Abstract
Compounds that exhibit the spin crossover effect are known to show a change of spin states through external stimuli. This reversible switching of spin states is accompanied by a change of the properties of the compound. Complexes, like iron (II)-triazole complexes, that exhibit this behavior at ambient temperature are often discussed for potential applications. In previous studies we synthesized iron (II)-triazole complexes and implemented them into electrospun nanofibers. We used Mössbauer spectroscopy in first studies to prove a successful implementation with maintaining spin crossover properties. Further studies from us showed that it is possible to use different electrospinning methods to either do a implementation or a deposition of the synthesized solid SCO material into or onto the polymer nanofibers. We now used a solvent in which both, the used iron (II)-triazole complex [Fe(atrz)3](2 ns)2 and three different polymers (Polyacrylonitrile, Polymethylmethacrylate and Polyvinylpyrrolidone), are soluble. This shall lead to a higher homogeneous distribution of the complex along the nanofibers. Mössbauer spectroscopy and other measurements are therefore in use to show a successful implementation without any significant changes to the complex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Some coordination compounds like iron (II)-triazole complexes are known to exhibit the spin crossover (SCO) phenomenon at ambient temperature [1]. This effect can generally be observed with different physical or chemical external stimuli [2]. Through this effect, these compounds are becoming increasingly interesting for several possible applications for example in novel optical devices and sensor systems [3]. However, addressing these complexes with the aforementioned stimuli can be a challenging task, therefore the implementation in a polymer matrix is especially interesting. This is why we first implemented triazole complexes into polymer nanofibers and used the electrospinning method [4, 5]. Further investigation showed that it was possible to determine the exact position of the complexes on top of the fibers. As the complexes were not soluble they formed beading structures but still showed similar SCO properties. Coaxial electrospinning made it further possible to obtain core-shell-like structures with SCO properties [6]. Electrospinning itself is a simple method to obtain polymer nanofibers with roughly a diameter above 100 nm out of a polymer solution. It has a high potential to be scaled up and be a multifaceted method in the industry [7]. In this fiber fabrication method, an electrical potential (between 5 and 30 kV) is applied between a collector and a droplet of the used polymer solution in a syringe needle. Due to the applied voltage, Coulomb forces act on the drop which exceeds the surface tension of the polymer solution. A fiber jet emerges then from the build-up Taylor cone. When the liquid dries in this process an electrically charged fiber remains on the used collector [6, 8, 9]. For our experiments, a rotating collector was used. To obtain a better homogeneous distribution of the complex inside the fibers we used [Fe(atrz)3](2 ns)2 which is known to be soluble in Dimethylformamide (DMF) and polymers that were also soluble in DMF [10]. This was also done to avoid the former mentioned beading structures. We measured Mössbauer spectra and other spectra like IR and UV/Vis of the obtained complexes and the composite fibers to determine which polymer complex combination enables successful implementation and to observe any possible damages to the complexes. In addition, it is shown in which case the SCO properties persist.
2 Materials and methods
2.1 Materials
The used FeII-triazole complex and composite materials were synthesized by using the following purchased chemicals without further purifying them: Iron(II)-Chloridetetrahydrate (FeCl2 \(\cdot \) 4H2O) (>99 %) from Sigma-Aldrich (St. Louis, MO, USA); l-Ascorbic acid (>99 %) from Carl Roth; 4-Amino-1,2,4-Triazole (99 %) purchased from Thermo Scientific (Waltham, MA, USA); Sodium 2-Naphthalenesulfonate (98 %) from Alfa Aesar (Haverhill, MA, USA); PMMA 350,000 Mw, PAN 150,000 Mw and PVP 40000 Mw from Sigma-Aldrich (St. Louis, MO, USA); and N,N-Dimethylformamide (DMF) from Carl Roth.
2.2 Methods
2.2.1 Synthesis of [Fe(atrz)3](2 ns)2
For the synthesis of [Fe(atrz)3](2 ns)2 it was first necessary to synthesise the corresponding Fe (II)-salt Fe(2 ns)2 \(\cdot \) 6 H2O. This was done as previously mentioned [6]. Therefore, to obtain Fe(2 ns)2 \(\cdot \) 6 H2O, 2.5 g of Sodium 2-Naphthalenesulfonate (10.85 mmol) was dissolved in 75 mL of H2O by heating up to 70 \({}^{\circ }\text {C}\) and stirring at 650 rpm. A dull solution was obtained. Separately, 1.08 g of FeCl2 \(\cdot \) 4 H2O (5.425 mmol) dissolved in 2.5 mL of H2O and then added to the Sodium 2-Naphthalenesulfonate solution. A white solid precipitated from the solution, which was then washed three times with 150 mL of water. The white solid was then dried in a desiccator under vacuum, and 2.01 g (yield: 3.45 mmol, 64.05 %) was obtained. The white precipitate was further analyzed via IR spectroscopy to confirm that Fe(2 ns)2 \(\cdot \) 6 H2O was obtained. MIR (in cm\(^{-1}\)): 612(m), 621(m), 645(m), 668(m), 736(w, broad), 758(s), 815(s), 906(w), 943(w), 964(w), 1033 (s), 1091 (m), 1181 (s, broad), 1347 (w), 1503 (m), 1592 (m), 1646 (s), 1670 (w), 1981 (w), 2364 (w, broad), 3061 (w), 3364 (s, broad).
Then, 1.4791 g of the obtained Fe (II)-salt (2.369 mmol) was dissolved in 6 mL of methanol. Separately, 0.6483 g of 4-Amino-1,2,4-Triazole (7.705 mmol) was dissolved in 5 mL of H2O. The solution of the Fe(II)-salt was then added to the 4-Amino-1,2,4-Triazole solution and was stirred for 2 h. Thereby, a pink precipitate was formed. The obtained solid was then purified by dispersing it in ethanol and centrifuging it three times at 6000 rpm for 10 min. The obtained solid was then dried in a desiccator, and 1.616 g was obtained (Yield: 88.8 %). Analytically found (calculated) with CHN elemental analysis for FeC26H26N12O6S2 \(\cdot \) 4 H2O (molar mass 794.597 g mol\(^{-1}\)): C, 38.90 (39.30); H, 4.07 (4.31); N, 21.13 (21.15) MIR (in cm\(^{-1}\)): 474 (m), 502 (m), 552 (s), 560 (s), 568 (s), 622 (s), 647 (m), 675 (s), 748 (s), 768 (w), 819 (s), 865 (s), 906 (s), 944 (m), 956 (m), 981 (w), 1032 (s), 1063 (m), 1093 (s), 1138 (s), 1184 (s), 1271 (s), 1346 (w), 1383 (w), 1446 (m), 1504 (w), 1544 (w), 1593 (w), 1628 (m,broad), 3011 (w), 3060 (m), 3073 (w), 3134 (w), 3163 (m), 3214 (w), 3283 (m,broad), 3498 (m,broad).
2.2.2 Electrospinning of PAN fibers with [Fe(atrz)3](2 ns)2
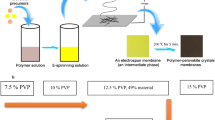
A solution was prepared by solving 0.395 g [Fe(atrz)3](2 ns)2 with 0.254 g l-Ascorbic acid in 7 ml DMF (N,N-Dimethylformamide) and sonicated for 1 h. In a separate solution, 1.012 g PAN is dissolved in 3 mL DMF and stirred overnight for 12 h to obtain a homogeneous solution. Following that both solutions were combined under heavy stirring. The solution was electrospun at 18 kV, with a pumping rate of 0.55 mLh\(^{-1}\), a collector speed of 10 ms\(^{-1}\), a needle diameter of 0.8 mm, and a needle to collector distance of 20 cm at room temperature. This sample is named Polymer complex composite 1 (PCC1). The resulting fiber was collected on an aluminum foil, as schematically shown in Fig. 1.
2.2.3 Electrospinning of PMMA fibers with [Fe(atrz)3](2 ns)2
A solution was prepared by solving 0.402 g [Fe(atrz)3](2 ns)2 with 0.251 g l-Ascorbic acid in 7 ml DMF and sonicated for 1 h. In a separate solution, 1.121 g PMMA is dissolved in 3 mL DMF and stirred overnight for 12 h to obtain a homogeneous solution. Following that both solutions were combined under heavy stirring. The solution was electrospun with the same conditions as the previous fiber. This sample is named Polymer complex composite 2 (PCC2).
2.2.4 Electrospinning of PVP fibers with [Fe(atrz)3](2 ns)2
A solution was prepared by solving 0.403 g [Fe(atrz)3](2 ns)2 with 0.245 g l-Ascorbic acid in 7mL DMF and sonicated for 1 h. In a separate solution, 2.022 g PVP is dissolved in 3 mL DMF and stirred overnight for 12 h to obtain a homogeneous solution. Following that both solutions were combined under heavy stirring. The solution was electrospun with the same conditions as the previous fiber. This sample is named Polymer complex composite 3 (PCC3).
2.3 Characterization
Infrared spectroscopy was performed to gain information about the molecular structure of the complex, polymer and composite. For this, a perkin elmer spectrum two was used with the ATR method between 500 and 4000 cm\(^{-1}\).
The UV/Vis was performed using a lambda 650S from Perkin Elmer from 800 to 250 nm in 1 nm steps. The fiber mats were placed in the reflectance sample holder of the 150 mm integrating sphere with a heater behind it to increase the temperature of the sample up to the spin transition, to determine if the spin transition still occurs. For the SEM images, a Carl Zeiss Supra 55VP was used. For the EDX measurement, an Oxford XMax 80 mm\(^{2}\) was used. The EDX was used to obtain information about the place of the complex in the fiber structure.
Mössbauer measurements were carried out in transmission geometry with a modified miniaturized Mössbauer Spectrometer MIMOS II (Space and Earth Science Instrumentation) at room temperature. The gamma radiation source were \(^{57}\)Co nuclei in a Rh matrix. The measurements were recorded with 14.4 kV and all isomer shifts were given relative to \(\upalpha \)-Fe.
3 Results
3.1 [Fe(atrz)3](2 ns)2
The measured Mössbauer spectrum of the complex before the implementation into the fibers is shown in Fig. 2. The Mössbauer spectrum shows a single clear signal which can be assigned to FeII in the LS state. The measured isomeric shift and quadrupole splitting are listed in Table 1. These measured values are characteristic for FeII-triazole complexes in the LS state which besides the measured IR bonds confirms a successful synthesis of the complex [11]. Even though the measured Mössbauer parameters deviate from literature values for this compound, it can still be assumed that the synthesis was successful [12]. The reason for this is the fact that the given parameters can vary with different water contents in these complexes [11]. In addition, a different synthesis was used for the obtained product, which could have resulted in a different size of the particles obtained and also potentially in a small deviation of the observed Mössbauer parameters. Furthermore, our product was washed three times with ethanol before drying in comparison to known synthesis of this system.
3.2 Electrospun PAN fibers with [Fe(atrz)3](2 ns)2
Through the elctrospinning process, we obtained a pink-colored fiber mat. In Fig. 4, the IR-spectra of the pristine PAN, the SCO compound, and the composite are shown. It is visible that all significant bands, such as the Nitrile-band (2250 cm\(^{-1}\) from the PAN or the fingerprint section of the complex are visible. Additionally, there are no significant shifts noticeable, leading to the assumption that the complex was still present after the electrospinning process.
Furthermore, a Mössbauer spectrum at ambient temperature was measured. The spectrum, shown in Fig. 3 shows the presence of iron(II), proving no oxidation of the complex happened. Two signals were visible in the measured Mössbauer Spectrum. From the measured isomeric shift and quadrupole splitting of both visible signals, which can be found in Table 1, it can be evaluated that the complex stayed intact. The values of both signals indicate iron(II) in HS (42 %) and LS state (58 %) without any signal which suggests oxidation. Therefore, it can be suggested that either some parts of the complex have reacted with the polymer matrix or that the interaction between the polymer and the complex promote a switching of spin states. Both assumptions are supported by the fact that the isomer shift changed and quadrupole splitting of the iron(II) LS signal increased in comparison to the measured value of the complex before the implementation into PAN through electrospinning. The implementation into the polymer significantly decreases the amount of iron in the sample resulting in a lower statistic for the measured signals which can also be stated for the other measured polymer complex composites. From the measured isomeric shift and quadrupole splitting, which values can be found in Table 1, it can be evaluated that the complex stayed intact, as both values are still in the common range for iron(II) triazoles complexes in the LS-state [11].
As the Mössbauer analysis showed us the presence of iron(II) in the LS state in the composite, UV/Vis measurements were done to investigate the spin crossover properties, as a color change occurs during the heating. Figure 4 shows the UV/Vis spectra in the range of 400 . to 700 nm. It is visible that two peaks, one at 480 nm and at 530 nm, are present in PCC1 in the low spin state and both diminish and a small peak forms at 500 nm when measuring at high temperatures. This can be explained by switching the HS and LS state. Therefore, it can be said that the switching still occurs but the color shift is not clearly visible to the human eye.
Lastly SEM and EXD measurements were performed for the sample, to determine the uniformity of the fibers and the distribution of the complex in the fiber structure. Figure 5 shows the results and we could estimate that the complex was in the whole fiber structure compared to previous results where non-solved complexes were used [6]. The fibers showed no beading in their structure and didn’t vary much in their size, with an average diameter of 220 nm.
3.3 Electrospun PMMA fibers with [Fe(atrz)3](2 ns)2
We obtained a pale-pink-colored fiber mat after the electropsinning process. As previously the IR-spectra of the pristine PMMA, the SCO compound, and the composite are shown in Fig. 7. The composite spectrum again shows significant bands of both educts, but the bands of the complex are much weaker, due to the strong PMMA band at 1700 cm\(^{-1}\).
The Mössbauer spectrum also was performed at room temperature to verify potential oxidation or side reactions. The measured spectrum, in Fig. 6, shows two iron(II)-species in the composite. The values for isomeric shift and quadrupole splitting show that a major part of the FeII was in high spin state after the implementation, with a relative area of over 90 % in comparison to the low spin state. Only a rest of the low-spin FeII seems to remain after the implementation. According to the Mössbauer spectrum it can be assumed that most of the complex decomposed during the process
The UV/Vis measurements, visible in Fig. 7, shows a small absorption maximum around 520 nm at room temperature, coming from the small amount of iron in the LS state. At high temperatures, the maximum diminishes leading to the assumption that the complex that stayed in the LS state is still capable of performing the spin state switching.
The SEM and EDX measurements just like for the previous sample show, are visible in Fig. 8, neat fiber without beading. The fibers have two different sizes with 612 nm and 198 nm, which is not ideal for continuous fiber production, but the EDX shows that the iron is distributed equally in the fiber structure.
3.4 Electrospun PVP fibers with [Fe(atrz)3](2 ns)2
We obtained a pink-colored fiber mat after the elctrospinning process. Similar to the previous samples, the IR spectra of pristine PVP, the SCO compound, and the composite are shown in Fig. 10. As for the previous composites, PCC3 also has all the significant bands of both educts, mainly the band at 1700 cm\(^{-1}\) from the PVP and the fingerprint region coming from the complex. The Mössbauer spectrum, performed at ambient temperatures, is visible in Fig. 9. Two signals were detected in the measured spectra that could be assigned to iron(II) in the LS and HS state by characteristic isomeric shifts and quadrupole splitting [11, 13]. Thereby, the majority of the iron(II) was in HS state and only roughly 37% could be assigned to iron(II) in the LS state. It can be assumed that the iron(II) in LS state is the complex that should have been implemented into the polymer. The HS part might be complex and further react with the polymer. The partly successful implementation is also proven by the measured UV/Vis spectrum in Figure UV in which a decrease of an absorption maximum can be observed after heating. This is caused by the spin crossover effect of the compound.
The SEM and EDX results are shown in Fig. 11. It is visible that neat fibers with no beading were produced. Through EDX it is visible that the Iron is well distributed among the fibers. The average fiber diameter is at 162 nm and therefore the smallest fiber from all composites.
4 Conclusion
In this study, we synthesized and characterized a soluble SCO compound, which we then combined with different polymers and produced nanofibers via electrospinning. All composites showed changes in the spin states distribution. The least amount of changes exhibites the composite with PAN as still more than half of the complex remained in the LS state. Additionally the other analytical methods further support the observation from the Mössbauer. PMMA on the other hand showed a strong shift, having over 90% of FeII in the HS state. This is further indicated by the pale pink color of the produced fiber mat. The product still showed weak SCO properties in the UV/VIS. From the Mössbauer from of the PVP composite it is visible that also a high percentage (about 63%) was in the HS state. Therefore, most of the complex must have reacted or interacted with the polymer. Only the remaining iron(II) in LS state seemed to be the original complex. This was also in this case proven by the measured UV/Vis in which a SCO was still visible. Possible interactions are ligand exchanges from the 2ns to, e.g. the nitrile of the PAN structure.
In summary, the best results were possible using PAN as polymer, as most of the complex was still in the LS state after the spinning process. The usage of soluble complexes that retain their abilities after the spinning process is a crucial step in developing new materials, as compared to insoluble and simply dispersed complexes, said soluble complexes are better distributed along the whole fiber [6]. The results lead to the assumption that the used complex either reacted with the polymer or showed an interaction with the polymer matrix, leading to an increase of HS state of the used complex. It is still crucial to keep the spin state as desired and therefore additional research needs to be done. In conclusion, it could be shown that solved molecular switches can be successfully integrated into different polymer fibers, to some degree, depending on the used polymer.
Data Availibility Statement
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary materials.
References
Roubeau, O.: Triazole-based one-dimensional spin-crossover coordination polymers. Chem. Eur. J. 18(48), 15230–15244 (2012)
Renz, F.: Physical and chemical induced spin crossover. In: Journal of physics: conference series, vol. 217, p. 012022 (2010). IOP Publishing
Khan, M.S., Farooq, H., Wittmund, C., Klimke, S., Lachmayer, R., Renz, F., Roth, B.: Polymer optical waveguide sensor based on fe-amino-triazole complex molecular switches. Polymers. 13(2), 195 (2021)
Bogue, R.: Nanosensors: a review of recent research. Sens. Rev. 29(4), 310–315 (2009)
Dreyer, B., Natke, D., Klimke, S., Baskas, S., Sindelar, R., Klingelhöfer, G., Renz, F.: Implementation of spin crossover compounds into electrospun nanofibers. Hyperfine Interact. 239, 1–8 (2018)
Kilic, M.S., Brehme, J., Pawlak, J., Tran, K., Bauer, F.W., Shiga, T., Suzuki, T., Nihei, M., Sindelar, R.F., Renz, F.: Incorporation and deposition of spin crossover materials into and onto electrospun nanofibers. Polymers. 15(10), 2365 (2023)
Nee, A.Y.C.: Handbook of Manufacturing Engineering and Technology. Springer, Hoboken (2014)
Baumgarten, P.K.: Electrostatic spinning of acrylic microfibers. J. Colloid. Interface Sci. 36(1), 71–79 (1971)
Taylor, G.I.: Electrically driven jets. Proc. R. Soc. London A. Math. Phys. Sci. 313(1515), 453–475 (1969)
Bräunlich, I., Sánchez-Ferrer, A., Bauer, M., Schepper, R., Knuüsel, P., Dshemuchadse, J., Mezzenga, R., Caseri, W.: Polynuclear iron (ii)–aminotriazole spincrossover complexes (polymers) in solution. Inorg. Chem. 53(7), 3546–3557 (2014)
Zhao, T., Cuignet, L., Dîrtu, M.M., Wolff, M., Spasojevic, V., Boldog, I., Rotaru, A., Garcia, Y., Janiak, C.: Water effect on the spin-transition behavior of fe (ii) 1, 2, 4-triazole 1d chains embedded in pores of mcm-41. J. Mater. Chem. C. 3(30), 7802–7812 (2015)
Koningsbruggen, P.J., Garcia, Y., Codjovi, E., Lapouyade, R., Kahn, O., Fournes, L., Rabardel, L.: Non-classical fe ii spin-crossover behaviour in polymeric iron (ii) compounds of formula [fe (nh 2 trz) 3] x 2 x h 2 o (nh 2 trz= 4-amino-1, 2, 4-triazole; x= derivatives of naphthalene sulfonate). J. Mater. Chem. 7(10), 2069–2075 (1997)
Varnek, V., Lavrenova, L.: Mössbauer study of the influence of ligands and anions of the second coordination sphere in fe (ii) complexes with 1, 2, 4-triazole and 4-amino-1, 2, 4-triazole on the temperature of the 1 a 1 to 5 t 2 spin transitions. J. Struct. Chem. 36(1), 104–111 (1995)
Acknowledgements
We thank the Nihei Laboratory of the Graduate School of Pure and Applied Sciences of the University of Tsukuba for the elemental analysis measurements. We furthermore thank the Deutsche Forschungsgemeinschaft (DFG), the Hannover School for Nanotechnology (HSN) for the financial support.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors received explicit financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standard
Not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brehme, J., Kilic, M.S., Pawlak, J. et al. Soluble molecular switches in electrospun nanofibers. Hyperfine Interact 245, 10 (2024). https://doi.org/10.1007/s10751-024-01842-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s10751-024-01842-z