Abstract
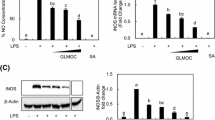
Gamma linolenic acid (GLA) is a member of the n-6 family of polyunsaturated fatty acids and can be synthesized from linoleic acid (LA) by the enzyme delta-6-desaturase. The therapeutic values of GLA supplementation have been documented, but the molecular mechanism behind the action of GLA in health benefits is not clear. In this study, we assessed the effect of GLA with that of LA on lipopolysaccharide (LPS)-induced inflammatory responses and further explored the molecular mechanism underlying the pharmacological properties of GLA in mouse RAW 264.7 macrophages. GLA significantly inhibited LPS-induced protein expression of inducible nitric oxide synthase, pro-interleukin-1β, and cyclooxygenase-2 as well as nitric oxide production and the intracellular glutathione level. LA was less potent than GLA in inhibiting LPS-induced inflammatory mediators. Both GLA and LA treatments dramatically inhibited LPS-induced IκB-α degradation, IκB-α phosphorylation, and nuclear p65 protein expression. Moreover, LPS-induced nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) nuclear protein–DNA binding affinity and reporter gene activity were significantly decreased by LA and GLA. Exogenous addition of GLA but not LA significantly reduced LPS-induced expression of phosphorylated extracellular signal-regulated kinase (ERK) 1/2 and c-Jun N-terminal kinase (JNK)-1. Our data suggest that GLA inhibits inflammatory responses through inactivation of NF-κB and AP-1 by suppressed oxidative stress and signal transduction pathway of ERK and JNK in LPS-induced RAW 264.7 macrophages.






Similar content being viewed by others
Abbreviations
- AP-1:
-
activator protein-1
- COX-2:
-
cyclooxygenase-2
- EMSA:
-
electrophoretic mobility shift assay
- ERK1/2:
-
extracellular signal-regulated kinase1/2
- GAPDH:
-
glyceraldehydes-3-phosphate dehydrogenase
- GLA:
-
gamma-linolenic acid
- GSH:
-
glutathione
- IL-1β:
-
interleukin-1β
- iNOS:
-
inducible form of NOS
- IS:
-
internal standard
- JNK:
-
c-Jun NH2-terminal kinase
- LA:
-
linoleic acid
- LPS:
-
lipopolysaccharide
- MAPK:
-
mitogen-activated protein kinase
- MTT:
-
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide
- NF-κB:
-
nuclear factor-κB
- NO:
-
nitric oxide
- NOS:
-
nitric oxide synthase
- PGE2 :
-
prostaglandin E2
- PMSF:
-
phenylmethylsulfonyl fluoride
- rcRNA:
-
recombinant RNA
- RT-PCR:
-
reverse transcriptase polymerase chain reaction
- SEAP:
-
secretory alkaline phosphatase
References
Korhonen, R., A. Lahti, H. Kankaanranta, and E. Moilanen. 2005. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 4: 471–479.
Park, J. Y., M. H. Pillinger, and S. B. Abramson. 2006. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin. Immunol. 119: 229–240.
Stylianou, E., and J. Saklatvala. 1998. Interleukin-1. Int. J. Biochem. Cell Biol. 30: 1075–1079.
Chen, F., V. Castranova, X. Shi, and L. M. Demers. 1999. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 45: 7–17.
Xie, Q. W., Y. Kashiwabara, and C. Nathan. 1994. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 269: 4705–4808.
Appleby, S. B., A. Ristimaki, K. Neilson, K. Narko, and T. Hla. 1994. Structure of the human cyclo-oxygenase-2 gene. Biochem. J. 302: 723–727.
Zenz, R., R. Eferl, C. Scheinecker, K. Redlich, J. Smolen, H. B. Schonthaler, L. Kenner, E. Tschachler, and E. F. Wagner. 2008. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res. Ther. 10: 201–210.
Das, U. N. 2007. Gamma-linolenic acid therapy of human glioma—a review of in vitro, in vivo, and clinical studies. Med. Sci. Monit. 13: RA119–RA131.
Kapoor, R., and Y. S. Huang. 2006. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 7: 531–534.
Fan, Y. Y., and R. S. Chapkin. 1998. Importance of dietary gamma-linolenic acid in human health and nutrition. J. Nutr. 128: 1411–1414.
Pham, H., K. Vang, and V. A. Ziboh. 2006. Dietary gamma-linolenate attenuates tumor growth in a rodent model of prostatic adenocarcinoma via suppression of elevated generation of PGE2 and 5S-HETE. Prostaglandins Leukot. Essent. Fat. Acids. 74: 271–282.
Kavanagh, T., P. E. Lonergan, and M. A. Lynch. 2004. Eicosapentaenoic acid and gamma-linolenic acid increase hippocampal concentrations of IL-4 and IL-10 and abrogate lipopolysaccharide-induced inhibition of long-term potentiation. Prostaglandins Leukot. Essent. Fat. Acids. 70: 391–397.
Furse, R. K., R. G. Rossetti, and R. B. Zurier. 2001. Gamma linolenic acid, an unsaturated fatty acid with anti-inflammatory properties, blocks amplification of IL-1 beta production by human monocytes. J. Immunol. 167: 490–496.
Johnson, M. M., D. D. Swan, M. E. Surette, J. Stegner, T. Chilton, A. N. Fonteh, and F. H. Chilton. 1997. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J. Nutr. 127: 1435–1444.
Fan, Y. Y., and R. S. Chapkin. 1993. Phospholipid sources of metabolically elongated gammalinolenic acid: conversion to prostaglandin E1 in stimulated mouse macrophages. J. Nutr. Biochem. 4: 602–607.
Zhao, G., T. D. Etherton, K. R. Martin, J. P. Vanden Heuvel, P. J. Gillies, S. G. West, and P. M. Kris-Etherton. 2005. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem. Biophys. Res. Commun. 336: 909–917.
Toborek, M., Y. W. Lee, R. Garrido, S. Kaiser, and B. Hennig. 2002. Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cells. Am. J. Clin. Nutr. 75: 119–125.
Denizot, F., and R. Lang. 1986. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 89: 271–277.
Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]-nitrate in biological fluids. Anal. Biochem. 126: 131–138.
Reed, D. J., J. R. Babson, P. W. Beatty, A. E. Brodie, W. W. Ellis, and D. W. Potter. 1980. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal. Biochem. 106: 55–62.
Lowry, O. H., N. J. Rosebrough, A. I. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
Belury, M. A., S. Y. Moya-Camarena, K. L. Liu, and J. P. Vanden Heuvel. 1997. Dietary conjugated linoleic acid induced peroxisome-specific enzyme accumulation and ornithine decarboxylase activity in mouse liver. J. Nutr. Biochem. 8: 579–584.
Vanden Heuvel, J. P., G. C. Clark, M. C. Kohn, A. M. Tritscher, W. F. Greenlee, G. W. Lucier, and D. A. Bell. 1994. Dioxin-responsive genes: examination of dose–response relationships using quantitative reverse transcriptase-polymerase chain reaction. Cancer Res. 54: 62–68.
Liu, K. L., W. C. Chen, R. Y. Wang, Y. P. Lei, L. Y. Sheen, and C. K. Lii. 2006. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress and NF-κB activation in RAW 264.7 macrophages. J. Agric. Food Chem. 54: 3472–3478.
de Lima, T. M., L. de Sa Lima, C. Scavone, and R. Curi. 2006. Fatty acid control of nitric oxide production by macrophages. FEBS Lett. 580: 3287–3295.
Tak, P. P., and G. S. Firestein. 2001. NF-kappa B: a key role in inflammatory diseases. J. Clin. Invest. 107: 7–11.
Fuhrmann, H., E. A. Miles, A. L. West, and P. C. Calder. 2007. Membrane fatty acids, oxidative burst and phagocytosis after enrichment of P388D1 monocyte/macrophages with essential 18-carbon fatty acids. Ann. Nutr. Metab. 51: 155–162.
Kaminska, B. 2005. MAPK signaling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta. 1754: 253–262.
Kang, J. S., Y. D. Yoon, K. H. Lee, S. K. Park, and H. M. Kim. 2004. Costunolide inhibits interleukin-1beta expression by down-regulation of AP-1 and MAPK activity in LPS-stimulated RAW 264.7 cells. Biochem. Biophys. Res. Commun. 313: 171–177.
Kim, H. G., D. H. Yoon, W. H. Lee, S. K. Han, B. Shrestha, C. H. Kim, M. H. Lim, W. Chang, S. Lim, S. Choi, W. O. Song, J. M. Sung, K. C. Hwang, and T. W. Kim. 2007. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol. 114: 307–315.
Hennig, B., W. Lei, X. Arzuaga, D. D. Ghosh, V. Saraswathi, and M. Toborek. 2006. Linoleic acid induces proinflammatory events in vascular endothelial cells via activation of PI3K/Akt and ERK1/2 signaling. J. Nutr. Biochem. 17: 766–772.
Dhillon, A. S., S. Hagan, O. Rath, and W. Kolch. 2007. MAP kinase signaling pathways in cancer. Oncogene. 26: 3279–3290.
Witztum, J. L. 1993. Role of oxidised low density lipoprotein in atherogenesis. Br. Heart. J. 69: S12–S18.
Richardson, S. J. 1993. Free radicals in the genesis of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 695: 73–76.
Rahman, I., J. Marwick, and P. Kirkham. 2004. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem. Pharmacol. 68: 1255–1267.
Borek, C. 2004. Dietary antioxidants and human cancer. Integr. Cancer Ther. 3: 333–341.
Gloire, G., S. Legrand-Poels, and J. Piette. 2006. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 72: 1493–1505.
Haddad, J. J., and H. L. Harb. 2005. L-gamma-Glutamyl-L-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol. Immunol. 42: 987–1014.
Sun, S., H. Zhang, B. Xue, Y. Wu, J. Wang, Z. Yin, and L. Luo. 2006. Protective effect of glutathione against lipopolysaccharide-induced inflammation and mortality in rats. Inflamm. Res. 55: 504–510.
Komatsu, W., K. Ishihara, M. Murata, H. Saito, and K. Shinohara. 2003. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic. Biol. Med. 34: 1006–1016.
Devi, M. A., and N. P. Das. 1994. Antiproliferative effect of polyunsaturated fatty acids and interleukin-2 on normal and abnormal human lymphocytes. Experientia. 50: 489–492.
Colquhoun, A., and R. I. Schumacher. 2001. gamma-Linolenic acid and eicosapentaenoic acid induce modifications in mitochondrial metabolism, reactive oxygen species generation, lipid peroxidation and apoptosis in Walker 256 rat carcinosarcoma cells. Biochim. Biophys. Acta. 1533: 207–219.
McGeer, P. L., and E. G. McGeer. 2004. Inflammation and the degenerative diseases of aging. Ann. N.Y. Acad. Sci. 1035: 104–116.
Acknowledgments
This research was funded by the National Science Council, Republic of China, under Grant NSC 95-2320-B-040-034 and by Chung Shan Medical University, under Grant CSMU 94-OM-B-003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Cheng-Shu Chang and HaiLun Sun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chang, CS., Sun, HL., Lii, CK. et al. Gamma-Linolenic Acid Inhibits Inflammatory Responses by Regulating NF-κB and AP-1 Activation in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Inflammation 33, 46–57 (2010). https://doi.org/10.1007/s10753-009-9157-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-009-9157-8