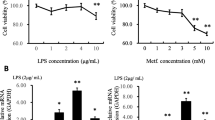
Abstract
Gamma-linolenic acid (GLA) and linoleic acid (LA), which are both n-6 unsaturated fatty acids, play vital roles in lipopolysaccharide (LPS)-induced inflammation. The multi-functional protein scavenger receptor CD36 has also been shown to participate in inflammation. However, the molecular mechanisms underlying the interactions between CD36 and GLA or LA in LPS-induced inflammation remain unclear. We used small interfering RNA and adenoviral systems to manipulate CD36 expression in primary goat mammary gland epithelial cells (pGMECs), and the results showed that nuclear factor kappa B (NF-κB) levels were significantly decreased by CD36 receptor signaling following treatment with GLA but not LA. GLA inhibited NF-κB activation in LPS-induced pGMECs. However, silencing CD36 or deleting its fatty acid-binding domain blocked the anti-inflammatory effects of GLA, resulting in an increase in NF-κB activation and disrupting its localization during LPS-induced inflammation. The activity of the cytokines IL-1β, IL-6, and TNF-α, which act downstream of NF-κB, was also modulated when CD34 expression was manipulated by the addition of GLA in LPS-induced pGMECs. Our data suggest that GLA, but not LA, may interact with the CD36 fatty acid-binding domain to regulate the activation and localization of NF-κB in LPS-induced pGMECs.





Similar content being viewed by others
REFERENCES
Chang, Cheng-Sue, Hai-Lun Sun, Chong-Kuei Lii, Haw-Wen Chen, Pei-Yin Chen, and Kai-Li Liu. 2010. Gamma-linolenic acid inhibits inflammatory responses by regulating NF-kappaB and AP-1 activation in lipopolysaccharide-induced RAW 264.7 macrophages. Inflammation 33: 46–57.
Nichols, Timothy C., Thomas H. Fischer, Efthymios N. Deliargyris, and Albert S. Baldwin Jr. 2001. Role of nuclear factor-kappa B (NF-kappa B) in inflammation, periodontitis, and atherogenesis. Annals of Periodontology 6: 20–29.
Penumetcha, M., M. Song, N. Merchant, and S. Parthasarathy. 2012. Pretreatment with n-6 PUFA protects against subsequent high fat diet induced atherosclerosis—potential role of oxidative stress-induced antioxidant defense. Atherosclerosis 220: 53–58.
Marion-Letellier, Rachel, Guillaume Savoye, Paul L. Beck, Remo Panaccione, and Subrata Ghosh. 2013. Polyunsaturated fatty acids in inflammatory bowel diseases: a reappraisal of effects and therapeutic approaches. Inflammatory Bowel Diseases 19: 650–661.
Tyagi, Anupama, Uday Kumar, Suryam Reddy, Vadakattu S. Santosh, Saazida B. Mohammed, Nasreen Z. Ehtesham, and Ahamed Ibrahim. 2012. Attenuation of colonic inflammation by partial replacement of dietary linoleic acid with alpha-linolenic acid in a rat model of inflammatory bowel disease. British Journal of Nutrition 108: 1612–1622.
Bernard, Laurence, Mohamad B. Montazer Torbati, Graulet Benoit, Leroux Christine, and Chilliard Yves. 2013. Long-chain fatty acids differentially alter lipogenesis in bovine and caprine mammary slices. Journal of Dairy Research 80: 89–95.
Kapoor, Rakesh, and Yung-Sheng Huang. 2006. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Current Pharmaceutical Biotechnology 7: 531–534.
Wahli, Walter, and Liliane Michalik. 2012. PPARs at the crossroads of lipid signaling and inflammation. Trends in Endocrinology and Metabolism 23: 351–363.
Sharif, Omar, Ulrich Matt, Simona Saluzzo, Karin Lakovits, Isabella Haslinger, Tanja Furtner, Bianca Doninger, and Sylvia Knapp. 2013. The scavenger receptor CD36 downmodulates the early inflammatory response while enhancing bacterial phagocytosis during pneumococcal pneumonia. Journal of Immunology 190: 5640–5648.
Baranova, Irina N., Roger Kurlander, Alexander V. Bocharov, Tatyana G. Vishnyakova, Zhigang Chen, Alan T. Remaley, Gyorgy Csako, Amy P. Patterson, and Thomas L. Eggerman. 2008. Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. Journal of Immunology 181: 7147–7156.
Baranova Irina, N., Tatyana G. Vishnyakova, Alexander V. Bocharov, Leelahavanichkul Asada, Kurlander Roger, Chen Zhigang, Ana C.P. Souza, Peter S.T. Yuen, Robert A. Star, Csako Gyorgy, Amy P. Patterson, and Thomas L. Eggerman. 2012. Class B scavenger receptor types I and II and CD36 mediate bacterial recognition and proinflammatory signaling induced by Escherichia coli, lipopolysaccharide, and cytosolic chaperonin 60. Journal of Immunology 188: 1371–1380.
Stuart, Lynda M., Jiusheng Deng, Jessica M. Silver, Kazue Takahashi, Anita A. Tseng, Elizabeth J. Hennessy, R. Alan, B. Ezekowitz, and Kathryn J. Moore. 2005. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. Journal of Cell Biology 170: 477–485.
Triantafilou, Martha, Frederick G.J. Gamper, Rowenna M. Haston, Mouratis Marios Angelos, Morath Siegfried, Hartung Thomas, and Triantafilou Kathy. 2006. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. Journal of Biological Chemistry 281: 31002–31011.
Tsai, Tsung-Huang, Shu-Fen Chen, Tai-Yu Huang, Chun-Fu Tzeng, Ann-Shyn Chiang, Yu Ru Kou, Tzong-Shyuan Lee, and Song-Kun Shyue. 2011. Impaired Cd14 and Cd36 expression, bacterial clearance, and Toll-like receptor 4-Myd88 signaling in caveolin-1-deleted macrophages and mice. Shock 35: 92–99.
Cao, Duoyao, Jun Luo, Dekun Chen, Huifen Xu, Huaiping Shi, Xiaoqi Jing, and Wenjuan Zang. 2016. CD36 regulates lipopolysaccharide-induced signaling pathways and mediates the internalization of Escherichia coli in cooperation with TLR4 in goat mammary gland epithelial cells. Scientific Reports 6: 23132.
Erdinest, Nir, Or Shmueli, Yoni Grossman, Haim Ovadia, and Abraham Solomon. 2012. Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Investigative Ophthalmology & Visual Science 53: 4396–4406.
Nozaki, Shuichi, Takao Tanaka, Shizuya Yamashita, Koichi Sohmiya, Tohru Yoshizumi, Fumio Okamoto, Yasushi Kitaura, Chikao Kotake, Hiroyuki Nishida, Atsuyuki Nakata, Tsutomu Nakagawa, Kengo Matsumoto, Kaoru Kameda-Takemura, Seiji Tadokoro, Yoshiyuki Kurata, Yoshiaki Tomiyama, Keishiro Kawamura, and Yuji Matsuzawa. 1999. CD36 mediates long-chain fatty acid transport in human myocardium: complete myocardial accumulation defect of radiolabeled long-chain fatty acid analog in subjects with CD36 deficiency. Molecular and Cellular Biochemistry 192: 129–135.
Coort Susan, L.M., Willems Jodil, Will A. Coumans, Ger J. van der Vusse, Bonen Arend, Jan F.C. Glatz, and Joost J.F.P. Luiken. 2002. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Molecular and Cellular Biochemistry 239: 213–219.
Wang, Z., J. Luo, W. Wang, W. Zhao, and X. Lin. 2010. Characterization and culture of isolated primary dairy goat mammary gland epithelial cells. Sheng Wu Gong Cheng Xue Bao = Chinese Journal of Biotechnology 26: 1123–1127.
Lin, Xian-zi, Jun Luo, Li-ping Zhang, Wei Wang, Heng-bo Shi, and Jiang-jiang Zhu. 2013. MiR-27a suppresses triglyceride accumulation and affects gene mRNA expression associated with fat metabolism in dairy goat mammary gland epithelial cells. Gene 521: 15–23.
Pantschenko, A.G., J. Woodcock-Mitchell, S.L. Bushmich, and T.J. Yang. 2000. Establishment and characterization of a caprine mammary epithelial cell line (CMEC). In Vitro Cellular and Developmental Biology - Animal 36: 26–37.
German, Tania, and Itamar Barash. 2002. Characterization of an epithelial cell line from bovine mammary gland. In Vitro Cellular and Developmental Biology - Animal 38: 282–292.
Shi, H.B., J. Luo, D.W. Yao, J.J. Zhu, H.F. Xu, H.P. Shi, and J.J. Loor. 2013. Peroxisome proliferator-activated receptor-gamma stimulates the synthesis of monounsaturated fatty acids in dairy goat mammary epithelial cells via the control of stearoyl-coenzyme A desaturase. Journal of Dairy Science 96: 7844–7853.
Baillie, A.G., C.T. Coburn, and N.A. Abumrad. 1996. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. Journal of Membrane Biology 153: 75–81.
Kuda, Ondrej, Terri A. Pietka, Zuzana Demianova, Eva Kudova, Josef Cvacka, Jan Kopecky, and Nada A. Abumrad. 2013. Sulfo-N-succinimidyl oleate (SSO) inhibits fatty acid uptake and signaling for intracellular calcium via binding CD36 lysine 164: SSO also inhibits oxidized low density lipoprotein uptake by macrophages. Journal of Biological Chemistry 288: 15547–15555.
Wang, Wei, Dejie Liang, Song Xiaojing, Tiancheng Wang, Cao Yongguo, Zhengtao Yang, and Zhang Naisheng. 2015. Magnolol inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. Inflammation 38: 16–26.
Okamura, Daryl M., Subramaniam Pennathur, Katie Pasichnyk, J.M. López-Guisa, Sarah Collins, Maria Febbraio, Jay Heinecke, and Allison A. Eddy. 2009. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. Journal of the American Society of Nephrology 20: 495–505.
LeMieux, Monique J., Nishan S. Kalupahana, Shane Scoggin, and Naima Moustaid-Moussa. 2015. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. Journal of Nutrition 145: 411–417.
Rogers, Lynette K., Christina J. Valentine, Michael Pennell, Markus Velten, Rodney D. Britt, Kelly Dingess, Xuilan Zhao, Stephen E. Welty, and Trent E. Tipple. 2011. Maternal docosahexaenoic acid supplementation decreases lung inflammation in hyperoxia-exposed newborn mice. Journal of Nutrition 141: 214–222.
Calder, Philip C. 2006. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. American Journal of Clinical Nutrition 83: 1505S–1519S.
Barabino, Stefano, Maurizio Rolando, Paola Camicione, Giambattista Ravera, Sabrina Zanardi, Sebastiano Giuffrida, and Giovanni Calabria. 2003. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea 22: 97–101.
Drover, V.A., M. Ajmal, F. Nassir, N.O. Davidson, A.M. Nauli, D. Sahoo, P. Tso, and N.A. Abumrad. 2005. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. The Journal of Clinical Investigation 115: 1290–1297.
Yuasa-Kawase, M., D. Masuda, T. Yamashita, R. Kawase, H. Nakaoka, M. Inagaki, K. Nakatani, K. Tsubakio-Yamamoto, T. Ohama, A. Matsuyama, et al. 2012. Patients with CD36 deficiency are associated with enhanced atherosclerotic cardiovascular diseases. Journal of Atherosclerosis and Thrombosis 19: 263–275.
Harmon, Gregory S., Michael T. Lam, and Christopher K. Glass. 2011. PPARs and lipid ligands in inflammation and metabolism. Chemical Reviews 111: 6321–6340.
Ray, Denise M., Filiz Akbiyik, Steven H. Bernstein, and Richard P. Phipps. 2005. CD40 engagement prevents peroxisome proliferator-activated receptor gamma agonist-induced apoptosis of B lymphocytes and B lymphoma cells by an NF-kappaB-dependent mechanism. Journal of Immunology 174: 4060–4069.
Piva, Roberto, Patrizia Gianferretti, Alessandra Ciucci, Riccardo Taulli, Giuseppe Belardo, and M. Gabriella Santoro. 2005. 15-Deoxy-delta 12,14-prostaglandin J2 induces apoptosis in human malignant B cells: an effect associated with inhibition of NF-kappa B activity and down-regulation of antiapoptotic proteins. Blood 105: 1750–1758.
Garcia-Bates, Tatiana M., Carolyn J. Baglole, Matthew P. Bernard, Thomas I. Murant, Patricia J. Simpson-Haidaris, and Richard P. Phipps. 2009. Peroxisome proliferator-activated receptor gamma ligands enhance human B cell antibody production and differentiation. Journal of Immunology 183: 6903–6912.
Croasdell, Amanda, Parker F. Duffney, Nina Kim, Shannon H. Lacy, Patricia J. Sime, and Richard P. Phipps. 2015. PPARgamma and the innate immune system mediate the resolution of inflammation. PPAR Research 2015: 549691.
Jiang, W.G., A. Redfern, R.P. Bryce, and R.E. Mansel. 2000. Peroxisome proliferator activated receptor-gamma (PPAR-gamma) mediates the action of gamma linolenic acid in breast cancer cells. Prostaglandins, Leukotrienes, and Essential Fatty Acids 62: 119–127.
Sato, Ayato, Kosuke Dodo, Makoto Makishima, Yuichi Hashimoto, and Mikiko Sodeoka. 2013. Synthesis and evaluation of 2,3-dinorprostaglandins: dinor-PGD1 and 13-epi-dinor-PGD1 are peroxisome proliferator-activated receptor alpha/gamma dual agonists. Bioorganic & Medicinal Chemistry Letters 23: 3013–3017.
Moore, Kathryn J., Evan D. Rosen, Michael L. Fitzgerald, Felix Randow, Lorna P. Andersson, David Altshuler, David S. Milstone, Richard M. Mortensen, Bruce M. Spiegelman, and Mason W. Freeman. 2001. The role of PPAR-gamma in macrophage differentiation and cholesterol uptake. Nature Medicine 7: 41–47.
Teboul, Lydia, Maria Febbraio, Danielle Gaillard, Amri Ez-Zoubir, Roy Silverstein, and Paul A. Grimaldi. 2001. Structural and functional characterization of the mouse fatty acid translocase promoter: activation during adipose differentiation. The Biochemical Journal 360: 305–312.
Toborek, Michal, Yong Woo Lee, Rosario Garrido, Simone Kaiser, and Bernhard Hennig. 2002. Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cells. American Journal of Clinical Nutrition 75: 119–125.
Fuhrmann, Herbert, Elizabeth A. Miles, Annette L. West, and Philip C. Calder. 2007. Membrane fatty acids, oxidative burst and phagocytosis after enrichment of P388D1 monocyte/macrophages with essential 18-carbon fatty acids. Annals of Nutrition & Metabolism 51: 155–162.
Pohl, Jürgen, Axel Ring, Ümine Korkmaz, Robert Ehehalt, and Wolfgang Stremmel. 2005. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Molecular Biology of the Cell 16: 24–31.
Kontrova, K., J. Zidkova, B. Bartos, V. Skop, J. Sajdok, L. Kazdova, K. Mikulik, P. Mlejnek, V. Zidek, and M. Pravenec. 2007. CD36 regulates fatty acid composition and sensitivity to insulin in 3T3-L1 adipocytes. Physiological Research 56: 493–496.
Zhu, Yaohong, Caroline Fossum, Mikael Berg, and Ulf Magnusson. 2007. Morphometric analysis of proinflammatory cytokines in mammary glands of sows suggests an association between clinical mastitis and local production of IL-1beta, IL-6 and TNF-alpha. Veterinary Research 38: 871–882.
Furse, Robert K., Ronald G. Rossetti, and Robert B. Zurier. 2001. Gammalinolenic acid, an unsaturated fatty acid with anti-inflammatory properties, blocks amplification of IL-1 beta production by human monocytes. Journal of Immunology 167: 490–496.
ACKNOWLEDGMENTS
We would like to thank the Special Fund for Agro-scientific Research in the Public Interest (Grant No. 201103038) for funding this work.
Author Contributions
All of the authors contributed to the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
ELECTRONIC SUPPLEMENTARY MATERIAL
Below is the link to the electronic supplementary material.
Supplementary Figure S1
(DOC 72 kb)
Supplementary Figure S2
(DOC 81 kb)
Supplementary Table S1
(DOC 68 kb)
Rights and permissions
About this article
Cite this article
Cao, D., Luo, J., Zang, W. et al. Gamma-Linolenic Acid Suppresses NF-κΒ Signaling via CD36 in the Lipopolysaccharide-Induced Inflammatory Response in Primary Goat Mammary Gland Epithelial Cells. Inflammation 39, 1225–1237 (2016). https://doi.org/10.1007/s10753-016-0358-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0358-7