Abstract
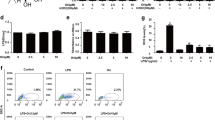
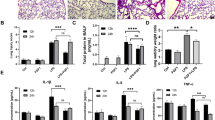
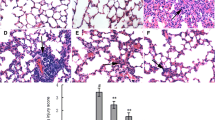
Artesunate, a derivative of artemisinin, has been reported to have anti-inflammatory property. However, few studies showed the protective effects of artesunate on lung injury. In this study, we aimed to investigate the effects of artesunate on LPS-induced lung injury in mice. The mice were treated with artesunate 1 h before or after LPS treatment. The effects of artesunate on lung MPO activity and malondialdehyde (MDA) content were detected. The lung wet/dry radio and the numbers of inflammatory cells in BALF were also measured. ELISA was used to evaluate the levels of TNF-α, IL-1β, and IL-6 in BALF. Western blot analysis was adapted to detect TLR4 and Nrf2 signaling pathways. The results showed that artesunate protected against LPS-induced ALI by decreasing the numbers of inflammatory cells, lung edema, MPO activity, and MDA content. Furthermore, artesunate significantly inhibited the levels of TNF-α, IL-1β, and IL-6. Artesunate also inhibited LPS-induced IL-6 and IL-8 production in the A549 cells. In addition, artesunate dose-dependently suppressed LPS-induced TLR4 expression and NF-κB activation. The expression of Nrf2 and HO-1 were also up-regulated by artesunate. The data suggest that artesunate possesses anti-inflammatory and anti-oxidant properties against LPS-induced ALI via inhibiting TLR4 signaling pathway and activating Nrf2 signaling pathway.








Similar content being viewed by others
References
Wheeler, A.P., and G.R. Bernard. 2007. Acute lung injury and the acute respiratory distress syndrome: a clinical review. The Lancet 369: 1553–1564.
BAUMANN, W.R., R.C. JUNG, M. KOSS, C.T. BOYLEN, L. NAVARRO, and O.P. SHARMA. 1986. Incidence and mortality of adult respiratory distress syndrome: a prospective analysis from a large metropolitan hospital. Critical Care Medicine 14: 1–4.
Enkhbaatar, P., and D.L. Traber. 2004. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clinical Science (London) 107: 137–143.
Kristof, A.S., P. Goldberg, V. Laubach, and S.N. Hussain. 1998. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. American Journal of Respiratory and Critical Care Medicine 158: 1883–1889.
Imai, Y., K. Kuba, G.G. Neely, R. Yaghubian-Malhami, T. Perkmann, G. van Loo, M. Ermolaeva, R. Veldhuizen, Y.H. Leung, H. Wang, et al. 2008. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133: 235–249.
Welbourn, C.R., and Y. Young. 1992. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages and inflammatory mediators. British Journal of Surgery 79: 998–1003.
Seki, H., K. Fukunaga, M. Arita, H. Arai, H. Nakanishi, R. Taguchi, T. Miyasho, R. Takamiya, K. Asano, A. Ishizaka, et al. 2010. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. Journal of Immunology 184: 836–843.
Zhang, X., P. Shan, S. Qureshi, R. Homer, R. Medzhitov, P.W. Noble, and P.J. Lee. 2005. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. Journal of Immunology 175: 4834–4838.
Wang, D., J. Shi, S. Lv, W. Xu, J. Li, W. Ge, C. Xiao, D. Geng, and Y. Liu. 2015. Artesunate attenuates lipopolysaccharide-stimulated proinflammatory responses by suppressing TLR4, MyD88 expression, and NF-kappaB activation in microglial cells. Inflammation 38: 1925–1932.
Lai, L.N., Y.X. Chen, X.X. Tian, X.J. Li, X.J. Zhang, J.W. Lei, Y.H. Bi, B.W. Fang, and X.L. Song. 2015. Artesunate alleviates hepatic fibrosis induced by multiple pathogenic factors and inflammation through the inhibition of LPS/TLR4/NF-kappa B signaling pathway in rats. European Journal of Pharmacology 765: 234–241.
Yang, Z., J. Ding, C. Yang, Y. Gao, X. Li, X. Chen, Y. Peng, J. Fang, and S. Xiao. 2012. Immunomodulatory and anti-inflammatory properties of artesunate in experimental colitis. Current Medicinal Chemistry 19: 4541–4551.
Wang, X.Q., H.L. Liu, G.B. Wang, P.F. Wu, T. Yan, J. Xie, Y. Tang, L.K. Sun, and C. Li. 2011. Effect of artesunate on endotoxin-induced uveitis in rats. Investigative Ophthalmology & Visual Science 52: 916–919.
Okorji, U.P., and O.A. Olajide. 2014. A semi-synthetic derivative of artemisinin, artesunate inhibits prostaglandin E2 production in LPS/IFNgamma-activated BV2 microglia. Bioorganic and Medicinal Chemistry 22: 4726–4734.
Lv, H., C. Zhu, Y. Liao, Y. Gao, G. Lu, W. Zhong, Y. Zheng, W. Chen, and X. Ci. 2015. Tenuigenin ameliorates acute lung injury by inhibiting NF-kappaB and MAPK signalling pathways. Respiratory Physiology & Neurobiology 216: 43–51.
Goodman, R.B., J. Pugin, J.S. Lee, and M.A. Matthay. 2003. Cytokine-mediated inflammation in acute lung injury. Cytokine and Growth Factor Reviews 14: 523–535.
Jiang, D., J. Liang, J. Fan, S. Yu, S. Chen, Y. Luo, G.D. Prestwich, M.M. Mascarenhas, H.G. Garg, D.A. Quinn, et al. 2005. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature Medicine 11: 1173–1179.
Ben, D.F., X.Y. Yu, G.Y. Ji, D.Y. Zheng, K.Y. Lv, B. Ma, and Z.F. Xia. 2012. TLR4 mediates lung injury and inflammation in intestinal ischemia-reperfusion. Journal of Surgical Research 174: 326–333.
Du, X., A. Poltorak, M. Silva, and B. Beutler. 1999. Analysis of Tlr4-mediated LPS signal transduction in macrophages by mutational modification of the receptor. Blood Cells, Molecules & Diseases 25: 328–338.
Fitzgerald, K.A., D.C. Rowe, B.J. Barnes, D.R. Caffrey, A. Visintin, E. Latz, B. Monks, P.M. Pitha, and D.T. Golenbock. 2003. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. Journal of Experimental Medicine 198: 1043–1055.
Zhang, B., Z.Y. Liu, Y.Y. Li, Y. Luo, M.L. Liu, H.Y. Dong, Y.X. Wang, Y. Liu, P.T. Zhao, F.G. Jin, and Z.C. Li. 2011. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. European Journal of Pharmaceutical Sciences 44: 573–579.
Chow, C.W., M.T. Herrera Abreu, T. Suzuki, and G.P. Downey. 2003. Oxidative stress and acute lung injury. American Journal of Respiratory Cell and Molecular Biology 29: 427–431.
Gonzalez, P.K., J. Zhuang, S.R. Doctrow, B. Malfroy, P.F. Benson, M.J. Menconi, and M.P. Fink. 1996. Role of oxidant stress in the adult respiratory distress syndrome: evaluation of a novel antioxidant strategy in a porcine model of endotoxin-induced acute lung injury. Shock 6(Suppl 1): S23–S26.
Urso, M.L., and P.M. Clarkson. 2003. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189: 41–54.
Leung, L., M. Kwong, S. Hou, C. Lee, and J.Y. Chan. 2003. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. Journal of Biological Chemistry 278: 48021–48029.
Chen, X.L., G. Dodd, S. Thomas, X. Zhang, M.A. Wasserman, B.H. Rovin, and C. Kunsch. 2006. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. American Journal of Physiology - Heart and Circulatory Physiology 290: H1862–H1870.
Ueda, K., T. Ueyama, K.I. Yoshida, H. Kimura, T. Ito, Y. Shimizu, M. Oka, Y. Tsuruo, and M. Ichinose. 2008. Adaptive HNE-Nrf2-HO-1 pathway against oxidative stress is associated with acute gastric mucosal lesions. American Journal of Physiology-Gastrointestinal and Liver Physiology 295: G460–G469.
Cho, H.Y., A.E. Jedlicka, S.P. Reddy, T.W. Kensler, M. Yamamoto, L.Y. Zhang, and S.R. Kleeberger. 2002. Role of NRF2 in protection against hyperoxic lung injury in mice. American Journal of Respiratory Cell and Molecular Biology 26: 175–182.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Deli Zhao and Jinling Zhang contribute equally to this article.
Rights and permissions
About this article
Cite this article
Zhao, D., Zhang, J., Xu, G. et al. Artesunate Protects LPS-Induced Acute Lung Injury by Inhibiting TLR4 Expression and Inducing Nrf2 Activation. Inflammation 40, 798–805 (2017). https://doi.org/10.1007/s10753-017-0524-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0524-6