Abstract
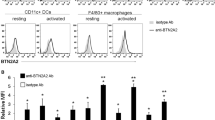
Recently, FGF21 was reported to play an important role in anti-inflammation. The aim of the study is to explore the mechanism for FGF21 alleviating inflammation of CIA. CIA mice were injected with FGF21 once a day for 28 days after first booster immunization. The results showed that FGF21 alleviates arthritis severity and decreases serum anti-CII antibodies levels in CIA mice. Compared with CIA model, the number of the splenic TH17 cells was significantly decreased in FGF21-treated mice. FGF21 treatment reduced the mRNA expression of IL-17, TNF-α, IL-1β, IL-6, IL-8, and MMP3 and increased level of IL-10 in the spleen tissue. The expression of STAT3 and phosphorylated STAT3 was suppressed in FGF21-treated group. The mRNA expression of RORγt and IL-23 also decreased. In conclusion, these findings suggest that the beneficial effects of FGF21 on CIA mice were achieved by down-regulating Th17-IL-17 axis through STAT3/RORγt pathway. Modulating of Th17-mediated inflammatory response may be one of the mechanisms for FGF21 attenuating inflammation in CIA.






Similar content being viewed by others
References
Choy, E. 2012. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 51(Suppl 5): v3–11.
Williams, R.O., M. Feldmann, and R.N. Maini. 2000. Cartilage destruction and bone erosion in arthritis: the role of tumour necrosis factor alpha. Annals of the Rheumatic Diseases 59: i75–80.
Durie, F.H., R.A. Fava, and R.J. Noelle. 1994. Collagen-induced arthritis as a model of rheu matoid arthritis. Clinical Immunology and Immunopathology 73(1): 11–8.
Bettelli, E., T. Korn, M. Oukka, and V.K. Kuchroo. 2008. Induction and effector functions of T(H)17 cells. Nature 453(7198): 1051–7.
Park, H., Z. Li, and X.O. Yangetal. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology 6(11): 1133–41.
Korn, T., E. Bettelli, and M. Oukka. 2009. IL-17 and Th17 cells. Annual Review of Immunology 27: 485–517.
Sato, K., A. Suematsu, K. Okamoto, A. Yamaguchi, Y. Morishita, Y. Kadono, et al. 2008. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. Rheumatol 35(3): 515–9.
Harrington, L.E., R.D. Hatton, and P.R. Manganetal. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinc from the T helper type 1 and 2 lineages. Nature Immunology 6(11): 1123–32.
Stamp, L.K., M.J. James, and L.G. Cleland. 2004. Interleukin-17: the missing link between T-cell accumulation and effector cell actions in rheumatoid arthritis. Immunology and Cell Biology 82(1): 1–9.
Nakae, S., A. Nambu, K. Sudo, and Y. Iwakura. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. Journal of Immunology 171: 6173–7.
Nakae, S., S. Saijo, R. Horai, K. Sudo, S. Mori, and Y. Iwakura. 2003. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proceedings of the National Academy of Sciences of the United States of America 100: 5986–90.
Zhao, Y., J.D. Dunbar, and A. Kharitonenkov. 2012. FGF21 as a therapeutic reagent (J). Advances in Experimental Medicine and Biology 728: 214–8.
Kharitonenkov, A., V.J. Wroblewski, A. Koester, Y.F. Chen, C.K. Clutinger, X.T. Tigno, et al. 2007. The metabolic state of diabetic monkeys is regulated by FGF-21. Endocrinology 148(2): 774–81.
Coskun, T., H.A. Bina, M.A. Schneider, J.D. Dunbar, C.C. Hu, Y. Chen, et al. 2008. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149: 6018–27.
Lee, M.S., S.E. Choi, E.S. Ha, S.Y. An, T.H. Kim, S.J. Han, et al. 2012. Fibroblast growth factor-21 protects human skeletal muscle myotubes from palmitate-induced insulin resistance by inhibiting stress kinase and NF-κB. Metabolism 61(8): 1142–51.
Gariani, K., G. Drifte, I. Dunn-Siegrist, J. Pugin, and F.R. Jornayvaz. 2013. Increased FGF21 plasma levels in humans with sepsis and SIRS. Endocrine Connections 2(3): 146–53.
Hulejová, H., L. Andrés Cerezo, M. Kuklová, O. Pecha, T. Vondráček, K. Pavelka, et al. 2012. Novel adipokine fibroblast growth factor 21 is increased in rheumatoid arthritis. Physiological Research 61: 489–94.
Yinhang, Yu., Li. Siming, Liu Yaonan, Tian Guiyou, Yuan Qingyan, et al. 2015. Fibroblast growth factor 21 (FGF21) ameliorates collagen-induced arthritis through modulating oxidative stress and suppressing nuclear factor-kappaβ pathway. International Immunopharmacology 25: 74–82.
Barnett, M.L., J.M. Kremer, E.W. St Clair, D.O. Clegg, D. Furst, M. Weisman, et al. 1998. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis and Rheumatism 41(2): 290–7.
Bevaart, L., M.J. Vervoordeldonk, and P.P. Tak. 2010. Collagen-induced arthritis in mice. Methods in Molecular Biology 602: 181–92.
Lubberts, E., M.I. Koenders, and W.B. van den Berg. 2005. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Research and Therapy 7: 29–37.
Chabaud, M., E. Lubberts, L. Joosten, W. van Den Berg, and P. Miossec. 2001. IL-17 derived from juxta-articular bone andsynovium contributes to joint degradation in rheumatoid arthritis. Arthritis Research 3: 168–177.
Lubberts, E., L.A. Joosten, B. Oppers, L. van den Bersselaar, C.J. Coenen-de Roo, J.K. Kolls, et al. 2001. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. Journal of Immunology 167(2): 1004–13.
Hwang, S.Y., and H.Y. Kim. 2005. Expression of IL-17 homologs and their receptors in the synovialcells of rheumatoid arthritis patients. Molecules and Cells 19(2): 180–4.
Kotake, S., N. Udagawa, N. Takahashi, K. Matsuzaki, K. Itoh, S. Ishiyama, et al. 1999. IL-17 in synovialfluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. Journal of Clinical Investigation 103(9): 1345–52.
Jovanovic, D.V., J.A. Di Battista, J. Martel-Pelletier, F.C. Jolicoeur, Y. He, M. Zhang, et al. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-βand TNF-α, by human. Macrophages The Journal of Immunology 160(7): 3513–21.
Katz, Y., O. Nadiv, and Y. Beer. 2001. Interleukin-17 enhances tumor necrosis factor-induced synthesis of interleukins 1, 6, and 8 in skin and IL-17 in rheumatoid arthritis in skin and synovial fibroblasts: a possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis and Rheumatism 44: 2176–84.
Beklen, A., M. Ainola, M. Hukkanen, C. Gürgan, T. Sorsa, and Y.T. Konttinen. 2007. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. Journal of Dental Research 86(4): 347–51.
LeGrand, A., B. Fermor, C. Fink, D.S. Pisetsky, J.B. Weinberg, T.P. Vail, et al. 2001. Interleukin-1, tumor necrosis factor, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E production in explants of human osteoarthritic knee menisci. Arthritis and Rheumatism 44: 2078–83.
Jones, C.E., and K. Chan. 2002. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. American Journal of Respiratory Cell and Molecular Biology 2(6): 748–53.
Fossiez, F., O. Djossou, P. Chomarat, L. Flores-Romo, S. Ait-Yahia, C. Maat, et al. 1996. T cell interleukin-17 induceomal cells to produce pro-inflammatory and hematopoietic cytokines. Journal of Experimental Medicine 183(6): 2593–603.
Agarwal, S., R. Misra, and A. Aggarwal. 2008. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. Rheumatol 35(3): 515–9.
Miossec, Pierre. 2003. Interleukin-17 in rheumatoid arthritis. Arthritis and Rheumatism 48(3): 594–601.
Ooi, J.D., R.K. Phoon, S.R. Holdsworth, and A.R. Kitching. 2009. IL-23, not IL-12, directs autoimmunity to the good pasture antigen. Journal of the American Society of Nephrology 20(5): 980–9.
Krause, A., N. Scaletta, J.D. Ji, and L.B. Ivashkiv. 2002. Rheumatoid arthritis synoviocyte survival is dependent on Stat3. Journal of Immunology 169: 6610–6.
Harris, T.J., J.F. Grosso, and H.R. Yen. 2007. Cutting edge: an in vivo requirement for STAT3 signaling in T17 development and 17-dependent autoimmunity. Journal of Immunology 179(7): 4313–7.
Yang, X.O., A.D. Panopoulos, R. Nurieva, S.H. Chang, D. Wang, S.S. Watowich, et al. 2007. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. Journal of Biological Chemistry 282(13): 9358–63.
Ivaylo, I.I., B.S. McKenzie, L. Zhou, C.E. Tadokoro, A. Lepelley, J.J. Lafaille, et al. 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17 + T helper cells. Cell 126(6): 1121–33.
Nicolas, Manel, Unutmaz Derya, and Dan R. Littman. 2008. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nature Immunology 9(6): 641–9.
Langrish, Claire L., Yi. Chen, Wendy M. Blumenschein, Jeanine Mattson, Beth Basham, et al. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. Journal of Experimental Medicine 201(2): 233–40.
Cho, M.L., J.W. Kang, Y.M. Moon, H.J. Nam, J.Y. Jhun, S.B. Heo, et al. 2006. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptorantagonist-deficient mice. Journal of immunology (Baltimore, Md:1950) 176(9): 5652–6.
Zhou, L., I.I. Ivanov, and R. Spolski. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunology 8: 967–74.
Putoczki, T.L., S. Thiem, A. Loving, et al. 2013. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 24(2): 257–71.
Grivennikov, S.I. 2013. IL-11: a prominent pro-tumorigenic member of the IL-6 family. Cancer Cell 24(2): 145–7.
Wang, L., T. Yi, M. Kortylewski, et al. 2009. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. Journal of Experimental Medicine 206(7): 1457–64.
Jarnicki, A., T. Putoczki, and M. Ernst. 2010. Stat3: linking inflammation to epithelial cancer—more than a “gut” feeling? Cell Div 5: 14.
Sironi, M., F. Breviario, P. Proserpio, et al. 1989. IL-1 stimulates IL-6 production in endothelial cells. Journal of Immunology 142(2): 549–53.
Kaplanski, G., N. Teysseire, C. Farnarier, et al. 1995. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia conorii via a cell-associated IL-1 alpha-dependent pathway. Journal of Clinical Investigation 96(6): 2839–44.
He, G., and M. Karin. 2011. NF-kappaB and STAT3—key players in liver inflammation and cancer. Cell Research 21(1): 159–68.
Bollrath, J., and F.R. Greten. 2009. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Reports 10(12): 1314–19.
Acknowledgments
This study was supported by The Scientific Research Foundation of Harbin University of Commerce for PhD (NO.92508177) and The National Natural Science Fund biologic science base improve program of research training and capacity (NO. J1210069/J0131)
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Sm., Yu, Yh., Li, L. et al. Treatment of CIA Mice with FGF21 Down-regulates TH17-IL-17 Axis. Inflammation 39, 309–319 (2016). https://doi.org/10.1007/s10753-015-0251-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0251-9