vinit, Sima Patel, Evgenii Tcyganov, and Dmitry I. Gabrilovich. 2016. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends in Immunology 37 (3): 208–220. https://doi.org/10.1016/j.it.2016.01.004.
Xiang, Xiaoyu, Yuelong Liu, Xiaoyin Zhuang, Shuangqin Zhang, Sue Michalek, Douglas D. Taylor, William Grizzle, and Huang Ge Zhang. 2010. TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. American Journal of Pathology 177: 1606–1610. https://doi.org/10.2353/ajpath.2010.100245.
Ray, Anuradha, Krishnendu Chakraborty, and Prabir Ray. 2013. Immunosuppressive MDSCs induced by TLR signaling during infection and role in resolution of inflammation. Frontiers in Cellular and Infection Microbiology 3. https://doi.org/10.3389/fcimb.2013.00052.
Yu, Xiaowen, Jie Zeng, and Jianping Xie. 2014. Navigating through the maze of TLR2 mediated signaling network for better mycobacterium infection control. Biochimie 102: 1–8. https://doi.org/10.1016/j.biochi.2014.02.012.
Gabay, Cem. 2006. Interleukin-6 and chronic inflammation. Arthritis Research and Therapy 8: S3. https://doi.org/10.1186/ar1917.
Lukens, John R., Jordan M. Gross, and Thirumala Devi Kanneganti. 2012. IL-1 family cytokines trigger sterile inflammatory disease. Frontiers in Immunology 3: 315. https://doi.org/10.3389/fimmu.2012.00315.
Popa, C., M. G. Netea, P. L. C. M. van Riel, J. W. M. van der Meer, and A. F. H. Stalenhoef. 2007. The role of TNF-in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. The Journal of Lipid Research 48: 751–762. doi:https://doi.org/10.1194/jlr.R600021-JLR200.
Kim, Ryungsa, Manabu Emi, Kazuaki Tanabe, and Koji Arihiro. 2006. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Research 66 (11): 5527–5536. https://doi.org/10.1158/0008-5472.CAN-05-4128.
Gargett, Tessa, Susan N. Christo, Timothy R. Hercus, Nazim Abbas, Nimit Singhal, Angel F. Lopez, and Michael P. Brown. 2016. GM-CSF signalling blockade and chemotherapeutic agents act in concert to inhibit the function of myeloid-derived suppressor cells in vitro. Clinical & Translational Immunology 5: e119. https://doi.org/10.1038/cti.2016.80.
Baniyash, Michal. 2006. Chronic inflammation, immunosuppression and cancer: New insights and outlook. Seminars in Cancer Biology 16 (1): 80–88. https://doi.org/10.1016/j.semcancer.2005.12.002.
Springer, Timothy A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 76 (2): 301–314. https://doi.org/10.1016/0092-8674(94)90337-9.
Garrood, T., L. Lee, and Costantino Pitzalis. 2006. Molecular mechanisms of cell recruitment to inflammatory sites: General and tissue-specific pathways. Rheumatology 45 (3): 250–260. https://doi.org/10.1093/rheumatology/kei207.
Highfill, Steven L., Paulo C. Rodriguez, Qing Zhou, Christine A. Goetz, Brent H. Koehn, Rachelle Veenstra, Patricia A. Taylor, A. Panoskaltsis-Mortari, J.S. Serody, D.H. Munn, J. Tolar, A.C. Ochoa, and B.R. Blazar. 2010. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 116: 5738–5747. https://doi.org/10.1182/blood-2010-06-287839.
Liechtenstein, Therese, Noemi Perez-Janices, Maria Gato, Fabio Caliendo, Grazyna Kochan, Idoia Blanco-Luquin, Frederick Arce, et al. 2014. A highly efficient tumor-infiltrating MDSC differentiation system for discovery of anti-neoplastic targets, which circumvents the need for tumor establishment in mice. Oncotarget 5: 7843–7857. https://doi.org/10.18632/oncotarget.2279.
Liu, Xing, Ming Yang, Ligang Chen, Lilei Peng, Qiang Ye, Shuzhan Zheng, and Zhongcai Fan. 2012. TNF-α and G-CSF induce CD62L and CD106 expressions on rat bone marrow-derived MSCs. Asian Biomedicine 6 (3): 453–458. https://doi.org/10.5372/1905-7415.0603.076.
Yang, Li, Jianhua Huang, Xiubao Ren, Agnieszka E. Gorska, Anna Chytil, Mary Aakre, David P. Carbone, Lynn M. Matrisian, Ann Richmond, P. Charles Lin, and Harold L. Moses. 2008. Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13: 23–35. https://doi.org/10.1016/j.ccr.2007.12.004.
Obermajer, Nataša, Ravikumar Muthuswamy, Kunle Odunsi, Robert P. Edwards, and Pawel Kalinski. 2011. PGE 2-induced CXCL 12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Research 71: 7463–7470. https://doi.org/10.1158/0008-5472.CAN-11-2449.
Hiratsuka, Sachie, Dan G. Duda, Yuhui Huang, Shom Goel, Tatsuki Sugiyama, Takashi Nagasawa, Dai Fukumura, and Rakesh K. Jain. 2011. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proceedings of the National Academy of Sciences of the United States of America 108: 302–307. https://doi.org/10.1073/pnas.1016917108.
Haribabu, Bodduluri, Ricardo M. Richardson, Ian Fisher, Silvano Sozzani, Stephen C. Peiper, Richard Horuk, Hydar Ali, and Ralph Snyderman. 1997. Regulation of human chemokine receptors CXCR4: Role of phosphorylation in desensitization and internalization. Journal of Biological Chemistry 272: 28726–28731. https://doi.org/10.1074/jbc.272.45.28726.
Maruyama, Akira, Hiroaki Shime, Yohei Takeda, Masahiro Azuma, Misako Matsumoto, and Tsukasa Seya. 2015. Pam2 lipopeptides systemically increase myeloid-derived suppressor cells through TLR2 signaling. Biochemical and Biophysical Research Communications 457: 445–450. https://doi.org/10.1016/j.bbrc.2015.01.011.
Chalmin, Fanny, Sylvain Ladoire, Grégoire Mignot, Julie Vincent, Mélanie Bruchard, Jean Paul Remy-Martin, Wilfrid Boireau, Alain Rouleau, Benoit Simon, David Lanneau, Aurélie de Thonel, Gabriele Multhoff, Arlette Hamman, François Martin, Bruno Chauffert, Eric Solary, Laurence Zitvogel, Carmen Garrido, Bernhard Ryffel, Christophe Borg, Lionel Apetoh, Cédric Rébé, and François Ghiringhelli. 2010. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. Journal of Clinical Investigation 120: 457–471. https://doi.org/10.1172/JCI40483.
Shime, Hiroaki, Akira Maruyama, Sumito Yoshida, Yohei Takeda, Misako Matsumoto, and Tsukasa Seya. 2017. Toll-like receptor 2 ligand and interferon-γ suppress anti-tumor T cell responses by enhancing the immunosuppressive activity of monocytic myeloid-derived suppressor cells. Oncoimmunology 7 (1): e1373231. https://doi.org/10.1080/2162402X.2017.1373231.
Bosisio, Daniela, Nadia Polentarutti, Marina Sironi, Sergio Bernasconi, Kensuke Miyake, Ginette R. Webb, Michael U. Martin, Alberto Mantovani, and Marta Muzio. 2002. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-y: A molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood 99 (9): 3427–3431.
Acknowledgments
The authors thank Theodore Micceri, Ph.D for critical reading of the manuscript.
Funding
This study was supported by the Grant No. AP05131710 “Pharmacological approaches to target myeloid-derived suppressor cells (MDSCs) for suppression of chronic inflammation as a stimulant of tumor growth in experimental models” of the Committee of Science of Ministry of Education and Science of the Republic of Kazakhstan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were carried out in compliance with the Guide for Care and Use of Laboratory Animals and approved by the Ethical Committee of M.A. Aitkhozhin’s Institute of Molecular Biology and Biochemistry, Almaty, Kazakhstan.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Perfilyeva, Y.V., Abdolla, N., Ostapchuk, Y.O. et al. Chronic Inflammation Contributes to Tumor Growth: Possible Role of l-Selectin-Expressing Myeloid-Derived Suppressor Cells (MDSCs). Inflammation 42, 276–289 (2019). https://doi.org/10.1007/s10753-018-0892-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0892-6