Abstract
M-type Barium hexaferrite is a famous hard magnetic material with a hexagonal crystal structure. La substituted Barium hexaferrite, Ba(1-x)La(x)Fe12O19, (where, x = 0.25 and 0.30) were synthesized using ball milling followed by sintering at 1300 °C for 5 hours. X-ray diffraction patterns reveal the formation of a single-phase magnetoplumbite structure of barium hexaferrite, which belongs to the P63/mmc space group. Mossbauer spectroscopic studies were carried out to study the magnetic phase, the effect of La doping on the valency of the Fe atom, and charge distribution in the prepared samples. Mossbauer spectra is fitted using 5 sextets, shows there is 5 sublattices present in the system. From the fitting of Mossbauer spectrum, it is observed that, there is no formation of Fe2+ ion even if the doping levels are as high as 30%, as it is expected that Fe3+ ion changes its valance to maintain the charge neutrality. However, there is an increase in s-electron density for number of sites with doping. Quadrupole splitting values shows spherical charge distribution for all the sublattices except 2b, due to asymmetric nature of bipyramidal site. Slightly varied magnetic hyperfine splitting values are observed for 12 k and 4f1 sites shows the effect of La substitution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hexaferrites are belongs to the ‘ferrite’ family and they possess hexagonal crystal structure. So, they are also known as ‘hexagonal ferrites’. They were discovered in 1950’s in Phillips laboratory [1]. They grabbed the global interest as they show permanent magnetic properties. They are still interesting to explore, since recently they showed room temperature multiferroic properties in Z-ferrites [2]. They also show high curie temperature, high electrical and thermal resistivity and high chemical stability [3].
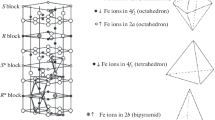
The M-type hexaferrites are the simplest and famous among the different types of hexaferrites. They have the chemical formula, MeFe12O19, where, ‘Me’ is the metal ion. Two such formula units are needed to form a single unit cell. M-type hexaferrites are composed of ‘S’ and ‘R’ block. Where, ‘S’ block is made up of 3 layers of oxygen ions with 4 oxygen in each layer and interstitial sites are occupied by Fe3+ ion. It has a cubic spinal structure. ‘R’ layer is made up of 4 layers of oxygen and one of the oxygen ion in the middle layer is replaced by metal ion such as Ba, Sr, or Pb etc. The interstitial sites created by oxygen ions are occupied by Fe3+ ions. The stacking sequence for single unit cell is SRS*R*, where, ‘*’ represent the 180° rotation along the c-axis. So, total 10 layers of oxygen is needed to form a unit cell. 5 different sub-lattices were formed, which were occupied by 12 Fe3+ ions viz., 12 k, 4f1, 4f2, 2a and 2b. Out of 12 Fe3+ ions, 6 were occupied in 12 k, 2 each in 4f1 and 4f2, and one each in 2a and 2b respectively [3,4,5].
S. Ounnunkad [6] prepared single phase Ba(1-x)La(x)Fe12O19, x = 0–0.20 via citrate combustion process and studied its structural and magnetic properties. X. Liu et al. [7] worked on La substituted M-type Sr ferrite and achieved single phase up to La content x = 0.30 and noticed different secondary phases above x = 0.30. Mossbauer spectroscopic results reveals that substitution of La3+ with Ba2+ sites result in valence change of Fe3+ from Fe2+ at 4f2 or 2a sites. Kumar S. et al. [8] synthesised La doped barium hexaferrite, where La content, x = 0–0.20 by the sol-gel technique. They achieved single phase magnetoplumbite structure, but they observed, La3+ substituted the Fe3+ ions than Ba2+ ions above x > 0.10. S. Verma et.al [9] synthesized Ba(1-x)La(x)Fe12O19, x = 0–0.20 by solid state method and studied magnetic properties. But the prepared samples show secondary phase along with the major magnetoplumbite structure for La substituted compositions(x > 0). These results show effect of La substitution on barium hexaferrite is not easy and not understood very well. Therefore, the present work, a high substitution of La(x = 0.25 and 0.30) were made on Barium hexaferrite using ball milling followed by heat treatment method to study its effect on structural as well as chemical properties of the barium hexaferrite.
2 Experimental methods
La substituted barium hexaferrite Ba(1-x)La(x)Fe12O19, (where x = 0.25 and 0.30) were synthesized using ball milling followed by heat treatment. The stoichiometric amount of precursors BaCO3, α-Fe2O3, and preheated La2O3 were weighed carefully and mixed using an agate mortar and pestle for about 3 hours. Mixed powders are ball milled in a planetary ball mill for 8 hours in an acetone media at a speed of 250 rpm. The ball-to-powder ratio was kept at 8:1. As milled powders were dried, pressed into pellets using a hydraulic press, and sintered at 1150 °C for 5 hours. The sintered pellets were crushed, ground to get fine powders, mixed with 1% PVA, and again pressed into pellets. These pellets were sintered at 1300 °C for 5 hours to get densified compacts.
Powder X-ray diffraction was carried out to study structural properties and analyse phase formation using a powder X-ray diffractometer (Brooker D2 Phaser) with Cu-Kα line as a source in the 2θ range 20° to 80°at scan rate 2°/min and step size 0.02.Mossbauer spectroscopic studies were carried out to analyse the chemical properties such as valency of Fe ion, formation of magnetic phase, charge distribution, magnetic hyperfine splitting values, etc., in the constant acceleration mode and velocity ranges from -12 mm/s to +12 mm/s.
3 Results and discussions
3.1 Structural studies: X-ray diffraction
The X-ray diffraction patterns of La substituted barium hexaferrite, Ba(1-x)La(x)Fe12O19,(x = 0.25 & x = 0.30) were shown in the Fig. 1. X-ray diffraction patterns were indexed with the JCPDF card number 00–043-0002. No unindexed peaks were observed, which shows that the single phase magnetoplumbite structure of barium hexaferrite is achieved for both La content x = 0.25 and x = 0.30 samples. It is also revealed that they crystallize in hexagonal crystal structure and belong to P63/mmc space group.
Crystallite size ‘D’ can be calculated using Sherrer’s formula [10],
where ‘K’ is a constant, ‘λ’ is the wavelength of the source, ‘β’ is full width half maximum and ‘θ’ is the angle.
The lattice parameters, cell volume, c/a ratio, and average crystallite size ‘D’ are listed in Table 1. The lattice parameters and cell volume ‘V’ slightly decreases for La content, x = 0.30 due to the smaller radii of La3+ ion (1.15Ǻ) than Ba2+(1.49Ǻ) ion.
3.2 Mossbauer spectroscopy
Mossbauer spectroscopy was carried out to study the chemical nature, charge distribution, and magnetic phase upon La substitution. Mossbauer spectroscopy was carried out in the velocity range -12 mm/s to +12 mm/s in a constant acceleration mode. The obtained spectrum is tried to fit using the software ‘MossA’ and shown in Fig. 2. Fitted Mossbauer parameters are listed in Table 2. 5 sextets are used to fit the observed data. These five sextets belong to the different sublattices, in which 4f1 is tetrahedral, 12 k, 4f2 and 2a are octahedral, and 2b is trigonal bipyramidal sites.
Isomer shift (IS)(δ)
All the observed isomer shift values are between 0.05 mm/s to 0.5 mm/s, which shows there is no formation of any Fe2+ ion in the system [11, 12]. Isomer shift values of all the sites of the x = 0.25 sample well match the previously reported literature except the 2b site. Lowering of IS values at 2b site shows the increase in s-electron density around the 2b site due to La3+ doping. However, pretty low isomer shift values of x = 0.30 samples are observed. This indicates the increase of s-electron density around 12 k, 4f2, 2a and 2b sites. The observation could be explained as follows.
To maintain the charge neutrality, the doping with La3+ to Ba2+ site, the charge composition occurs with different possibilities. Every ‘x’ amount of La3+ substitution creates a ‘+x’ excess charge per chemical formula (Ba(1-x)La(x)Fe12O19). The conversion of Fe3+ to Fe2+ is most expected. i.e., Fe3+ +e− ➔Fe2+.
To elaborate, 10 chemical formula units have 120Fe3+ ions. Out of 120 Fe3+ ions, conversion of a ‘10x’ amount of Fe2+ ions from Fe3+ ions happen. For instance, for x = 0.30, 10 chemical formula units result in 3 Fe2+ ions. Further, another possibility is the creation of Fe3+ vacancies. i.e., 10 chemical formula units result in 1 Fe3+ vacancy [13]. Similarly, the creation of oxygen vacancies is also possible in the charge compensation process. By observing the IS values, as stated before, there is no formation of Fe2+ions. So, the first possibility could be ruled out. However, one extra electron per formula unit will be created from La3+ substitution, and hopping between Fe3+ to Fe2+ creates one more extra electron [14]. These two extra electrons might be compensated by creating oxygen vacancy (by introducing positive charges into the system from oxygen vacancy). The oxygen vacancies increase the local charge density (s-electron density). The observed results for both samples show an increase in s-electron density, indicating the possibility of the above charge compositing mechanism. The possibility of creating Fe3+ vacancies can’t be ignored, but the presence of the same cannot be detected using the Mossbauer spectrometer.
Quadrupole splitting (QS)
Quadrupole splitting values is the measure of charge distribution in the system. All the observed QS values in both samples lie between 0 and 1 mm/s except 2b site, which shows the spherical charge distribution. Large values of the 2b site is due to the asymmetric charge distribution nature of the trigonal bipyramidal site [15].
Magnetic hyperfine splitting (BHF)(Heff)
BHF values in both the sample show slightly increased values for 12 k site and slightly decreased values for 4f1 sites. BHF values for all the sites are approximately same as reported values. The variation in 12 k and 4f1 sites may due to substitution of La ion, which might cause perturbation in the magnetic field splitting of Fe atom.
4 Conclusions
The x-ray diffraction measurements revealed that a single phase magnetoplumbite structure is formed for both La content x = 0.25 and 0.30. From Mossbauer spectroscopy of x = 0.25 and x = 0.30, we can conclude that there is no formation of Fe2+ ion even if the substitution levels of La are as high as 25% and 30%. However, s-electron density increases around different sites increase with doping. An increase in s-electron density indicates the possibility of the formation of oxygen vacancies, and it increases with doping. The spherical charge distribution is observed except 2b site. Magnetic hyperfine splitting values are slightly varied for 12 k and 4f1 sites.
Data availability
To access any set of data, any one could contact/write to either the first author or the communicating author.
References
Went, J.J.: Ferroxdure. a class of new permanent magnet materials. Phil. Tech. Rev. 13, 194–208 (1952)
Kitagawa, Y., Hiraoka, Y., Honda, T., Ishikura, T., Nakamura, H., Kimura, T.: Low-field magnetoelectric effect at room temperature. Nat. Mater. (2010). https://doi.org/10.1038/nmat2826
Kojima, H.: Fundamental properties of hexagonal ferrites with magnetoplumbite structure. In: Wohlfarth, E.P. (ed.) Ferromagnetic Materials: a Handbook on the Properties of Magnetically Ordered Substances, pp. 303–391. North-Holland, Amsterdam (1982)
Standley, K.J.: Oxide Magnetic Materials, 2nd edn. Oxford University Press, London (1972)
Pullar, R.C.: Hexagonal ferrites: a review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. (2012). https://doi.org/10.1016/j.pmatsci.2012.04.001
Ounnunkad, S.: Improving magnetic properties of barium hexaferrites by La or Pr substitution. Solid State Commun. (2006). https://doi.org/10.1016/j.ssc.2006.03.020
Liu, X., Zhong, W., Yang, S., Yu, Z., Gu, B., Du, Y.: Influences of La3+ substitution on the structure and magnetic properties of M-type strontium ferrites. J. Magn. Magn. Mater. (2002). https://doi.org/10.1016/S0304-8853(01)00914-3
Kumar, S., Manglam, M.K., Supriya, S., Satyapal, H.K., Singh, R.K., Kar, M.: Lattice strain mediated dielectric and magnetic properties in La doped barium hexaferrite. J. Magn. Magn. Mater. (2019). https://doi.org/10.1016/j.jmmm.2018.10.085
Verma, S., Sharma, P., Pandey, O.P., Paesano, A., Sun, A.C.: Structure and magnetic properties of Ba1-xLaxFe12O19 prepared by Ba1-xLaxFe2O4. IEEE Trans. Magn. (2014). https://doi.org/10.1109/TMAG.2013.2278373
Brandon, D., Kaplan, W.D.: Microstructural characterization of Materials (2nd Eds). Wiley pub. (2008)
Rensen, J.G., Van Wieringen, J.S.: Anisotropic Mössbauer fraction and crystal structure of BaFe12O19. Solid State Commun. (1969). https://doi.org/10.1016/0038-1098(69)90502-X
Long, G.J., Grandjean, F.: Mössbauer Spectroscopy Applied to Magnetism and Materials Science, vol. 1. Springer Science & Business Media, Berlin (2013)
Silva, L.M., da Silva, R.B., Silva, R.L., Morales, M.A., de Araújo, J.H.: Improvement of (BH) max in Ba-hexaferrite doped with La and co. Ceram. Int. (2022). https://doi.org/10.1016/j.ceramint.2022.04.306
Alam, M.S., Kagomiya, I., Kakimoto, K.I.: A comprehensive study of structure, oxygen vacancy, and electrical properties of Mg2+ introduced in barium hexaferrite synthesized via spark plasma sintering. J. Solid State Chem. (2022). https://doi.org/10.1016/j.jssc.2022.122976
Ovchinnikov, V.V.: Mössbauer Analysis of Alloys’ Atomic and Magnetic Structure. Cambridge Int Science Publishing (2006)
Acknowledgements
The Author (Nishkala K.R.) is very thankful for Manipal Academy of Higher Education, Manipal for T.M.A. Pai Fellowship (financial support); LACAME 2022 organizers for registration fee waiver; Dr. Sudheendra Rayaprol, UGC-DAE CSR, Mumbai centre for X-Ray diffraction measurements; Dr. V.R. Reddy, UGC-DAE CSR, Indore centre for Mossbauer spectroscopy measurements.
Funding
1. Open access funding provided by Manipal Academy of Higher Education, Manipal.
2. For Author, Nishkala K.R., Manipal Academy of Higher Education, Manipal provided T.M.A. Pai Fellowship.
3. For Author Nishkala K.R. Registration fee waiver was given the to attend LACAME 2022 conference from the organisers of the conference.
Author information
Authors and Affiliations
Contributions
K R, Nishkala: Conceptualisation, Writing-original draft.
Daivajna, Mamatha D: Supervision.
Mutalik, Srinivas: Resources (providing Ball mill Facility).
Rao, Rajat Radhakrishna: Resources (Helping to use Ball Mill Facility).
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
There is no competing interests to disclose.
Financial interests
There is no financial Interests that could affect the research work presented.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Proceedings of the XVII Latin American Conference on the Applications of the Mössbauer Efect (LACAME) is a biennial Conference that has been held since 1988. This XVII LACAME was held in Vitória, Espírito Santo, Brazil, from November 06 - 11, 2022
Edited by Edson C. Passamani
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nishkala, K.R., Rao, R.R., Mutalik, S. et al. Effect of La substitution on the structural and chemical properties of Barium hexaferrite via Mossbauer spectroscopy. Hyperfine Interact 244, 7 (2023). https://doi.org/10.1007/s10751-023-01818-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s10751-023-01818-5