Abstract
Little is known of the macrobenthos supported within stingray feeding pits. Compared to adjacent unpitted areas during low tide, macrobenthic abundance and biodiversity within the stingray pits might be expected to be (i) greater, the water-retaining pits functioning like rock pools; (ii) no different, since macrofaunal recolonisation can occur very rapidly; or (iii) less, consequent on the substratum changes that typify depressions in soft sediments. In both (i) and (iii) differences in composition of the supported assemblages would be expected, though not in (ii). To differentiate between these alternative hypotheses, faunal characteristics within intertidal stingray pits were compared to those in the adjacent background sandflat in Moreton Bay, Queensland, where the prey of the rays are the decapod crustaceans Trypaea and Mictyris that otherwise structure the benthic system. Results generally (though not totally) support hypothesis (ii), it being consistently found that feeding pits supported less macrobenthic abundance than the surrounding sandflat but subequal taxon density, evenness and patchiness of their faunas, and their taxonomic compositions were very similar. Such feeding pits undoubtedly structure many intertidal sandflats and increase both their topographical complexity and their habitat diversity, but this is not reflected in increased macrobenthic biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Along warm temperate and tropical coasts, stingrays are common predators of marine macrobenthic polychaetes, molluscs, and crustaceans (Jacobsen & Bennett, 2013). They use several feeding modes but one in particular causes significant impact on the surface sediment: the ray’s jaws are forcibly protruded into the sea bed, accompanied by a powerful jet of water, and prey accompanied by associated soft sediment is sucked up, the suspended sediment being redeposited on the surface downstream (Tillett et al., 2008; Fisher et al., 2011). This creates a characteristically shaped and in relatively quiet waters a long-lasting, feeding pit (Cross & Curran, 2000; Takaeuchi & Tamaki, 2014) (Fig. 1).
Along the lagoonal side of North Stradbroke Island/Minjerribah within Moreton Bay/Quandamooka, Queensland, such up to 20 cm deep pits created by Hemitrygon fluviorum (Ogilby), Maculabatis toshi (Whitley) and especially Neotrygon australiae Last, White & Serét (Pierce et al., 2009) visibly dominate the band of intertidal sand between some mean sea level (MSL) (the downshore limit of the Avicennia + Rhizophora mangrove fringe) and low water neap tide level (LWN) (near the usual local upshore limit of the intertidal Nanozostera seagrass beds). This sandflat is otherwise structured by crustaceans, i.e. by the burial shafts of the soldier crab Mictyris longicarpus Latreille and the burrows of the callianassid ghost shrimp/yabby Trypaea australiensis Dana, upon both of which at least some of the stingrays are likely to feed (Tillett et al., 2008; Pardo et al., 2015).
These feeding pits have received much scientific attention, especially in respect of the occurrence in them of other fish and meiofauna. Krück et al. (2009) and Chargulaf et al. (2011), for example, have studied their role in sheltering juvenile fish and prawns during low tide, and Chargulaf & Tibbetts (2015) suggested that their supported meiofaunal assemblages provide a source of food to those juvenile nekton. The speed with which meiofaunal recolonisation takes place and the length of time that the pits last as discrete structures have also been analysed in considerable detail (Thrush et al., 1991; Cross & Curran, 2000; O’Shea et al., 2012; Flowers et al., 2020). The effect of stingray pits on the distribution and abundance of the benthic macrofauna, however, has received much less attention, as indeed has the macrofauna of tropical and subtropical soft sediments in general (Gray, 2002; Carvalho et al., 2023).
It has been pointed out that often such pits contain the only standing water on a sandflat at low tide and therefore form the soft-shore equivalent of hard-shore rockpools (Meager et al., 2005) that can considerably enhance local biodiversity (Chee et al., 2020). Hence, they might be expected to contribute a significant resource to non-desiccation-resistant marine animals during low water, resulting then in increased local habitat heterogeneity (McPhee, 2017) and greater abundance and diversity than on the background sandflat for at least some components of the macrofauna. They might thus play a similar role to the biodiversity refuges provided by ‘pockmarks’ on the seabed (Webb et al., 2009) (‘craters on the seafloor, presumably by the expulsion of fluids from the sediments’). Such a potential outcome, however, runs counter to observations that, at least for meiofauna, newly formed pits are denuded of fauna but then recover their former abundance and diversity over time until they do not differ from the background sandflat (Cross & Curran, 2004), i.e. that pits will contain the same or lower than background animal abundance and diversity. Which of these two scenarios is the characteristic state for the macrobenthos has not been determined. One of the few relevant studies is that of Thrush et al. (1991) who compared the occurrence of two benthic assemblages, one dominated by polychaetes the other by bivalves, in and between stingray pits near Auckland, New Zealand. They found an equivalent situation to that for the meiofauna to prevail, i.e. that there were little or no consistent significant differences between the inhabitants of pits and those of the surrounding unpitted sediment because of rapid recolonisation by the macrofauna, which occurs via active migration or as a result of passive advection (Savidge & Taghon, 1988). The only animal that was possibly more common in their pits being an epibenthic crustacean, the cumacean Colurostylis.
It is also clear that, like depressions in soft sediments in general (Van Blaricom, 1982; McGrorty & Reading, 1984), the nature of the sediment within pits on intertidal flats differs from that surrounding them (Toniolo et al., 2021), fine inorganic and organic particles characteristically accumulating (Yager et al., 1993; D’Andrea et al., 2002), including callianassid faecal pellets (Frankenberg et al., 1967) (note the accumulation of Trypaea australiensis pellets—the dark surface material—in the pits shown in Fig. 1). Granted this tendency of depressions to fill with fine particles and the importance of prevailing particle size spectra, sediment compaction, etc. to infauna (Sassa & Yang, 2019; Armonies, 2021), a third potential outcome is failure of recolonisation by the local macrofauna and/or a change in faunal assemblage supported during the recolonisation interval. The same result will occur if the recolonisation rate of the dominant benthic taxa is slow (Zajac, 2004).
Macrofaunal assemblage structure of pools on rocky shores is known to differ from that of surrounding areas (Metaxas and Scheibling, 1993), and the present study seeks to broaden the available database on macrofaunal colonisation of the equivalent pits of soft-sediment systems beyond Thrush et al.’s (1991) New Zealand study of stingray pits. It tests the null hypothesis that there is no significant difference in abundance and biodiversity of the macrofauna within the pits and in the general MSL-LWN horizon on the sandflats.
Methods
Study area, sample collection, and processing
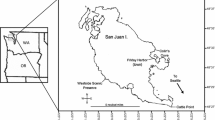
Macrofaunal sampling was conducted over a period of six weeks during the 2023 austral spring within the relatively pristine Eastern Banks region of the oligohaline, mesotidal, and subtropical Moreton Bay Marine Park (Dennison & Abal, 1999; Gibbes et al., 2014). Three replicate stations, some 150–200 m apart, were worked between LWN and MSL across a sandflat off the township of Dunwich/Gumpi near the southern end of the Rainbow Channel, centred on 27°29′39″S,153°24′02″E (Fig. 2). Such expanses of fine- to medium-grained, tidal-delta, quartz sand characterise the zone between the upper mangrove and lower seagrass beds all along the Rainbow Channel (Laycock, 1978). The macrobenthos of these three stations were known from pilot sampling to support somewhat different variants of the local sandflat fauna, being dominated by the haustorioid amphipod crustacean Urohaustorius metungi Fearn-Wannan, by the galeommatoid bivalve mollusc Mysella sp., and the third with no overwhelming dominant. At each station, 20 replicate water-filled stingray pits were sampled at the point of low tide each by a cylindrical core sample of ca. 0.083 m internal diameter (0.0054 m2 area) and 0.2 m depth, as were 20 associated replicate non-pitted areas without a standing water cover. In each case, these samples were taken in the form of two groups of 10 cores 10–20 m apart. What were clearly recently created pits were avoided; all sampled ones no longer having visible mounds of displaced sediment, nor any reduced sand at the surface (cf. the insert in Fig. 1).
This sampling procedure collects the smaller (usually < 5 mm) and more numerous members of the macrofauna that constitute the large majority of invertebrate biodiversity (Bouchet et al., 2002; Albano et al., 2011), although not the meiofauna nor much scarcer megafauna (in any event no such megafauna was observed). Warwick et al. (2006) have shown that different spatial patterning rules may apply to meiofauna and macrofauna. The soldier crab Mictyris longicarpus was excluded from the analysis. These crabs do not inhabit a fixed location but bury beneath the sandflat surface for the duration of high water and then emerge to wander over the surface, travelling up to 450 m before reburying again elsewhere when threatened or up to some 4 h later (Dittmann 1998). By chance, some samples can be dominated by these wandering crabs, whereas at another point in time or space they might contain none. In such circumstances, exclusion seemed most appropriate.
Collection and treatment of core samples followed the same procedure as earlier studies of macrobenthic assemblages associated with the Rainbow Channel intertidal (Barnes, 2017, 2023, and references therein). Cores were collected during daylight hours and were gently sieved through 710 µm mesh on site. Retained material from each core was placed in a 30 × 25 cm translucent tray over an A3 LED pad in which the living fauna was located by visual examination until no further animal could be observed. All samples were processed immediately after collection. Animals were identified to the lowest taxonomic level that could be achieved with certainty, with all organismal nomenclature being as listed in the World Register of Marine Species (www.marinespecies.org), accessed November 2023. Several taxa, however, including polyclads, nemertines, and various polychaete and peracaridan groups, although relatively important in Moreton Bay have not yet been investigated systematically in or near southern Queensland (Davie & Phillips, 2008; Davie et al., 2010). Consequently, members of several groups were treated as morphotaxa, an operationally appropriate procedure to detect spatial patterns of biodiversity and differential abundance (Dethier & Schoch, 2006; Pos et al., 2014) or were mostly identified only to genus, as relevant. Although this incurs a high probability of failing to distinguish any closely similar forms, experience of taxonomic resolution/sufficiency in other soft-sediment macrobenthic studies (e.g. Warwick, 1988; Tataranni et al., 2009; Brind’Amour et al., 2014) indicates that operating at various levels from species up to family all produce similar conclusions.
Analyses
Numbers of each component zoobenthic morphotaxon within the pits and in the adjacent unpitted sandflat surface were subjected to similarity analysis, and assemblage metrics were derived and compared via PAST 4.11 software (Hammer et al., 2001) or Microsoft Excel for Mac 16.77 with the StatPlus:mac Pro 8.0.4 add-on, all metrics being based on animal numerical abundance. Univariate metrics assessed were those known to be of major importance in the assessment of local-scale biodiversity (Blowes et al., 2022), i.e. (1) overall faunal numbers, (2) observed numbers of morphotaxa, i.e. Hill’s N0 [‘species density’ sensu (Gotelli & Colwell, 2001)], and (3) relative evenness (= equitability) of taxon abundances (Pielou’s J). In addition, (4) Gatti et al. (2020) AED biodiversity index incorporating, besides N0 taxon density, Hill–Shannon N1, and Hill-Simpson N2 metrics (Roswell et al., 2021) was also assessed, as was (5) local patchiness in assemblage abundance (as estimated by Lloyd’s Ip patchiness). Comparison of magnitudes of metrics used Wilcoxon matched pairs tests and one-way ANOVA. Statistical significance of the Wilcoxon test was obtained by Monte Carlo simulation with 9999 iterations.
Multivariate statistical comparison of assemblage composition used hierarchical clustering analysis of S17 Bray–Curtis similarity carried out on both untransformed and on standardised taxon abundances (i.e. all samples adjusted to the same total abundance to eliminate variation in local density), ANOSIM, PerMANOVA, and IndVal, all with 9999 permutations.
Results
The number of animals occurring in the stingray pits was always lower than in the adjacent exposed sandflat, both per individual core sample (ANOVA F1,118 = 8.26; P = 0.0048) and per set of 10 cores per station (Wilcoxon P = 0.028): on average, they totalled only 63% of the sandflat value The number of taxa per unit area in each habitat type did not differ significantly (ANOVA F1,118 = 0.97, P = 0.33; Wilcoxon test P = 0.63), however, and neither did values of evenness and of the AED index (Wilcoxon P = 0.075 and = 0.6, respectively) (Table 1). Abundance was distributed patchily across both habitat types (P = 0.0001), particularly in the stingray pits.
The adjacent stingray pit and sandflat assemblages at two of the sites were significantly different (ANOSIM R = 0.102, P = 0.0028 & R = 0.273, P = 0.0001; PerMANOVA F = 2.5, P = 0.0026 and F = 6.6, P = 0.0001), not least because of the differential abundance of the dominant pit forms Urohaustorius and Mysella, respectively, in the two habitat types at each site, but not at the third (ANOSIM R = 0.027, P = 0.18; PerMANOVA F = 1.32, P = 0.19). In any event, all values of ANOSIM R were very small and Bray–Curtis similarities were high at > 60% based on taxon abundances and > 65% on the standardised data. Fifty-four taxa were recorded in total. Both faunas were dominated by the polychaete Spio, the amphipods Urohaustorius, Doowia, and Eriopisella and the bivalves Mysella and Eumarcia, which together comprised > 70% of the total macrobenthic numbers. Doowia, Tritia, and Mysella occurred subequally in both habitats; nevertheless, IndVal identified Doowia and Mysella as the two taxa most indicative of the pits, whilst Urohaustorius and Goniada characterised the sandflat. The relative proportions of major categories of benthos in the two systems are shown in Table 2. No taxon occurred abundantly in only one of the two habitat types; of those occurring at > 30 m−2 only the uncommon hermit crab Diogenes sp. occurred in the pits but was not recorded on the flats and only the amphipod? Kamaka showed the converse pattern. Figure 3B shows the extent to which the stingray pit and background sand faunas varied in parallel across the intertidal flat stations.
Discussion
Despite their ease of access and their prevalence, little is known of the ecology of the sandflats on Moreton Bay shores and of their invertebrate fauna (Skilleter, 1998; Barnes, 2017), but there is no reason to think that those at Dunwich are atypical in any respect. Reworking the Rainbow Channel sandflat data of Barnes and Barnes (2012), the fauna of the Dunwich sand is certainly very similar in composition to those to the north at Capembah and those to the south at Deanbilla (mean Bray–Curtis similarity of 55%). The dominance of Urohaustorius in particular is characteristic of other areas along the central east coast of Australia (e.g. Dexter, 1983).
It is clear from the results obtained that in no sense did the stingray pits there constitute a specific biodiversity asset, provide a refuge for macrofaunal invertebrates (except for a few nekton and possibly for Diogenes), or support a benthic fauna of differing richness or essential nature from that in the surrounding sandflats. This also applies at local scale in that taxonomically the pit faunas were more similar to those in the immediately adjacent exposed sand than they were to pit faunas in other regions of the sampling site.
This result would seem to confirm the observations of Thrush et al. (1991) in New Zealand. Stingray pits are small (much smaller than most rock pools and very much smaller than the submarine ‘pockmarks’ described by Webb et al. (2009) and this ensures a large perimeter per unit area or volume for animals to pass across to recolonise them. It is odd, however, that the two groups of animals that would be thought least likely to be able rapidly to invade newly created pits, bivalves and sedentary polychaetes, are the two that were most numerous there and indeed comprised higher proportions of the fauna than in the sandflats. The distinctive tubes of Spiochaetopterus were a highly visible feature of some pits (although most of the tubes were unoccupied, possibly because the worms retreated into the sediment below the cored 20 cm) and live animals occurred at a density of 28 m−2, but that worm was present at only a third of that density on the flats. The two most mobile background sandflat components, i.e. those that might be considered most likely to recolonise rapidly, the peracarid crustaceans Urohaustorius, Eriopisella, Limnophora, etc. and errant predatory worms, particularly Goniada, however, displayed the greatest reduction in pit versus sandflat comparisons.
This contrasts with the situation described by Drolet and Barbeau (2009) for tide pools on three mudflats in the Bay of Fundy, Canada, where the dominant benthic amphipod Corophium volutator (Pallas) consistently occurred at higher density in the pools than on the adjacent flats during low tide. Decreased risk of predation and hence increased survival in such pools has been generally suggested to be the explanation for the occurrence there of juvenile nekton (Kneib, 1987), but this was not the case for the Corophium volutator (Drolet and Barbeau, 2009). Neither did it result from active habitat selection, but to differential rates of emigration: those Corophium volutator that found themselves in a pool tending to remain there, whereas those on the unpitted flats were more mobile. Unfortunately, there have been no studies of differential predation rates by fish or birds on intertidal stingray pits or other sand/mudflat pools that might help to untangle the complex manner in which macrobenthic abundance and biodiversity are distributed intertidally (Dewenter et al., 2023).
Although whilst feeding stingrays modify the habitat more than many carnivores and their depressions may remain long after the activity creating them has ceased, their effect on the system in essence does not differ from the general nature of predation. Prey items are taken, and the taxa concerned recolonise denuded areas. The rates of recolonisation will depend in part on powers of dispersal, but for most marine benthos this can be accomplished rapidly. For a few taxa, perhaps like the epifaunal Diogenes in the present case, the miniature ponds created can permit activity to be continued during low tide, but this might be expected to attract the attention of avian predators (the hermit crab’s strong shell may be relevant here), so most macrobenthos either retreat into the sediment or retreat with the tide. To understand the reasons for the individual patterns displayed by specific infaunal crustaceans and polychaetes, however, it will require much greater understanding of their individual ecologies than we have at present.
Data availability
The faunal abundance data on which this paper is based are contained in the Supplementary data file.
References
Albano, P. G., B. Sabelli & P. Bouchet, 2011. The challenge of small and rare species in marine biodiversity surveys: microgastropod diversity in a complex tropical coastal environment. Biodiversity and Conservation 20: 3223–3237. https://doi.org/10.1007/s10531-011-0117-x.
Armonies, W., 2021. Who lives where? Macrobenthic species distribution over sediment types and depth classes in the eastern North Sea. Helgoland Marine Research 75: 8. https://doi.org/10.1186/s10152-021-00552-1.
Barnes, R. S. K., 2017. Patterns of benthic invertebrate biodiversity in intertidal seagrass in Moreton Bay, Queensland. Regional Studies in Marine Science 15: 17–25. https://doi.org/10.1016/j.rsma.2017.07.003.
Barnes, R. S. K., 2023. Seagrass macrobenthic biodiversity does not vary in conformity with a leaky-lagoonal confinement gradient. Marine Environmental Research 185: 105897. https://doi.org/10.1016/j.marenvres.2023.105897.
Barnes, R. S. K. & M. K. S. Barnes, 2012. Shore height and differentials between macrobenthic assemblages in vegetated and unvegetated areas of an intertidal sandflat. Estuarine, Coastal and Shelf Science 106: 112–120. https://doi.org/10.1016/j.ecss.2012.05.011.
Blowes, S. A., G. N. Daskalova, M. Dornelas, et al., 2022. Local biodiversity change reflects interactions among changing abundance, evenness and richness. Ecology. https://doi.org/10.1002/ecy.3820.
Bouchet, P., P. Lozouet, P. Maestrati & V. Heros, 2002. Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biological Journal of the Linnean Society 75: 421–436. https://doi.org/10.1046/j.1095-8312.2002.00052.x.
Brind’Amour, A., P. Laffargue, J. Morin, S. Vaz, A. Foveau & H. Le Bris, 2014. Morphospecies and taxonomic sufficiency of benthic megafauna in scientific bottom trawl surveys. Continental Shelf Research 72: 1–9. https://doi.org/10.1016/j.csr.2013.10.015.
Carvalho, S., Z. Alsaffar, J. Ellis, H. Alghamdi & J. Cúrdia, 2023. Broad-scale spatial distribution patterns of soft-sediment macrobenthic communities in the Red Sea. Frontiers in Marine Science 10: 1072342. https://doi.org/10.3389/fmars.2023.1072342.
Chargulaf, C.A., K.A. Townsend & I.R. Tibbetts, 2011. Community structure of soft sediment pool fishes in Moreton Bay, Australia. Journal of Fish Biology 78:479-494. https://doi.org/10.1111/j.1095-8649.2010.02866.x
Chargulaf, C. A. & I. R. Tibbetts, 2015. Spatial and temporal variation of meiofauna community structure in soft-sediment pools around Moreton Bay, Australia. Australian Journal of Zoology 63: 204–213. https://doi.org/10.1071/ZO14063.
Chee, S. Y., J. L. S. Wee, C. Wong, J. C. Yee, Y. Yusup & A. Mujahid, 2020. Drill-cored artificial rock pools can promote biodiversity and enhance community structure on coastal rock revetments at reclaimed coastlines of Penang, Malaysia. Tropical Conservation Science 13: 1–15. https://doi.org/10.1177/1940082920951912.
Cross, R. E. & M. C. Curran, 2000. Effects of feeding pit formation by rays on an intertidal meiobenthic community. Estuarine, Coastal and Shelf Science 51: 293–298. https://doi.org/10.1006/ecss.2000.0682.
Cross, R. E. & M. C. Curran, 2004. Recovery of meiofauna in intertidal feeding pits created by rays. Southeastern Naturalist 3: 219–230. https://doi.org/10.1656/1528-7092(2004)003[0219:ROHIIF]2.0.cCO;2.
D’Andrea, A. F., R. C. Allen & G. R. Lopez, 2002. Organic matter flux and reactivity on a South Carolina sandflat: the impacts of porewater advection and macrobiological structure. Limnology and Oceanography 47: 1056–1070. https://doi.org/10.4319/lo.2002.47.4.1056.
Davie, P. J. F. & J. A. Phillips, 2008. The Marine Fauna and Flora of Moreton Bay Workshop: a focus on species. In: Davie, P.J.F. & J.A. Phillips (eds), Proceedings of the Thirteenth International Marine Biological Workshop. The Marine Fauna and Flora of Moreton Bay, Queensland. Volume 1. Memoirs of the Queensland Museum – Nature 54(1): 1–2.
Davie, P. J. F., I. W. Brown & D. G. Mayer, 2010. Assessment of long-term temporal changes in the macrobenthic communities south of Peel Island, Moreton Bay, Queensland. In: Davie, P.J.F. & J.A. Phillips (eds), Proceedings of the Thirteenth International Marine Biological Workshop. The Marine Fauna and Flora of Moreton Bay, Queensland. Volume 3. Memoirs of the Queensland Museum – Nature 54(3): 401–435.
Dennison, W. C. & E. G. Abal, 1999. Moreton Bay Study: A Scientific Basis for the Healthy Waterways Campaign, SE Queensland Regional Water Quality Management Strategy, Brisbane:
Dethier, M. N. & G. C. Schoch, 2006. Taxonomic sufficiency in distinguishing natural spatial patterns on an estuarine shoreline. Marine Ecology Progress Series 306: 41–49. https://doi.org/10.3354/meps306041.
Dewenter, J., J. Yong, P. Schupp, S. Moorthi, K. Lõhmus, I. Krönke, D. Pieck & S. Rodhe, 2023. Abundance, biomass and species richness of macrozoobenthos along an intertidal elevation gradient. Authorea. https://doi.org/10.22541/au.168084557.76191565/v1.
Dexter, D. M., 1983. A guide to the sandy beach fauna of New South Wales. Wetlands 3: 94–104.
Dittmann, S., 1998. Behaviour and population structure of soldier crabs Mictyris longicarpus (Latreille): Observations from a tidal flat in tropical north Queensland, Australia. Senckenbergiana Maritima 28: 177–184. https://doi.org/10.1007/BF03043148.
Drolet, D. & M. A. Barbeau, 2009. Differential emigration causes aggregation of the amphipod Corophium volutator (Pallas) in tide pools on mudflats of the upper Bay of Fundy, Canada. Journal of Experimental Marine Biology and Ecology 370: 41–47. https://doi.org/10.1016/j.jembe.2008.11.012.
Fisher, R. A., G. C. Call & R. D. Grubbs, 2011. Cownose ray (Rhinoptera bonasus) predation relative to bivalve ontogeny. Journal of Shellfish Research 30: 187–196. https://doi.org/10.2983/035.030.0126.
Flowers, K. I., M. R. Heithaus & Y. P. Papastamatiou, 2020. Buried in the sand: Uncovering the ecological roles and importance of rays. Fish and Fisheries 22: 105–127. https://doi.org/10.1111/faf.12508.
Frankenberg, D., S. L. Coles & R. E. Johannes, 1967. The potential trophic significance of Callianassa major fecal pellets. Limnology and Oceanography 12: 113–120. https://doi.org/10.4319/lo.1967.12.1.0113.
Gatti, R. C., N. Amoroso & A. Monaco, 2020. Estimating and comparing biodiversity with a single universal metric. Ecological Modelling 424: 109020. https://doi.org/10.1016/j.ecolmodel.2020.109020.
Gibbes, B., A. Grinham, D. Neil, A. Olds, P. Maxwell, R. Connolly, T. Weber, N. Udy & J. Udy, 2014. Moreton Bay and its estuaries: a sub-tropical system under pressure from rapid population growth. In Wolanski, E. (ed), Estuaries of Australia in 2050 and Beyond Springer, Dordrecht: 203–222.
Gotelli, N. J. & R. K. Colwell, 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391. https://doi.org/10.1046/j.1461-0248.2001.00230.x.
Gray, J. S., 2002. Species richness of marine soft sediment. Marine Ecology Progress Series 244: 285–297. https://doi.org/10.3354/meps244285.
Hammer, Ø., D. A. T. Harper & P. D. Ryan, 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1): 9.
Jacobsen, I. P. & M. B. Bennett, 2013. A comparative analysis of feeding and trophic level ecology in stingrays (Rajiformes; Myliobatoidei) and electric rays (Rajiformes; Torpedinoidei). PLoS ONE 8: e71348. https://doi.org/10.1371/journal.pone.0071348.
Kneib, R. T., 1987. Predation risk and use of intertidal habitats by young fishes and shrimp. Ecology 68: 379–386. https://doi.org/10.2307/1939269.
Krück, N. C., C. A. Chargulaf, U. Saint-Paul & I. R. Tibbetts, 2009. Early post-settlement habitat and diet shifts and the nursery function of tidepools during Sillago spp. recruitment in Moreton bay, Australia. Marine Ecology Progress Series 384: 207–219. https://doi.org/10.3354/meps07992.
Laycock, J. W., 1978. North Stradbroke Island. Papers, Department of Geology, University of Queensland 8: 89–96.
McGrorty, S. & C. J. Reading, 1984. The rate of infill and colonization by invertebrates of borrow pits in the Wash (S.E. England). Estuarine, Coastal and Shelf Science 19: 303–319. https://doi.org/10.1016/0272-7714(84)90027-1.
McPhee, D., 2017. Environmental History and Ecology of Moreton Bay, CSIRO, Clayton:
Meager, J. J., I. Williamson & C. R. King, 2005. Factors affecting the distribution, abundance and diversity of fishes of small, soft-substrata tidal pools within Moreton Bay, Australia. Hydrobiologia 537: 71–80. https://doi.org/10.1007/s10750-004-2308-x.
Metaxas, A. & R. E. Scheibling, 1993. Community structure and organization of tidepools. Marine Ecology Progress Series 98: 187–198. https://doi.org/10.3354/meps098187.
O’Shea, O. R., M. Thums, M. van Keulen & M. Meekan, 2012. Bioturbation by stingrays at Ningaloo Reef, Western Australia. Marine and Freshwater Research 63: 189–197. https://doi.org/10.1071/MF11180.
Pardo, S. A., K. B. Burgess, D. Teixeira & M. B. Bennett, 2015. Local-scale resource partitioning by stingrays on an intertidal flat. Marine Ecology Progress Series 533: 205–218. https://doi.org/10.3354/meps11358.
Pierce, S. J., S. A. Pardo & M. B. Bennett, 2009. Reproduction of the blue-spotted maskray Neotrygon kuhlii (Myliobatoidei: Dasyatidae) in south-east Queensland, Australia. Journal of Fish Biology 74: 1291–1308. https://doi.org/10.1111/j.1095-8649.2009.02202.x.
Pos, E., J. E. G. A. Andino, D. Sabatier, et al., 2014. Are all species necessary to reveal ecologically important patterns? Ecology and Evolution 4: 4626–4636. https://doi.org/10.1002/ece3.1246.
Roswell, M., J. Dushoff & R. Winfree, 2021. A conceptual guide to measuring species diversity. Oikos 130: 321–338. https://doi.org/10.1111/oik.07202.
Sassa, S. & S. Yang, 2019. Role of geoenvironmental dynamics in the biodiversity of sandy beaches and sandflats: The ecohabitat chart and its ecological implications. Estuarine, Coastal and Shelf Science 219: 278–290. https://doi.org/10.1016/j.ecss.2019.02.002.
Savidge, W. B. & G. L. Taghon, 1988. Passive and active components of colonization following two types of disturbance on intertidal sandflat. Journal of Experimental Marine Biology and Ecology 115: 137–155. https://doi.org/10.1016/002-0981(88)90099-8.
Skilleter, G. A., 1998. Ecology of benthic invertebrates in Moreton Bay. In Tibbetts, I. R., W. J. Hall & W. C. Dennison (eds), Moreton Bay and Catchment University of Queensland, Brisbane: 365–394.
Takeuchi, S. & A. Tamaki, 2014. Assessment of benthic disturbance associated with stingray foraging for ghost shrimp by aerial survey over an intertidal sandflat. Continental Shelf Research 84: 139–157. https://doi.org/10.1016/j.csr.2014.05.007.
Tataranni, M., F. Maltagliati, A. Floris, A. Castelli & C. Lardicci, 2009. Variance estimates and taxonomic resolution: an analysis of macrobenthic spatial patterns at different scales in a western Mediterranean coastal lagoon. Marine Environmental Research 67: 219–229. https://doi.org/10.1016/j.marenvres.2009.02.003.
Thrush, S. F., R. D. Pridmore, J. E. Hewitt & V. J. Cummings, 1991. Impact of ray feeding disturbances on sandflat macrobenthos: do communities dominated by polychaetes or shellfish respond differently? Marine Ecology Progress Series 69: 245–252. https://doi.org/10.3354/meps069245.
Tillett, B. J., I. R. Tibbetts & D. L. Whithead, 2008. Foraging behaviour and prey discrimination in the bluespotted maskray Dasyatis kuhlii. Journal of Fish Biology 73: 1554–1561. https://doi.org/10.1111/j.1095-8649.02022.x.
Toniolo, M. A., C. Seitz & G. M. E. Perillo, 2021. Origin and evolution of tidal depressions in a tidal flat and their role in carbon sequestration in the Bahía Blanca Estuary (Argentina). Marine Geology 436: 106467. https://doi.org/10.1016/j.margeo.2021.106467.
Van Blaricom, G. R., 1982. Experimental analysis of structural regulation in a marine sand community exposed to oceanic swell. Ecological Monographs 52: 283–305. https://doi.org/10.2307/2937332.
Warwick, R. M., 1988. Analysis of community attributes of the macrobenthos of Frierfjord/Langesundfjord at the taxonomic level higher than species. Marine Ecology Progress Series 46: 167–170. https://doi.org/10.3354/meps046167.
Warwick, R. M., S. L. Dashfield & P. J. Somerfield, 2006. The integral structure of a benthic infaunal assemblage. Journal of Experimental Marine Biology and Ecology 330: 12–18. https://doi.org/10.1016/j.jembe.2005.12.013.
Webb, K. E., D. K. A. Barnes & S. Planke, 2009. Pockmarks: refuges for marine benthic biodiversity. Limnology and Oceanography 54: 1776–1788. https://doi.org/10.4319/lo.2009.54.5.1776.
Yager, P., A. R. M. Nowell & P. A. Jumars, 1993. Enhanced deposition to pits: a local food source for benthos. Journal of Marine Research 51: 209–236. https://doi.org/10.1357/0022240933223819.
Zajac, R. N., 2004. Macrofaunal responses to pit-mound patch dynamics in an intertidal mudflat: local versus patch-type effects. Journal of Experimental Marine Biology and Ecology 313: 297–315. https://doi.org/10.1016/j.jembe.2004.08.011.
Acknowledgements
We are indebted to the Quandamooka Yoolooburrabee Aboriginal Corporation and the Quandamooka Aboriginal Land and Sea Management Agency for permission to carry out field research within the native title area of the Quandamooka People and to the Queensland Parks and Wildlife Service for permission to sample in a Habitat Protection Zone of the Moreton Bay Marine Park under permit MPP19-002133. The study was conducted from the Moreton Bay Research Station, Dunwich, and we are most grateful to its staff especially Kevin Townsend and Martin Wynne for their hospitality and support.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
RSKB participated in the Conceptualization, methodology, investigation, formal analysis, writing of the manuscript, and visualisation. LGC contributed to Investigation.
Corresponding author
Ethics declarations
Competing interests
The authors have no financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Iacopo Bertocci
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barnes, R.S.K., Cottrell, L.G. Do stingray feeding pits enhance intertidal macrobenthic biodiversity?. Hydrobiologia 851, 3403–3412 (2024). https://doi.org/10.1007/s10750-024-05504-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05504-7