Abstract
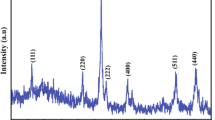
Trivalent iron sulfide (Fe2 S 3) particles were synthesized using a modified polyol method. These particles exhibited a needle-like shape (diameter = 10-50 nm, length = 350-1000 nm) and generated a clear XRD pattern. Mössbauer spectra of the product showed a paramagnetic doublet at room temperature and distributed hyperfine magnetic splitting at low temperature. The Curie temperature of this material was determined to be approximately 60 K. The data suggest that the Fe2 S 3 had a structure similar to that of maghemite (γ-Fe2 O 3) with a lattice constant of a = 10.6 Å. The XRD pattern calculated from this structure was in agreement with the experimental pattern and the calculated hyperfine magnetic field was also equivalent to that observed in the experimental Mössbauer spectrum.
Similar content being viewed by others
References
Chadha, A., Sharma, R.K., Stinespring, C.D., Dadyburjor, D.B.: Iron sulfide catalysts for coal liquefaction prepared using a micellar technique. Ind. Eng. Chem. Res. 35, 2916–2919 (1996)
Hu, H., Bai, J., Zhu, H., Wang, Y., Guo, S., Chen, G.: Catalytic liquefaction of coal with highly dispersed Fe2S3 impregnated in situ. Energy Fuels 15, 830–834 (2001)
Boehm, H.P., Flaig, E.: Iron(III) sulfide. Angew. Chem. Int. Ed. 5, 963–963 (1966)
Yamaguchi, S.: Magnetic iron sulfide of the γ- Al2O3 type. J. Appl. Phys. 44, 1929–2 (1973)
Yamaguchi, S., Wada, H.: Nachweis des Eisensulfids vom Gamma- Al2O3-typ mit Hilfe der Elektronenbeugung. Z. Anal. Chem. 266, 341–342 (1973)
Yamaguchi, S., Wada, H.: Bildung des ferromagnetischen Fe2S3. Z. Anorg. Allg. Chem. 397, 222–224 (1973)
Sugiura, C.: Sulfur K x-ray absorption spectra of FeS, FeS2, and Fe2S3. J. Chem. Phys. 74, 215–4 (1981)
Stiller, A.H., McCormick, B.J., Russell, P.: Existence and stability of a simple sulfide of iron (III). J. Amer. Chem. Soc. 100, 2553–2554 (1978)
Onufrienok, V.V.: Metastable iron sulfides. Inorg. Mater. 41, 650–653 (2005)
Lyubutin, I.S., Starchikov, S.S., Lin, C.-R., Lu, S.-Z., Shaikh, M.O., Funtov, K.O., Dmitrieva, T.V., Ovchinnikov, S.G., Edelman, I.S., Ivantsov, R.: Magnetic, structural, and electronic properties of iron sulfide Fe3S4 nanoparticles synthesized by the polyol mediated process. J Nanopart Res. 15, 1397 (2013)
Shimizu, R., Yamada, Y., Kobayashi, Y.: Liquid phase synthesis of iron sulfide particles. J. Radioanal. Nucl. Chem. 303, 1473–1476 (2014)
Shimizu, R., Kubono, I., Kobayashi, Y., Yamada, Y.: Iron (III) sulfide particles produced by a polyol method. Hyperfine Interact. 231, 115–121 (2015)
Izumi, F., Momma, K.: Three-dimensional visualization in powder diffraction. Solid State Phenom. 130, 15–20 (2007)
Momma, K., Izumi, F.: VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 44, 1272–1276 (2011)
Schwarz, K.: DFT calculations of solids with LAPW and WIEN2k. J. Solid State Chem. 176, 319–328 (2003)
Sumiyama, K., Hirose, Y., Nakamura, Y.: Structural and magnetic properties of nonequilibrium disordered Fe-Al alloys produced by facing target type DC sputtering. J. Phys. Soc. Jpn. 59, 2963–2970 (1990)
Scherrer, A.: The Scherrer formula for X-ray particles size determination. Phys. Rev. 56, 978–982 (1939)
Izumi, F., Momma, K.: Three-dimensional visualization in powder diffraction. Solid State Phenom. 130, 15–20 (2007)
Yamamoto, N.: The particle size dependence of the Néel temperature of α-FeOOH fine particles. Bulletin of the Institute for Chemical Research, Kyoto University 46, 283–288 (1969)
Inoue, M., Hirasawa, I.: The relationship between crystal morphology and XRD peak intensity on CaSO4 ∙ 2H2O. J. Cryst. Growth 380, 169–175 (2013)
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Proceedings of the International Conference on the Applications of the Mössbauer Effect (ICAME 2017), Saint-Petersburg, Russia, 3-8 September 2017
Edited by Valentin Semenov
Rights and permissions
About this article
Cite this article
Kubono, I., Nishida, N., Kobayashi, Y. et al. Mössbauer spectra of iron (III) sulfide particles. Hyperfine Interact 238, 91 (2017). https://doi.org/10.1007/s10751-017-1465-z
Published:
DOI: https://doi.org/10.1007/s10751-017-1465-z