Abstract
Water diversion for hydropower plants is one of the leading causes of habitat alteration and biotic homogenization. The impacts of small hydropower plants on fish communities are usually assessed using taxonomic composition and structure indicators. Size-based indicators are useful tools to evaluate the effects of environmental factors and anthropogenic perturbations on riverine ecosystems. In this study, we investigated the impact of water diversion on fish community body size structure in 16 small hydropower plants in the upper Ter River basin (NE Iberian Peninsula). We tested the use of multiple size-based metrics under different sampling intensities (one- vs. four-pass electrofishing) and compared control vs. diverted stream stretches. Results revealed that water diversion had a negative effect on fish community average and median length, while size spectrum and size diversity metrics showed no responses to the impact. Altitude was positively related with body size metrics and was the strongest natural driver affecting them. Moreover, we found that all the size-based metrics exhibited consistent values under different sampling efforts. Our findings suggest that size-based metrics could be useful indicators for bioassessment of river flow alteration and that one-pass electrofishing was robust enough to characterize the stream fish community size structure in our study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flow alteration due to large dams (≥ 15 m high or with reservoir volume capacity exceeding 3 million m3) (WDC, 2000) and small instream barriers for hydropower plants have been described as one of the main drivers of ecosystem degradation and biodiversity loss in freshwater ecosystems (Poff & Zinnermann, 2010; Dudgeon, 2019). The effects of large hydropower plants (≥ 30 MW) (according to U.S Department of Energy) in rivers have been much more documented than those of small hydropower plants (< 10 MW) (Santos et al., 2012; Couto & Olden, 2018), even though the latter’s impacts on habitat and hydrology may be similar to or stronger than those produced by large hydropower plants (Kibler & Tullos, 2013; Couto et al., 2021). The high occurrence of small instream barriers (WCD, 2000; Belletti et al., 2020) and the rising trend in small hydropower plant construction to supply non-oil-dependent energy (Couto & Olden, 2018) demand more research focused on the assessment of small hydropower plants’ impacts on river ecosystems.
Small hydropower plants have important consequences on river ecosystems including flow regime alteration due to water diversion (Anderson et al., 2015). The streamflow depletion caused by water diversion results in shallower water habitats and a loss of fish refuges downstream of the weir, causing negative impacts on fish community composition and structure (e.g., Kubečka et al., 1997; Tiemann, 2004; Anderson et al., 2006; Slawski et al., 2008; Alexandre & Almeida, 2010; Mueller et al., 2011). Taxonomical metrics that evaluate the community composition and structure (e.g., richness, abundance, diversity, guild/trait group) are commonly used approaches to assess the response of communities to the presence of low-head dams associated to run-of-river small hydropower plants (Fencl et al., 2017; Pereira et al., 2020). However, biological methods that provide a direct link to trophic structure and respond to hydrological pressures and flow modification are demanded (Reyjol et al., 2014; European Commission, 2016).
Body size is defined as a key trait in ecology that integrates metabolic and trophic traits and can evaluate ecosystem functionality (Woodward et al., 2005). Body size structure can be used as an alternative to characterize the structure of fish communities, especially in situations of low taxonomic richness, such as fish communities in Mediterranean streams or headwater reaches (Murphy et al., 2013; Maceda-Veiga et al., 2022) or sites with a paucity of taxonomical or functional knowledge (Benejam et al., 2018). Some of the species-based responses to water diversion can revert to the individual body size, potentially altering the trophic structure of the whole fish community. For instance, previous studies have observed a decrease of large-sized individuals and/or species or an increase in small-sized individuals in diverted reaches (Kubečka et al., 1997; Walters & Post, 2008; Benejam et al., 2016a; Merciai et al., 2017; Boddy et al., 2020). Another detected response is a reduction in fish density and biomass of native species, such as brown trout (Salmo trutta Linnaeus, 1758) (Almodóvar and Nicola., 1999). Even though fish size structure has been extensively used to evaluate ecological impacts on marine (Blanchard et al., 2017) and lake (Emmrich et al., 2011) fish communities, research that apply multiple size-based metrics to evaluate the impacts on stream fish communities are still arising, and to our knowledge, just few studies have already assessed the impact of water diversion for small hydropower plants in stream fish assemblages applying a size-based approach (Walters and Post, 2008; Boddy et al., 2020).
Three main size-based approaches are widely used among aquatic ecologists and can inform different aspects of the community body size structure regardless of species information. First, simple-size metrics as average body length have been shown to respond to anthropogenic perturbations on stream fish (Walters & Post, 2008; Murphy et al., 2013; Benejam et al., 2016a). A second approach is the community size spectrum, which describes the relationship between body size and the abundance or biomass of the individuals in a community (Sheldon, 1972; Platt & Denman, 1977). The size spectrum slope, intercept, and linearity can be considered indicators of the ecological health of ecosystems (Petchey & Belgrano, 2010). The slope can be used as a descriptor of the relative abundance of small- and large-sized individuals in the community and can be related to key ecosystem functions such as the trophic transfer efficiency (Mehner et al., 2018). The intercept can be interpreted as the total production of the community and is a proxy of the community carrying capacity (Sprules & Barth, 2016). The linearity can be an indicator of community stability and can be used to measure the disturbance when compared to a reference (non-impacted) state (Benoît & Rochet, 2004). These three size spectrum metrics have responded to anthropogenic pressures in stream communities including changes in land use urbanization (Benejam et al., 2016b; Sutton & Jones., 2019), flow alteration (Kopf et al., 2018; Vila-Martínez et al., 2019; Boddy et al., 2020), nutrient concentration (Murry & Farrell., 2014; Arranz et al., 2021), or biological invasions (Kopf et al., 2018; Vila-Martínez et al., 2019; Arranz et al., 2021). Third, functional size diversity and evenness have also been applied to evaluate anthropogenic impacts in rivers, such as changes in land and urban cover (Benejam et al., 2016b; Sutton & Jones, 2019; Jiménez-Prado & Arranz, 2021), by accounting for the intraspecific size variability within a community. Taken together, using multiple size-based metrics to represent the fish size structure at the community level could contribute to potentially assessing the impacts of water diversion on the fish trophic structure.
The present study aimed to evaluate the effects of water diversion by small hydropower plants on stream fish communities by using multiple size-based metrics under different sampling intensities. To do this, we used a fish data documenting 36 fish surveys in the upper Ter River basin (NE Iberian Peninsula) sampled in 16 small hydropower plants in 2010. Specifically, we evaluated the impact of water diversion on fish body size structure over the influence of environmental gradients (Objective 1) and the influence of different sampling efforts on the fish size-based metrics (Objective 2). We hypothesized that the size structure of the fish community would be altered in impacted sites with respect to non-impacted sites due to water diversion (Kubečka et al., 1997; Benejam et al., 2016a; Boddy et al., 2020) (Hypothesis 1). This alteration would result in (i) a lower average, median, and maximum fish body length; (ii) a steepening of size spectrum slope, a decline in community carrying capacity (Y-axis size spectrum intercept), and a decrease in community stability (size spectrum linearity); and (iii) a decrease on functional size diversity (less size diversity and evenness). Moreover, we hypothesized that the body size structure of fish communities will be robust regardless of the sampling effort intensity, resulting in no significant changes in size-based metrics when using successive electrofishing passes (Sutton & Jones, 2019) (Hypothesis 2).
Materials and methods
Study site
The Ter River (Catalonia, NE Iberian Peninsula) rises in the eastern Pyrenees mountains and flows into the Mediterranean Sea. It is partially subject to a snow-fed regime and influenced by the continental wet Mediterranean climate in its upper section and by Mediterranean climate fluctuations in the lower regions (Boix et al., 2010; Benejam et al., 2016a). The drainage area is artificially divided by the Sau and Susqueda reservoirs into two subbasins: the upper Ter basin (1799 km2) and the lower Ter basin (1156 km2). The study site is located in the upper Ter River basin where the river has good water quality (Espinosa et al., 2021) but holds a long-standing thread of instream barriers that alters the flow regime, increasing the risk of non-compliance with Water Framework Directive on environmental flows (ACA, 2010). Most of the weirs and barriers of the upper Ter basin were built in the 19th and early twentieth century and were associated with small hydroelectric plants that supplied energy for textile industries, small urban areas, and industrial colonies. Currently, there are 133 documented barriers in the upper Ter basin (ACA, 2022) and 85 operating small hydropower plants that provide electric energy to be commercialized (ACA, 2010). The main criterion for the selection of the small hydropower plants for the study was the presence of a control stream stretch to be compared with the diverted stream stretch (see further details in Study design). We excluded any study site under the influence of discharge from wastewater treatment plants’ effluents. Moreover, we assured that no stocked populations of brown trout were present in the study sites. Although recreational fishing may influence the population size structure of brown trout (Almodóvar & Niccola, 2004), we did not evaluate the effects of recreational fishing because most sites occurred in protected freshwater areas where fishing without release is forbidden (ACA, 2022b; DOGC, 2023). Only three sites at low altitudes (< 639 m.a.s.l) were located in areas not excluded from fishing (site 13, 14, and 15) (Fig. 1a). Moreover, the population size of brown trout in these sites was considerably low (between 3 and 17 individuals in control stretches and between 5 and 26 individuals in diverted stretches) by the fact that brown trout occur in the limit of their geographical distribution (Aparicio, 2016).
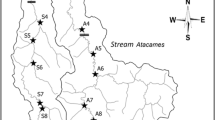
a Location of Ter River in Europe (in blue) and geographical position of the small hydropower plants within the Ter River Basin (Catalonia, NE Iberian Peninsula). The small hydropower plants are marked in orange dots and are mainly located in the headwater parts of the upper Ter River Basin, upstream Sau and Susqueda reservoirs (the blue arrow indicates the flow direction), b General schematic representation of the sampling design in each site (small hydropower plant). The sampled stream stretches were located at non-impacted reaches (control sampled stretches) and water diversion-impacted reaches (diverted sampled stretches) for each small hydropower plant. Control sampled stretches (100 m) were located in reaches with natural flows, usually upstream of the weir and always above the impounded reach. Diverted sampled stretches (100 m) were downstream of the weir, in water-depleted reach, below the scour pool formed at weir base and above tailrace outlet, to avoid potential hydropeaking effects
In total, we selected 16 small hydropower plants in several headwater tributaries and the mainstem of the Ter River (Fig. 1a). All were run-of-river hydropower plants without reservoirs and with small weirs (< 15 m) created to divert water through a channel to supply energy for electricity production. The altitudinal distribution ranges from 434 to 1,246 m.a.s.l. The average weir height across all small hydropower plants was 3.3 m (from 1.1 to 9 m). Water diversion causes an important reduction in streamflow below weirs in which the water-depleted reaches had an average length of 2 km (from 0.5 to 7 km) (Table of the characteristics of the small hydropower plants and weirs on Online Resource 1). All the hydropower plants selected for the study generate less than 10 MW (from 37 to 2847 kW), considered small hydropower plants (but this threshold may vary among countries, Couto & Olden, 2018).
Study design
Two stream stretches, one control and one diverted, were sampled at each of the 16 small hydropower plants. The control stream stretches were located out of the influence of the small hydropower plants, in natural flow reaches. The diverted stream stretches were sampled in the water-depleted section, situated below the scour pool formed at weir base but above the tailrace outlet, where water is returned after electricity production, to avoid the potential hydropeaking effect (Fig. 1b). In addition, at four small hydropower plants (1, 2, 3, and 5) one control or diverted sampling stretch was included upstream and downstream of the weir (Table of the characteristics of the sampled stream stretches on Online Resource 2). These additional sampling stream stretches were added when the control or diverted reach was long enough to perform an extra sampling stretch while maintaining similar habitat condition (Benejam et al. 2016a). We treated these additional samplings as independent control and diverted samples to increase sample size and statistical power in further statistical analyses. Thus, in total there were 18 control stretches and 18 diverted stretches. In each sampled stream stretch, the average depth (cm) was estimated from measures of depth in 10 sections along the 100-m sampled stream stretches.
Fish sampling
One sampling was carried out in each stream stretch in 2010, from July to September, to avoid the influence of spawning events. The fish sampling consisted of 100-m transects from downstream to upstream using the electrofishing technique (250–350 V, 2–3 A, fully rectified triphasic DC). A single electrofishing pass without block nets was conducted at most of the sites, following the European standard (CEN, 2005). The sampling was performed using one or two anodes according to the stream width. All stream stretches were sampled by the same electrofishing crew, which maintained a consistent sampling effort. Consequently, sampling stretches with larger areas required more time to sample than those with smaller total area. Stunned fish were collected with dip nets; all caught individuals were identified to species, measured (fork length, mm), and weighed (0.1 g precision). Fish were returned to the same stream stretch where they were sampled.
To assess the influence of sampling effort on the fish community size structure, we performed a removal fish sampling method (Peterson et al., 2004) in a subset of 16 stream stretches: 7 control and 9 diverted stretches (Table of the characteristics of the sampled stream stretches on Online Resource 2). The method requires 3–4 consecutive electrofishing passes (with 30-min non-fishing intervals) in a restricted area blocked by nets (5 mm mesh size), and applying the same time/effort for each electrofishing pass. The caught fish were identified, measured, and weighed separately for each electrofishing pass. The application of the removal method allowed to compare the size metrics (average, median, and maximum length, slope, intercept, and linearity of the size spectrum, and functional size diversity and evenness) across the first, second, third, and fourth electrofishing passes. This procedure aimed to determine whether increasing sampling effort could entail changes on body size metrics.
Calculation of size-based metrics
We integrated several body size measures for fish weight and length to ensure that the variation in fish size structure along the environmental gradient is comprehensively captured. We employed fish lengths for calculating size diversity metrics because previous research demonstrated significant response of length size diversity to environmental gradients (Emmrich et al. 2011). Instead, we used fish body mass for calculating size spectrum parameters because it is a metric more related to energy fluxes (Andersen, 2019). Simple-size metrics were represented by the geometric average length (mm), median length (mm), and maximum length (mm) in each fish community. We also calculated the community fish size spectrum for each stream stretch. To do this, we first classified the fish body mass into seven size classes following a geometric series of 2 (Table of size classes on Online Resource 3). The width of the size classes increased progressively, so the larger they were, the wider the size class. Given that the electrofishing method is less efficient to catch small-sized individuals, we grouped the smallest individuals into the first size class, as has been done in other fish studies in the same geographical area (Arranz et al., 2021). Because the width of the size classes is not constant across the size distribution, we normalized the fish abundance of each size class by dividing it by the width of each size interval (Sprules & Barth, 2016). The size spectrum parameters were obtained by an ordinary-least-square (OLS) regression between the log2 fish normalized abundance (Y-axis) and the log2 midpoint of individual size classes (X-axis) (formally referred to as Normalized Abundance Size Spectrum—NASS; Sprules & Barth, 2016). Statistical artifacts emerged from the strong correlation between intercept and slope (Sprules & Barth, 2016). To remove slope effects from intercept effects, the ordinary-least-square regression for each location was centered in the midpoint of the mass range (X-axis): the midpoints of the size intervals used in the calculations were fixed at X = 0. This transformation allowed us to calculate the slopes of the regression without any constraints on the intercept (Sprules & Barth, 2016). We obtained the slope (b), the Y-axis intercept (or midpoint height), and the coefficient of regression (R2) for each sampled community. Finally, we calculated two functional size diversity metrics in each stream stretch. The size diversity (µ) and size evenness (Je) were determined using the length of fish individuals following the method described by Quintana and colleagues (Quintana et al., 2008, 2016). These metrics provide insights into the body size distribution and equitability within the community. Higher values of µ indicate that the fish community will be represented by a high size diversity and high values of Je indicate that the body size distribution follows a homogeneous distribution (Quintana et al. 2016).
Statistical analysis
We applied Generalized Linear Mixed Models (GLMMs) to test the water diversion impacts on fish body size structure (Objective 1). Stream stretches were nested within small hydropower plants, so we used GLMMs to account for the hierarchical structure of fish data. The GLMMs can allow to treat stream stretches as independent observations while controlling the variation that it could be within the hydropower plants. We also incorporated the influence of environmental gradients in the GLMM because changes in size structure can also be affected by environmental factors. Therefore, the fixed part of the model was composed of the two continuous predictors, altitude (m.a.s.l) and mean depth (m), and the two discrete predictors, stream order and water diversion impact. We tested the collinearity for each predictor by using the variance inflation factor (vif values) and the collinearity was low (< 3), indicating that these predictors could be applied to the analysis. We carried out a previous standardization of the continuous predictors to standardize the units for comparison among model coefficients. Additionally, we checked for correlation within variables to reduce the number of variables and their multicollinearity in the models, but all predictors showed low correlation values (Pearson’s correlation < 0.7). We also tested spatial autocorrelation by evaluating the relationship between body size metrics and the geographical distance among sites using Mantel correlogram tests with 999 permutations (Smouse et al., 1986). Spatial autocorrelation was performed with the package ecodist (Legendre & Fortin, 1989) through the R environment. In all cases, we did not detect any significant effect of the spatial autocorrelation on all size-based measures (Mantel correlogram test; p-value < 0.05). Then, we performed eight GLMMs for each size-based metric that corresponded to (i) simple-size metrics (i.e., length average, median, and maximum), (ii) size spectrum metrics (i.e., slope, intercept, and linearity), and (iii) functional size diversity metrics (i.e., size diversity and evenness). We considered small hydropower plant as a random effect in the model to treat control stretches and diverted stretches as independent stream locations. For each size-based metric, we compared different GLMMs by removing variables one by one from the fixed part (i.e., stepwise backward selection) while maintaining the random part. The final (best) fixed structure was determined as the model with the lowest AIC value (Akaike, 1974) (Details on the candidate models on Online Resource 4). Finally, we performed a Kruskal‒Wallis nonparametric test to compare the size-based metrics according to the sampling effort (from one electrofishing pass to four passes) (Objective 2). All statistical analyses were performed using R version 4.3.1 software (R Core Team 2023).
Results
Community composition and size structure
A total of five fish species were identified with a total abundance of 6,029 individuals in the 36 sampled stream stretches (18 control and 18 diverted stream stretches). The fish community composition was represented by two native species, brown trout (60.5% of the total abundance) and Mediterranean barbel (Barbus meridionalis Risso, 1826; 7.1% of the total abundance). But the fish community was also compounded by three non-native species Phoxinus sp. (27.1%), Barbatula sp. (5.3%), and common carp (Cyprinus carpio Linnaeus, 1758, 0.1%). Brown trout was present in all the samples and was the only species found in 23 of the 36 sampled stream stretches. Mediterranean barbel was at 16 of the stream stretches, followed by Phoxinus sp. and Barbatula sp. at 12 and 11 stream stretches, respectively. The least frequently occurring species was common carp, which was sampled in only two locations. All stream stretches that belonged to headwater tributaries had a community entirely represented by native species, while non-native species appeared in most of the mainstem stretches located in lower sections of the study site (Fig. 2 and Size variability among species on Online Resource 5).
Fish counts by species in each sampling stream stretch, along the altitudinal gradient. Sites are sorted by altitude ranging from site 1 at 1246 m.a.s.l to site 16 at 434 m.a.s.l and indicating control and diverted stretches. (Barbatula and Phoxinus are not named at species level because its determination is pending of genetic studies in some catchments of NE Iberian Peninsula)
Size-based metrics differed among the stream stretches. Community average lengths ranged from 50.5 to 177.2 mm (112.9 ± 31.4 mm), community median lengths ranged from 41.0 to 172.0 mm (104.2 ± 35.5 mm), and community maximum lengths ranged from 138.3 to 485.0 mm (258.7 ± 60.3 mm) (Values for each size-based metric on Online Resource 6). The size spectrum parameters also showed high spatial variability in the size spectrum slopes from -4.3 to -1.0 (-2.0 ± 0.5) and size spectrum intercept from -4.17 to 0.8 (-1.6 ± 1.0), but almost all stream stretches presented high size spectrum linearity from 0.5 to 0.99 (0.8 ± 1.1), validating the robustness of our approach (Values for each size-based metric on Online Resource 6). Finally, functional size measures varied consistently with size diversity ranging from 1.1 to 2.8 (2.0 ± 0.3) and size evenness ranging from 0.7 to 1.0 (0.8 ± 0.1) (Values for each size-based metric on Online Resource 6).
Drivers of community size structure
Our results showed that the flow reduction due to water diversion had a significant negative effect on the average (β = -0.206; t-value = -2.189; p-value < 0.01; 95% CI [− 0.395, − 0.017]) and median fish length (β = -0.248; t-value = -2.435; p-value < 0.01; 95% CI [− 0.453, − 0.043]), suggesting that fish on diverted stream stretches presented smaller sizes (Table 1 and Fig. 3). However, the results did not show any significant effect of water diversion on maximum length or other size-based metrics including the size spectrum (slopes, intercept, and linearity) and functional size diversity (size diversity and size evenness) (Table 1).
Concerning the effects of the environmental variables on community size structure, our analysis revealed that altitude was significantly positively associated with most of the size-based metrics (Table 1). Fish community average length (β = 0.681; t-value = 4.649; p-value < 0.001; 95% CI [0.386, 0.975]), median length (β = 0.634; t-value = 4.251; p-value < 0.001; 95% CI [0.333, 0.934]), and maximum length (β = 0.474; t-value = 2.491; p-value ≤ 0.05; 95% CI [0.091, 0.858]) significantly increased with altitude (Table 1 and Fig. 4), suggesting that communities at higher altitudes presented higher abundances of large-sized individuals than those in lower altitudes. Altitude also affected the size spectrum slopes (β = 0.344; t-value = 2.127; p-value ≤ 0.05; 95% CI [0.015, 0.673]) with flattered slopes at higher altitude, indicating a relative increase of large-sized individuals at higher altitudes (Table 1 and Fig. 4). Finally, size spectrum linearity (β = 0.464; t-value = 3.117; p-value < 0.01; 95% CI [0.164, 0.765]) positively responded to altitude, indicating that communities at higher altitudes were more stable and well-sized structured (Table 1 and Fig. 4). Furthermore, functional size diversity and the size spectrum intercept did not present any evidence to be influenced by altitude. Regarding the other analyzed environmental variables, our results did not reveal any evidence that stream order and mean depth influence any of the studied size-based metrics (Table 1).
Assessing sampling effort
The frequency distributions of size-based metrics showed minimal differences when comparing increasing sampling intensity (from one to four electrofishing passes) (Fig. 5). Moreover, we found no significant differences for any of the size-based metrics when applying the Kruskal–Wallis test to compare from one to four electrofishing passes (Table 2), suggesting that there was no influence of sampling effort on the determination of the fish community size structure. These results indicate that one-pass electrofishing could be enough to determine the size structure of the studied communities.
Frequency distributions of size-based metrics according to the number of passes from electrofishing for the subset of 16 stream locations where more than one electrofishing pass was carried out. Y-axes are the number of electrofishing passes and X-axes indicate the values of each size-based metric. Colors on distributions indicate the number of electrofishing passes (from one to four passes; from blue, yellow, orange to brown, respectively) when fish individuals were caught
Discussion
The novelty of this work arises from the use of multiple size-based metrics, instead of quantifying the impacts of water diversion on community structure and taxonomic composition (e.g., Kubečka et al., 1997; Cumming, 2004; Anderson et al., 2006; Poulet, 2007; Jumani et al., 2018). Our results showed that water diversion had a negative effect on fish community size structure as evidenced by a decrease in community average and median fish lengths in diverted stream stretches (Hypothesis 1-i). However, size spectra and functional size diversity showed no response to water diversion, contrary to our hypothesis (Hypothesis 1-ii, iii). Furthermore, results indicated that the altitudinal gradient was the most important natural driver shaping the community size structure, with a higher proportion of small-bodied individuals inhabiting the lower sampled stream stretches. Finally, the values of size-based metrics did not differ under different sampling efforts, suggesting that community size structure could be characterized with one-pass electrofishing sampling, matching with Hypothesis 2.
Our results were consistent with previous research assessing the impact of water diversion in fish body size (Kubečka et al., 1997; Walters & Post, 2008; Benejam et al., 2016a; Merciai et al., 2017), suggesting that community average length may be a useful indicator for this impact. The decline in community average body length in diverted stream stretches could be explained by the modifications in stream hydromorphology. Diverted stream stretches in the study area presented less maximum water depth, less fish refuges, lower abundance of riffles, higher abundance of pools, and more streambed exposition (Benejam et al., 2016a), which are characteristics frequently associated to water diversion (Kubečka et al., 1997; Gillette et al., 2005; Anderson et al., 2006; Mueller et al., 2011; Kuriqi et al., 2021). Water diversion can cause low-flow conditions that involve a reduction of aquatic physical habitat, habitat heterogeneity, resource availability, and changes on water velocity that can lead to less opportunities to large-bodied individuals (Rolls et al., 2012; Lake, 2003; Walters, 2016). For example, previous works reported that the survival of larger individuals of wild brook trout [Salvelinus fontinalis (Mitchill 1814)] was reduced in extreme summer low-flow conditions in small tributaries while smaller individuals were not affected (Xu et al., 2010). When the available aquatic habitat is reduced, large-bodied individuals are more likely to experience respiratory stress because they have more oxygen demands (Magalhães, 1993; Elliott, 2000). Moreover, large-bodied fish can appear more vulnerable to predators such as mammals or birds in shallower habitats where large fish have difficulties to find refugia (Power, 1987; Harvey & Stewart, 1991; Magalhães, 1993). Besides, the higher mobility of large-bodied individuals potentially enables them to move to other reaches when flow permanence is reduced (Young, 1994; Davey & Kelly, 2007). The loss of large-bodied fish could be also associated to an increase of small-sized individuals’ abundance due to a decrease on fish predation pressure. For example, in headwater streams from Australia, small native species expanded or contracted their geographical distributions corresponding with the absence or presence of the predator non-native brown trout, which totally disappeared in streams with flow reduction (Closs & Lake, 1996). These results could also be related with the environmental filtering theory (Weiher et al., 1998). Previous works found that water velocity and habitat structural complexity act as universal environmental filters (Bower & Winemiller, 2019), and that the filters that tend to favor small body sizes (e.g., shallow water depths and scarcity of large shelters) are predominantly responsible for shaping species distributions in more isolated small streams (1–4 Strahler order) (Schmera et al., 2017; Oliveira et al., 2022). Taken together, large-bodied individuals may be less able to succeed in diverted stream stretches, which could be reflected in our results through a reduction of fish community average and median length. Yet, our results showed a lack of response to water diversion in size spectrum and size diversity metrics. This suggests that although these size-based metrics can reflect changes related to trophic structure, the impact of water diversion may be insufficient to cause changes on the trophic structure. In contrast, previous research found that habitat alteration in diverted reaches caused the loss of large-sized species (brown trout) but an increase of small-bodied fishes’ abundances that led to steeper size spectrum slopes (Boddy et al., 2020). These changes in size spectrum slopes were explained because, despite basal resource not being affected by flow reduction, the total absence of brown trout (top predator) caused an alteration of trophic interactions (Boddy et al., 2020).
Altitude was the strongest natural driver that affected most of the size-based metrics. Stream fish communities are structured along environmental gradients (Osborne & Wiley, 1992; Magalhães et al., 2002; Benejam et al., 2018). The relationship between altitude and body size is usually negative because headwaters can support small-sized individuals, while lower sectors, deeper, more stable, and productive, can sustain older age groups and large-sized individuals (Schlosser, 1982; Larscheid & Hubert, 1992). In our study, the positive relationship between altitude and body size metrics could be explained by the high relative abundance of small-bodied non-native species such as Phoxinus sp. or Barbatula sp. in lower stream stretches and the absence of these fish species in upper stream stretches, where only brown trout was present. Phoxinus sp. can reach 100 mm as maximum length (Aparicio, 2016; in our dataset, a mean of 54.4 ± 12.8 mm) and Barbatula sp. 100–120 mm (Aparicio, 2016; in our dataset, a mean of 63.9 ± 11.1 mm), while brown trout in NE Iberian Peninsula can reach sizes up to 700 mm but just in large, productive rivers, lakes, and reservoirs (Aparicio, 2016; in our dataset, a mean of 130.5 ± 49.9 mm).
We cannot ignore the potential influence of cumulative effects of small hydropower plants, as it has been studied for large dams. These effects are reflected in hydrological alteration, habitat loss, and water quality degradation (Alexandre & Almeida, 2010; Kibler & Tullos, 2013; Timpe & Kaplan, 2017; Jumani et al., 2020; Morden et al., 2022). Another associated impact is the habitat fragmentation, limiting organisms’ dispersal, fish migrations, and reducing populations’ connectivity. Those effects can lead to genetic loss, local extinction and may alter life-history strategies and ecosystem functioning (Santucci, et al., 2005; Sheer & Steel, 2006; Lange et al., 2018; Couto et al., 2021). However, in our study site, there exists a strong correlation between the accumulated number of small hydropower plants and altitude (Pearson’s correlation = 0.91; p-value < 0.001). This high correlation does not allow us to separate the single effects of altitude from the cumulative effects, as we are unable to make comparisons with stream sites unaffected by small hydropower plants in an undammed stream. Concurrently to cumulative effects, the potential increasing anthropogenic pressures in stream stretches at lower altitudes (e.g., changes in land use or pollution) could revert on the body size patterns and condition of fish individuals (Benejam et al., 2016b; Sutton & Jones, 2019). Previous work with the same data base and study site found that the condition of brown trout was lower in diverted stream stretches and decreased in lower sites (Benejam et al., 2016a). The upper sections of the catchment, with less potential anthropogenic pressures, could support less disturbed habitats that favor stable communities characterized by high abundance of small individuals and low abundance of larger individuals. But the cumulative effects of downstream barriers may have played a role in the reduction of small-sized individuals at higher stream stretches in the study site. We found higher values of size spectrum linearity at higher sites, indicating that the fish community was more stable, and this is related to less perturbed habitats. Contrarily, the lower values of linearity on the lower sites could be a reflect of a more perturbed or less stable habitat (Benoît et al., 2004; Arranz et al., 2019). Non-salmonid non-native species tend to primarily colonize rivers with low-altitude and low-discharge in the southern part of the European Alps region (Niedrist et al., 2023), as we found in our study site. The higher abundance of small-sized non-native individuals could be also a consequence of habitat impoverishment, especially as a response to low flows since non-native fish often are tolerant species and occur in more degraded sites, as dammed rivers (Marchetti et al., 2004; Maceda-Veiga et al., 2017). Moreover, the presence of weirs in the mainstem and tributaries and the loss of discharge due to water diversion can block or disrupt upstream fish movements (Ovidio & Philippart, 2002; Lake, 2003; Gosset et al., 2006; Gavioli et al. 2018). Such movement constraints could be increased when there are cumulated barriers; while fish may be able to overcome single barriers, passage over multiple barriers is not guaranteed because it is energetically costly (Jones et al., 2021). Thus, the small-bodied non-native species, which have limited abilities to overcome obstacles (Ordeix et al., 2011; Jones et al., 2021), are more likely to be found in lower sections of the basin, thus contributing to the alteration of the natural patterns of body size structure along environmental gradients.
We found that all size-based metrics did not vary under different sampling effort. This suggest that, although electrofishing sampling can show biases associated to individual body size (Bayley & Dowling, 1993; Anderson, 1995; Dolan & Miranda, 2003; Peterson et al., 2004), the size structure of the studied fish communities could be described with one-pass electrofishing. Similar results were found in Ontario streams, where the mean size decreased at increasing sampling intensity but not the size range, the size diversity, and the size evenness (Sutton & Jones, 2019). Also, in New Zealand headwater streams, there was a high correlation when comparing size range and maximum size for one and three electrofishing passes, suggesting that single-pass electrofishing was valid and robust for measuring size structure (Fraley et al., 2018). Other studies have found that the efficiency of electrofishing was not influenced by body size under appropriate environmental conditions including conductivity or depth in streams (Allard, 2014) and temperature in reservoirs (Bodine et al., 2011). On the other hand, other authors detected that a single electrofishing pass is insufficient to accurately represent the community size structure, particularly when comparing streams with different conductivity (Pusey et al., 1998). Other works have proposed models to account for size bias in electrofishing, as this can affect estimates of biomass (Richter et al., 2022). Taking into consideration that the European Community guidelines advise that size-based indicators should be included in river monitoring (European Commission, 2016), our results in headwater stream stretches showed evidence that one-pass sampling can fully represent fish community size structure. This would be beneficial in fish biomonitoring allowing to cover large temporal and spatial variability and reducing costs and efforts. However, these results are restricted to be applied to streams with similar size and abiotic attributes, where capture efficiency would be comparable.
Conclusion
Run-of-river hydropower plants are affecting a large number of streams and rivers. Consequently, water diversion in streams is causing significant modifications to river habitats and fish communities (Carlisle et al., 2011; Dudgeon et al., 2019). Under global change scenarios with increasing drought episodes, water diversion could exacerbate the negative effects of flow reduction in fish community size structure, leading to negative impacts on both biodiversity and ecosystem processes (Xenopoulos et al., 2005; Darwall & Smith, 2006; Ledger et al., 2013; Carosi et al., 2019). Thus, by incorporating body size approaches together with taxonomical approaches, we can gain comprehensive understanding of how small hydropower plants can impact river ecological status and identify potential mechanisms behind changes in food web structure. However, further research in trophic relationships is needed to provide insights to better understand the mechanisms involved in the impact of small hydropower plants on fish communities. Implementing body size approaches for river assessment can offer useful complementary tools to quantify and understand the ecosystem response to water diversion and habitat fragmentation, improving the success of stream conservation and restoration programs.
Data availability
The dataset generated during and/or analyzed during the current study is available from the corresponding author on reasonable request.
References
ACA (Agència Catalana de l’Aigua), 2010. Report of environmental sustainability the zonal plan for implementing environmental flows in the upper basin of Ter River. Barcelona: Generalitat de Catalunya.
ACA (Agència Catalana de l’Aigua), 2022. Report of Longitudinal connectivity. Structures present in the rivers of the Internal Basins of Catalonia and improvement actions.
ACA (Catalan Water Agency), 2022b. Management plan for the Catalan river basin district 2022–2027.
Akaike, H., 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control 19: 716–723. https://doi.org/10.1109/TAC.1974.1100705.
Alexandre, C. M. & P. R. Almeida, 2010. The impact of small physical obstacles on the structure of freshwater fish assemblages. River Research and Applications 26: 977–994. https://doi.org/10.1002/rra.1308.
Allard, L., G. Grenouillet, K. Khazraie, L. Tudesque, R. Vigouroux & S. Brosse, 2014. Electrofishing efficiency in low conductivity neotropical streams: towards a non-destructive fish sampling method. Fisheries Management and Ecology 21: 234–243. https://doi.org/10.1111/fme.12071.
Almodóvar, A. & G. G. Nicola, 1999. Effects of a small hydropower station upon brown trout Salmo trutta L. in the River Hoz Seca (Tagus basin, Spain) one year after regulation. Regulated Rivers: Research & Management 15: 477–484. https://doi.org/10.1002/(SICI)1099-1646(199909/10)15:5%3c477::AID-RRR560%3e3.0.CO;2-B.
Almodóvar, A. & G. G. Nicola, 2004. Angling impact on conservation of Spanish stream-dwelling brown trout Salmo trutta. Fisheries Management and Ecology 11: 173–182. https://doi.org/10.1111/j.1365-2400.2004.00402.x.
Andersen, K. H., 2019. Fish ecology, evolution, and exploitation: a new theoretical synthesis, Monographs in population biology, Princeton University Press, Princeton:
Anderson, C. S., 1995. Measuring and Correcting for Size Selection in Electrofishing Mark-Recapture Experiments. Transactions of the American Fisheries Society 124: 663–676. https://doi.org/10.1577/1548-8659(1995)124%3c0663:MACFSS%3e2.3.CO;2.
Anderson, E. P., M. C. Freeman & C. M. Pringle, 2006. Ecological consequences of hydropower development in Central America: impacts of small dams and water diversion on neotropical stream fish assemblages. River Research and Applications 22: 397–411. https://doi.org/10.1002/rra.899.
Anderson, D., H. Moggridge, P. Warren & J. Shucksmith, 2015. The impacts of “run-of river” hydropower on the physical and ecological condition of rivers. Water and Environment Journal 29: 268–276. https://doi.org/10.1111/wej.12101.
Aparicio, E., 2016. Peixos continentals de Catalunya. Lynx editions.
Arranz, I., C. H. Hsieh, T. Mehner & S. Brucet, 2019. Systematic deviations from linear size spectra of lake fish communities are correlated with predator–prey interactions and lake-use intensity. Oikos 128: 33–44. https://doi.org/10.1111/oik.05355.
Arranz, I., S. Brucet, M. Bartrons, C. García-Comas & L. Benejam, 2021. Fish size spectra are affected by nutrient concentration and relative abundance of non-native species across streams of the NE Iberian Peninsula. Science of Total Environment 795: 148792. https://doi.org/10.1016/j.scitotenv.2021.148792.
Bayley, P. B. & D. C. Dowling, 1993. The effect of habitat in biasing fish abundance and species richness estimates when using various sampling methods in streams. Polskie Archiwum Hydrobiologii 40: 5–14.
Belletti, B., C. Garcia de Leaniz, J. Jones, S. Bizzi, L. Börger, G. Segura, A. Castelletti, W. Van de Bund, K. Aarestrup, J. Barry, K. Belka, A. Berkhuysen, K. Birnie-Gauvin, M. Bussettini, M. Carolli, S. Consuegra, E. Dopico, T. Feierfeil, S. Fernández, P. Fernandez Garrido, E. Garcia-Vazquez, S. Garrido, G. Giannico, P. Gough, N. Jepsen, P. E. Jones, P. Kemp, J. Kerr, J. King, M. Łapińska, G. Lázaro, M. C. Lucas, L. Marcello, P. Martin, P. McGinnity, J. O’Hanley, R. Olivo del Amo, P. Parasiewicz, M. Pusch, G. Rincon, C. Rodriguez, J. Royte, C. T. Schneider, J. S. Tummers, S. Vallesi, A. Vowles, E. Verspoor, H. Wanningen, K. M. Wantzen, L. Wildman & M. Zalewski, 2020. More than one million barriers fragment Europe’s rivers. Nature 588: 436–441. https://doi.org/10.1038/s41586-020-3005-2.
Benejam, L., S. Saura-Mas, M. Bardina, C. Solà, A. Munné & E. García-Berthou, 2016a. Ecological impacts of small hydropower plants on headwater stream fish: from individual to community effects. Ecology of Freshwater Fish 25: 295–306. https://doi.org/10.1111/eff.12210.
Benejam, L., F. Teixeira-de Mello, M. Meerhoff, M. Loureiro, E. Jeppesen & S. Brucet, 2016b. Assessing effects of change in land use on size related variables of fish in subtropical streams. Canadian Journal of Fisheries and Aquatic Sciences 73: 547–556. https://doi.org/10.1139/cjfas-2015-0025.
Benejam, L., I. Tobes, S. Brucet & R. Miranda, 2018. Size spectra and other size-related variables of river fish communities: systematic changes along the altitudinal gradient on pristine Andean streams. Ecological Indicators 90: 366–378. https://doi.org/10.1016/j.ecolind.2018.03.012.
Benoît, E. & M. J. Rochet, 2004. A continuous model of biomass size spectra governed by predation and the effects of fishing on them. Journal of Theoretical Biology 226: 9–21. https://doi.org/10.1016/S0022-5193(03)00290-X.
Blanchard, J. L., R. F. Heneghan, J. D. Everett, R. Trebilco & A. J. Richardson, 2017. From bacteria to whales: using functional size spectra to model marine ecosystems. Trends in Ecology & Evolution 32: 174–186. https://doi.org/10.1016/j.tree.2016.12.003.
Boddy, N. C., K. M. Fraley, H. J. Warburton, P. G. Jellyman, D. J. Booker, D. Kelly & A. R. McIntosh, 2020. Big impacts from small abstractions: The effects of surface water abstraction on freshwater fish assemblages. Aquatic Conservation: Marine and Freshwater Ecosystems 30: 159–172. https://doi.org/10.1002/aqc.3232.
Bodine, K. A., D. L. Buckmeier, J. W. Schlechte & D. E. Shoup, 2011. Effect of electrofishing sampling design on bias of size-related metrics for blue catfish in reservoirs. American Fisheries Society Symposium 77: 607–620.
Boix, D., E. García-Berthou, S. Gascón, L. Benejam, E. Tornés, J. Sala, J. Benito, A. Munné, C. Solà & S. Sabater, 2010. Response of community structure to sustained drought in Mediterranean rivers. Journal of Hydrology 383: 135–146. https://doi.org/10.1016/j.jhydrol.2010.01.014.
Bower, L. M. & K. O. Winemiller, 2019. Fish assemblage convergence along stream environmental gradients: an intercontinental analysis. Ecography 42: 1691–1702. https://doi.org/10.1111/ecog.04690.
Carlisle, D. M., D. M. Wolock & M. R. Meador, 2011. Alteration of streamflow magnitudes and potential ecological consequences: a multiregional assessment. Frontiers in the Ecology and the Environment 9: 264–270. https://doi.org/10.1890/100053.
Carosi, A., R. Padula, L. Ghetti & M. Lorenzoni, 2019. Endemic freshwater fish range shifts related to global climate changes: A long-term study provides some observational evidence for the Mediterranean area. Water 11: 2349. https://doi.org/10.3390/w11112349.
CEN, 2005. CEN Water Quality - Sampling of Fish With Electricity, 2005. (European standard - EN 14011:2003, Brussels
Closs, G. E. & P. S. Lake, 1996. Drought, differential mortality and the coexistence of a native and an introduced fish species in a south east Australian intermittent stream. Environmental Biology of Fishes 47: 17–26. https://doi.org/10.1007/BF00002376.
Couto, T. B. & J. D. Olden, 2018. Global proliferation of small hydropower plants–science and policy. Frontiers in Ecology and the Environment 16: 91–100. https://doi.org/10.1002/fee.1746.
Couto, T. B., M. L. Messager & J. D. Olden, 2021. Safeguarding migratory fish via strategic planning of future small hydropower in Brazil. Nature Sustainability 4: 409–416. https://doi.org/10.1038/s41893-020-00665-4.
Cumming, G., 2004. The impact of low-head dams on fish species richness in Wisconsin, USA. Ecological Applications 14: 1495–1506.
Darwall, W. R. T. & K. Smith, 2006. The status and distribution of freshwater fish endemic to the Mediterranean basin, IUCN: International Union for Conservation of Nature.
Davey, A. J. & D. J. Kelly, 2007. Fish community responses to drying disturbances in an intermittent stream: a landscape perspective. Freshwater Biology 52: 1719–1733. https://doi.org/10.1111/j.1365-2427.2007.01800.x.
DOGC (Diari Oficial de la Generalitat de Catalunya) number 8859, 2023. Resolution ACC/483/2023 by which fishing is ordered in the continental waters of Catalonia during the 2023 season.
Dolan, C. R. & L. E. Miranda, 2003. Immobilization thresholds of electrofishing relative to fish size. Transactions of the American Fisheries Society 132: 969–976. https://doi.org/10.1577/T02-055.
Dudgeon, D., 2019. Multiple threats imperil freshwater biodiversity in the Anthropocene. Current Biology 29: R960–R967. https://doi.org/10.1016/j.cub.2019.08.002.
Elliott, J. M., 2000. Pools as refugia for brown trout during two summer droughts: trout responses to thermal and oxygen stress. Journal of Fish Biology 56: 938–948. https://doi.org/10.1111/j.1095-8649.2000.tb00883.x.
Emmrich, M., S. Brucet, D. Ritterbusch & T. Mehner, 2011. Size spectra of lake fish assemblages: responses along gradients of general environmental factors and intensity of lake-use. Freshwater Biology 56: 2316–2333. https://doi.org/10.1111/j.1365-2427.2011.02658.x.
Espinosa, C., M. Abril, È. Bretxa, M. Jutglar, S. Ponsá, N. Sellarès, L. Vendrell-Puigmitjà, L. Llenas, M. Ordeix & L. Proia, 2021. Driving Factors of Geosmin Appearance in a Mediterranean River Basin: The Ter River Case. Frontiers in Microbiology 12: 741750. https://doi.org/10.3389/fmicb.2021.741750.
European Commission, Directorate-General for Environment, Ecological flows in the implementation of the Water Framework Directive. Guidance document No 31, Publications Office, 2016. https://data.europa.euhttps://doi.org/10.2779/775712
Fencl, J. S., M. E. Mather, J. M. Smith & S. M. Hitchman, 2017. The blind men and the elephant examine biodiversity at low-head dams: Are we all dealing with the same dam reality? Ecosphere 8: e01973. https://doi.org/10.1002/ecs2.1973.
Fraley, K. M., H. J. Warburton, P. G. Jellyman, D. Kelly & A. R. McIntosh, 2018. Responsiveness of fish mass–abundance relationships and trophic metrics to flood disturbance, stream size, land cover and predator taxa presence in headwater streams. Ecology of Freshwater Fish 27: 999–1014. https://doi.org/10.1111/eff.12410.
Gavioli, A., M. Mancini, M. Milardi, V. Aschonitis, E. Racchetti, P. Viaroli & G. Castaldelli, 2018. Exotic species, rather than low flow, negatively affect native fish in the Oglio River, Northern Italy. River Research and Applications 34: 887–897. https://doi.org/10.1002/rra.3324.
Gillette, D. P., J. S. Tiemann, D. R. Edds & M. L. Wildhaber, 2005. Spatiotemporal patterns of fish assemblage structure in a river impounded by low-head dams. Copeia 2005: 539–549. https://doi.org/10.1643/CE-04-135R2.
Gosset, C., J. Rives & J. Labonne, 2006. Effect of habitat fragmentation on spawning migration of brown trout (Salmo trutta L.). Ecology of Freshwater Fish 15: 247–254. https://doi.org/10.1111/j.1600-0633.2006.00144.x.
Harvey, B. C. & A. J. Stewart, 1991. Fish size and habitat depth relationships in headwater streams. Oecologia 87: 336–342.
Jiménez-Prado, P. & I. Arranz, 2021. The response of fish size and species diversity to environmental gradients in two Neotropical coastal streams. Hydrobiologia 848: 4419–4432. https://doi.org/10.1007/s10750-021-04654-2.
Jones, P. E., T. Champneys, J. Vevers, L. Börger, J. C. Svendsen, S. Consuegra, J. Jones, C. Garcia de Leaniz & C., 2021. Selective effects of small barriers on river-resident fish. Journal of Applied Ecology 58(7): 1487–1498. https://doi.org/10.1111/1365-2664.13875.
Jumani, S., S. Rao, N. Kelkar, S. Machado, J. Krishnaswamy & S. Vaidyanathan, 2018. Fish community responses to stream flow alterations and habitat modifications by small hydropower projects in the Western Ghats biodiversity hotspot, India. Aquatic Conservation: Marine and Freshwater Ecosystems 28: 979–993. https://doi.org/10.1002/aqc.2904.
Jumani, S., M. J. Deitch, D. Kaplan, E. P. Anderson, J. Krishnaswamy, V. Lecours & M. R. Whiles, 2020. River fragmentation and flow alteration metrics: a review of methods and directions for future research. Environmental Research Letters 15: 123009. https://doi.org/10.1088/1748-9326/abcb37.
Kibler, K. M. & D. D. Tullos, 2013. Cumulative biophysical impact of small and large hydropower development in Nu River, China. Water Resources Research 49: 3104–3118. https://doi.org/10.1002/wrcr.20243.
Kopf, R. K., P. Humphries, N. R. Bond, N. C. Sims, R. J. Watts, R. M. Thompson, S. Hladyz, J. D. Koehn, A. J. King, N. McCasker & S. McDonald, 2018. Macroecology of fish community biomass–size structure: effects of invasive species and river regulation. Canadian Journal of Fisheries and Aquatic Sciences 76: 109–122. https://doi.org/10.1139/cjfas-2017-0544.
Kubečka, J., J. Matena & J. & P. Hartvich, 1997. Adverse ecological effects of small hydropower stations in the Czech Republic: 1. Bypass plants. Regulated Rivers: Research & Management 13: 101–113. https://doi.org/10.1002/(SICI)1099-1646(199703)13:2%3c101::AID-RRR439%3e3.0.CO;2-U.
Kuriqi, A., A. N. Pinheiro, A. Sordo-Ward, M. D. Bejarano & L. Garrote, 2021. Ecological impacts of run-of-river hydropower plants—Current status and future prospects on the brink of energy transition. Renewable and Sustainable Energy Reviews 142: 110833. https://doi.org/10.1016/j.rser.2021.110833.
Lake, P. S., 2003. Ecological effects of perturbation by drought in flowing waters. Freshwater Biology 48: 1161–1172.
Lange, K., P. Meier, C. Trautwein, M. Schmid, C. T. Robinson, C. Weber & J. Brodersen, 2018. Basin-scale effects of small hydropower on biodiversity dynamics. Frontiers in Ecology and the Environment 16: 397–404. https://doi.org/10.1002/fee.1823.
Larscheid, J. G. & W. A. Hubert, 1992. Factors influencing the size structure of brook trout and brown trout in southeastern Wyoming mountain streams. North American Journal of Fisheries Management 12: 109–117. https://doi.org/10.1577/1548-8675(1992)012%3c0109:FITSSO%3e2.3.CO;2.
Ledger, M. E., L. E. Brown, F. K. Edwards, A. M. Milner & G. Woodward, 2013. Drought alters the structure and functioning of complex food webs. Nature Climate Change 3: 223–227. https://doi.org/10.1038/nclimate1684.
Legendre, P. & M. J. Fortin, 1989. Spatial pattern and ecological analysis. Vegetatio 80:107–138. https://www.jstor.org/stable/20038425
Maceda-Veiga, A., R. Mac Nally & A. de Sostoa, 2017. The presence of non-native species is not associated with native fish sensitivity to water pollution in greatly hydrologically altered rivers. Science of the Total Environment 607: 549–557. https://doi.org/10.1016/j.scitotenv.2017.07.010.
Maceda-Veiga, A., R. Mac Nally, A. de Sostoa & J. D. L. Yen, 2022. Patterns of species richness, abundance and individual-size distributions in native stream-fish assemblages invaded by exotic and translocated fishes. Science of the Total Environment. 838: 155953.
Magalhães, M. F., 1993. Effects of season and body-size on the distribution and diet of the Iberian chub Leuciscus pyrenaicus in a lowland catchment. Fish Biology 42: 875–888. https://doi.org/10.1111/j.1095-8649.1993.tb00397.x.
Magalhães, M. F., P. Beja, C. Canas & M. J. Collares-Pereira, 2002. Functional heterogeneity of dry-season fish refugia across a Mediterranean catchment: the role of habitat and predation. Freshwater Biology 47: 1919–1934. https://doi.org/10.1046/j.1365-2427.2002.00941.x.
Marchetti, M. P., T. Light, P. B. Moyle & J. H. Viers, 2004. Fish invasions in California watersheds: testing hypotheses using landscape patterns. Ecological Applications 14: 1507–1525. https://doi.org/10.1890/03-5173.
Mehner, T., B. Lischke, K. Scharnweber, K. Attermeyer, S. Brothers, U. Gaedke, S. Hilt & S. Brucet, 2018. Empirical correspondence between trophic transfer efficiency in freshwater food webs and the slope of their size spectra. Ecology 99: 1463–1472.
Merciai, R., C. Molons-Sierra, S. Sabater & E. García-Berthou, 2017. Water abstraction affects abundance, size-structure and growth of two threatened cyprinid fishes. PLoS ONE 12: e0175932. https://doi.org/10.1371/journal.pone.0175932.
Morden, R., A. Horne, N. R. Bond, R. Nathan & J. D. Olden, 2022. Small artificial impoundments have big implications for hydrology and freshwater biodiversity. Frontiers in Ecology and the Environment 20: 141–146. https://doi.org/10.1002/fee.2454.
Mueller, M., J. Pander & J. Geist, 2011. The effects of weirs on structural stream habitat and biological communities. Journal of Applied Ecology 48: 1450–1461. https://doi.org/10.1111/j.1365-2664.2011.02035.x.
Murphy, C. A., F. Casals, C. Solà, N. Caiola, A. de Sostoa & E. García-Berthou, 2013. Efficacy of population size structure as a bioassessment tool in freshwaters. Ecological Indicators 34: 571–579. https://doi.org/10.1016/j.ecolind.2013.06.007.
Murry, B. A. & J. M. Farrell, 2014. Resistance of the size structure of the fish community to ecological perturbations in a large river ecosystem. Freshwater Biology 59: 155–167. https://doi.org/10.1111/fwb.12255.
Niedrist, G. H., A. Hilpold & P. Kranebitter, 2023. Unveiling the rise of non-native fishes in eastern alpine mountain rivers: Population trends and implications. Journal of Fish Biology. https://doi.org/10.1111/jfb.15508.
Oliveira, F. J. M., D. P. Lima Junior & L. M. Bini, 2022. Body size explains patterns of fish dominance in streams. Hydrobiologia 849: 2241–2251. https://doi.org/10.1007/s10750-022-04860-6.
Ordeix, M., Q. Pou-Rovira, N. Sellarès, M. Bardina, A. Casamitjana, C. Solà & A. Munné, 2011. Fish pass assessment in the rivers of Catalonia (NE Iberian Peninsula). A case study of weirs associated with hydropower plants and gauging stations. Limnetica 30: 405–426. https://doi.org/10.23818/limn.30.29.
Osborne, L. L. & M. J. Wiley, 1992. Influence of tributary spatial position on the structure of warmwater fish communities. Canadian Journal of Fisheries and Aquatic Sciences 49: 671–681. https://doi.org/10.1139/f92-076.
Ovidio, M, & J. C. Philippart, 2002. The impact of small physical obstacles on upstream movements of six species of fish: synthesis of a 5-year telemetry study in the River Meuse basin. In Aquatic Telemetry: Proceedings of the Fourth Conference on Fish Telemetry in Europe: 55–69. Springer Netherlands.
Pereira, H. R., L. F. Gomes, H. D. O. Barbosa, F. M. Pelicice, J. C. Nabout, F. B. Teresa & L. C. G. Vieira, 2020. Research on dams and fishes: determinants, directions, and gaps in the world scientific production. Hydrobiologia 847: 579–592. https://doi.org/10.1007/s10750-019-04122-y.
Petchey, O. L. & A. Belgrano, 2010. Body-size distributions and size-spectra: universal indicator of ecological status? Biology Letters 6: 434–437. https://doi.org/10.1098/rsbl.2010.0240.
Peterson, J. T., R. F. Thurow & J. W. Guzevich, 2004. An evaluation of multipass electrofishing for estimating the abundance of stream-dwelling salmonids. Transactions of the American Fisheries Society 133: 462–475. https://doi.org/10.1577/03-044.
Platt, T. & K. Denman, 1977. Organisation in the pelagic ecosystem. Helgoländer Wissenschaftliche Meeresuntersuchungen 30: 575–581. https://doi.org/10.1007/BF02207862.
Poff, N. L. & J. K. H. Zimmerman, 2010. Ecological responses to altered flow regimes: A literature review to inform the science and management of environmental flows. Freshwater Biology 55: 194–205. https://doi.org/10.1111/j.1365-2427.2009.02272.
Poulet, N., 2007. Impact of weirs on fish communities in a piedmont stream. River Research and Applications 23: 1038–1047. https://doi.org/10.1002/rra.1040.
Power, M. E., 1987. Predator avoidance by grazing fishes in temperate and tropical streams: importance of stream depth and prey size. In Kerfoot, C. & A. Sih (eds), Predation (W University Press of New England, Hanover: 333–351.
Pusey, B. J., M. J. Kennard, J. M. Arthur & A. H. Arthington, 1998. Quantitative sampling of stream fish assemblages: Single-vs multiple-pass electrofishing. Australian Journal of Ecology 23: 365–374. https://doi.org/10.1111/j.1442-9993.1998.tb00741.x.
Quintana, X. D., S. Brucet, D. Boix, R. López-Flores, S. Gascón, A. Badosa, J. Sala, R. Moreno-Amich & J. J. Egozcue, 2008. A nonparametric method for the measurement of size diversity with emphasis on data standardization. Limnology and Oceanography-Methods 6: 75–86. https://doi.org/10.4319/lom.2008.6.75.
Quintana, X. D., J. J. Egozcue, O. Martínez-Abella, R. López-Flores, S. Gascón, S. Brucet & D. Boix, 2016. Update: A non-parametric method for the measurement of size diversity, with emphasis on data standardization. The measurement of the size evenness. Limnology and Oceanography: Methods 14: 408–413. https://doi.org/10.1002/lom3.10099.
R Core Team, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL (Morris et al., 2022; Piggott et al., 2015; Schweiger et al., 2010; Su et al., n.d.)
Reyjol, Y., C. Argillier, W. Bonne, A. Borja, A. D. Buijse, A. C. Cardoso, M. Daufresne, M. Kernan, M. T. Ferreira, S. Poikane, N. PratA, L. Solheim, S. Stroffek, P. Usseglio-Polatera, B. Villeneuve & W. van de Bund, 2014. Assessing the ecological status in the context of the European Water Framework Directive: where do we go now? Science of the Total Environment 497: 332–344. https://doi.org/10.1016/j.scitotenv.2014.07.119.
Richter, I. A., H. C. Henrique Giacomini, D. T. De Kerckhove, D. A. Jackson & N. E. Jones, 2022. Correcting for size bias in electrofishing removal samples. Ecological Modelling 467: 109929. https://doi.org/10.1016/j.ecolmodel.2022.109929.
Rolls, R. J., C. Leigh & F. Sheldon, 2012. Mechanistic effects of low-flow hydrology on riverine ecosystems: ecological principles and consequences of alteration. Freshwater Science 31: 1163–1186. https://doi.org/10.1899/12-002.1.
Santos, J. M., A. Silva, C. Katopodis, P. Pinheiro, A. Pinheiro, J. Bochechas & M. T. Ferreira, 2012. Ecohydraulics of pool-type fishways: Getting past the barriers. Ecological Engineering 48: 38–50. https://doi.org/10.1016/j.ecoleng.2011.03.006.
Santucci, V. J., Jr., S. R. Gephard & S. M. Pescitelli, 2005. Effects of multiple low-head dams on fish, macroinvertebrates, habitat, and water quality in the Fox River, Illinois. North American Journal of Fisheries Management 25: 975–992. https://doi.org/10.1577/M03-216.1.
Schlosser, I. J., 1982. Trophic structure, reproductive success, and growth rate of fishes in a natural and modified headwater stream. Canadian Journal of Fisheries and Aquatic Sciences 39: 968–978. https://doi.org/10.1139/f82-132.
Schmera, D., D. Árva, P. Boda, E. Bódis, Á. Bolgovics, G. Borics, A. Csercsa, C. Deák, E. Á. Krasznai, B. A. Lukács, P. Mauchart, A. Móra, P. Sály, A. Specziár, K. Süveges, I. Szivák, P. Takács, M. Tóth, G. Várbíró, A. E. Vojtkó & T. Erős, 2017. Does isolation influence the relative role of environmental and dispersal-related processes in stream networks? An empirical test of the network position hypothesis using multiple taxa. Freshwater Biology 63: 74–85. https://doi.org/10.1111/fwb.12973.
Sheer, M. B. & E. A., Steel, 2006. Lost watersheds: barriers, aquatic habitat connectivity, and salmon persistence in the Willamette and Lower Columbia River basins. Transactions of the American Fisheries Society 135: 1654–1669. https://doi.org/10.1577/T05-221.1.
Sheldon, R. W., A. Prakash & W. H. Sutcliffe Jr., 1972. The size distribution of particles in the ocean. Limnology and Oceanography 17: 327–340. https://doi.org/10.4319/lo.1972.17.3.0327.
Slawski, T. M., F. M. Veraldi, S. M. Pescitelli & M. J. Pauers, 2008. Effects of tributary spatial position, urbanization, and multiple low-head dams on warmwater fish community structure in a Midwestern stream. North American Journal of Fisheries Management 28: 1020–1035. https://doi.org/10.1577/M06-186.1.
Smouse, P. E., J. C. Long & R. R. Soka, 1986. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Systematic Zoology 35: 627–632. https://doi.org/10.2307/2413122.
Sprules, W. G. & L. E. Barth, 2016. Surfing the biomass size spectrum: some remarks on history, theory, and application. Canadian Journal of Fisheries and Aquatic Sciences 73: 477–495. https://doi.org/10.1139/cjfas-2015-0115.
Sutton, I. A. & N. E. Jones, 2019. Measures of fish community size structure as indicators for stream monitoring programs. Canadian Journal of Fisheries and Aquatic Sciences 77: 824–835. https://doi.org/10.1139/cjfas-2019-0268.
Tiemann, J. S., D. P. Gillette, M. L. Wildhaber & D. R. Edds, 2004. Effects of Lowhead dams on riffle-dwelling fishes and macroinvertebrates in a midwestern river. Transactions of the American Fisheries Society 133: 705–717. https://doi.org/10.1577/T03-058.1.
Timpe, K. & D. Kaplan, 2017. The changing hydrology of a dammed Amazon. Science Advances 3: e1700611. https://doi.org/10.1126/sciadv.1700611.
Vila-Martínez, N., N. Caiola, C. Ibáñez, L. Benejam & S. Brucet, 2019. Normalized abundance spectra of fish community reflect hydro-peaking on a Mediterranean large river. Ecological Indicators 97: 280–289. https://doi.org/10.1016/j.ecolind.2018.10.014.
Walters, A. W., 2016. The importance of context dependence for understanding the effects of low-flow events on fish. Freshwater Science 35: 216–228. https://doi.org/10.1086/683831.
Walters, A. W. & D. M. Post, 2008. An experimental disturbance alters fish size structure but not food chain length in streams. Ecology 89: 3261–3267. https://doi.org/10.1890/08-0273.1.
Weiher, E., G. D. P. Clarke & P. A. Keddy, 1998. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 81: 309–322. https://doi.org/10.2307/3547051.
Woodward, G., B. Ebenman, M. Emmerson, J. M. Montoya, J. M. Olesen, A. Valido & P. H. Warren, 2005. Body size in ecological networks. Trends in Ecology & Evolution 20: 402–409. https://doi.org/10.1016/j.tree.2005.04.005.
World Commission on Dams (WCD), 2000. Dams and Development, Earthscan Publications Ltd, London:
Xenopoulos, M. A., D. M. Lodge, J. Alcamo, M. Märker, K. Schulze & D. P. Van Vuuren, 2005. Scenarios of freshwater fish extinctions from climate change and water withdrawal. Global Change Biology 11: 1557–1564. https://doi.org/10.1111/j.1365-2486.2005.001008.x.
Xu, C. L., B. H. Letcher & K. H. Nislow, 2010. Size-dependent survival of brook trout Salvelinus fontinalis in summer: effects of water temperature and stream flow. Journal of Fish Biology 76: 2342–2369. https://doi.org/10.1111/j.1095-8649.2010.02619.x.
Young, M. K., 1994. Mobility of brown trout in south-central Wyoming streams. Canadian Journal of Zoology 72: 2078–2083. https://doi.org/10.1139/z94-278.
Acknowledgements
We thank S. Saura-Mas, A. Saperas, J. Guillamet, E. Navarro, and A. Vilajeliu for assistance in field work. This project was financed by the Catalan Water Agency (ACA), Government of Catalonia (CTN1001569) and the Spanish Ministry of Science, Innovation and Universities [Grant Number RTI2018-095363-B-I00]. IA is granted by the program Atracción del Talento (Comunidad de Madrid; project FRESCO; Grant Number 2022-T1/AMB-24100).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Ministerio de Universidades, RTI2018-095363-B-I00, Lluís Benejam.
Author information
Authors and Affiliations
Contributions
RG, LB, MO, and IA conceived the idea. RG and IA analyzed the data. RG wrote the first version and all authors reviewed previous versions of the manuscript. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest. The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Fish samplings were carried out after obtaining approval of local agencies. Fish were sampled following the European Committee for Standardization protocol, and caught fish were released at the same site.
Additional information
Handling editor: Fernando Mayer Pelicice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gurí, R., Benejam, L., Ordeix, M. et al. Assessing water diversion impacts on the fish community size structure in headwater stream stretches. Hydrobiologia 851, 3413–3431 (2024). https://doi.org/10.1007/s10750-024-05507-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05507-4