Abstract
Recent research highlighted the need to include experimental estimates of tolerance limits to varying environmental conditions when investigating what factors limit species distributions. However, most niche approaches are only based on the statistical dependence between environmental and occurrence data. Here, we combined field data with survival experiments to assess the role of salinity as a limiting factor in the distribution of two species of exotic ostracods from the Iberian Peninsula. Vizcainocypria viator is a free-living species associated with rice fields and Ankylocythere sinuosa is a commensal of the red swamp crayfish (Procambarus clarkii). Experiments and field data indicate that the distribution of V. viator is limited by adult survival at low and high salinities (below electrical conductivity of 0.6 mS/cm and above 10 mS/cm). In the case of A. sinuosa, the analysis of field data shows that its prevalence is negatively affected by high salinity, whereas experiments indicate an optimal survival at high salinities (conductivity above 10.2 mS/cm), thus suggesting that high salinity may impact A. sinuosa distribution indirectly through affecting host traits (e.g. reduced activity). The habitat of close ancestors (marine versus non-marine respectively for A. sinuosa and V. viator) most likely explains the contrasting differences in salinity tolerance between both ostracod species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The geographic range of a species is particularly difficult to estimate (Grinnell, 1917). It is defined as “the fraction of geographical space where a species is present and interacts non-ephemerally with the ecosystem” (Zunino & Palestrini, 1991). The concept involves both the location or type of habitat used by individuals, and the form in which they occur, depending on their life cycle (e.g. butterfly larvae on a host plant or ostracod diapausing eggs in the sediment of a dry pond). Geographic distributions are dynamic over time, undergoing contractions and expansions (Tomiolo & Ward, 2018; Fitt et al., 2019). Many species are experiencing significant range reductions, with habitat alteration being one of the main causes of these declines (Sala et al., 2000; Barnosky et al., 2011). On the other hand, the current globalisation trend has allowed many other species to occupy large geographic extensions outside their native range, aided by anthropogenic introductions followed by rapid geographic expansion (Davis, 2009; Mestre et al., 2020). In addition, climate change is driving range shifts in different taxa (e.g. Bridle et al., 2014), also frequently favouring invasive species expansions or amplifying their impacts in a synergistic way (Dukes, 2010).
The geographical distribution of a species and its dynamics can be affected by multiple factors including abiotic, biotic, demographic, spatial and temporal (Pulliam, 2000; Wiens & Graham, 2005; Colwell & Rangel, 2009; Holt, 2009). Firstly, species distributions are constrained by their instrinsic ranges of tolerance to environmental conditions (e.g. temperature, humidity, salinity, etc.; De Candolle, 1855; Good, 1931; MacArthur, 1972; Holt, 2009). Second, interactions with other species may facilitate or hinder the presence of a species in a given location, or influence its dispersal capacity (McGill et al., 2006; Soberón, 2007; HilleRisLambers et al., 2013; Mestre et al., 2020). For example, the geographic distribution of a parasite may be limited by the distribution of its hosts (Colwell et al., 2012), and the spatial distributions of orchids are strongly influenced by their interactions with pollinating insects (Štípková et al., 2020). Third, species experience demographic fluctuations of a stochastic nature (not linked to environmental variation), which can influence local extinction-colonisation dynamics (Pulliam, 2000; Pearson & Dawson, 2003; Huntley et al., 2010). Fourth, dispersal dynamics in space can generate incongruities between the actual and potential distribution of a species (Colwell & Rangel, 2009). On the one hand, a species may be absent from sites with optimal habitats due to dispersal limitations (Kubisch et al., 2014). On the other hand, a species may inhabit unsuitable places due to a permanent flow of immigration from nearby favourable habitats, a phenomenon known as “demographic rescue” (Kanarek et al., 2015). Fifth, a species may occupy a habitat seasonally during favourable periods, and disappear during periods of environmental harshness, through migration or diapause (i.e. “temporary dispersal”; Plue & Cousins, 2013; Wisnoski et al., 2019). Finally, evolution may play a very important role in the geographic distribution of species. Across the range occupied by a species, local populations may differ in their tolerances to environmental conditions due to phenotypic plasticity or genetic differences driven by local adaptation (Pereira et al., 2017; Bennett et al., 2019). Local adaptation is considered one of the possible mechanisms of geographical expansion of species (Lee-Yaw et al., 2018; Mestre et al., 2020).
The development of new tools, such as ecological niche models and geographic information systems have allowed to better estimate the geographic distribution of species in relation to environmental gradients (Elith et al., 2006). However, obtaining the data needed to apply such models is not always a straightforward task. Due to the complexity of processes associated with geographic distributions, the tolerance limits of species to environmental variables (i.e. their ecological niche; Hutchinson, 1978) cannot be solely inferred from correlations based on geographic distribution data. It requires the design of laboratory survival experiments under controlled conditions (Holt, 2009). Survival experiments are basic approaches for testing ecological and evolutionary theories, largely related to the concept of ecological niche (Soberón & Peterson, 2005). At a more practical level, they allow predictions of species distributions when occurrence data are unavailable or limited, and increase the predictive capacity of models with existing data (Peterson & Soberón, 2012; Kotta et al., 2019). In this study, we combine the analysis of occurrence data with tolerance experiments under laboratory conditions to assess the role of salinity in shaping the geographic distribution of two exotic ostracod species from the Iberian Peninsula.
Freshwater ostracods are among the most frequent invertebrate groups within inland water bodies. Sexual dimorphism is common in podocopid ostracods, in most cases large differences are found in the external features of the shells, and males are usually smaller than females (Cohen & Morin, 1990; Meisch, 2000). Ostracods have proved to be a very useful group in ecological studies, due to their wide distribution, small size, high developmental speed and ease of keeping them alive in aquaria under controlled conditions. For these reasons, they are particularly suitable organisms for addressing questions requiring laboratory experiments (Ganning, 1971; Martens, 1985; Mesquita-Joanes et al., 2012). Ostracods play a very important role in the structure of small aquatic systems (Diner et al., 1986) and are sensitive organisms for ecotoxicological testing (Havel & Talbott, 1995). The presence of ostracods in freshwater ecosystems is conditioned by the physico-chemical characteristics of the water. Hydroperiod, temperature and salinity are amongst the most influential factors in the distribution and abundance of ostracods (De Deckker, 1981; Neale, 1988; Aladin, 1993; Horne, 1993). In general, the species richness of microcrustaceans is altered by changes in salinity (Jensen et al., 2010). In ostracods, salinity affects the osmotic regulation of individuals and the balance between calcification and ionic regulation (Aladin, 1993; Mezquita et al., 1999). For this reason, a relationship exists between the distribution of ostracod species and the ionic composition of the water, due to their need to calcify their shells. Ostracods have evolved different osmotic regulation mechanisms to tolerate changes in salt content (Mesquita-Joanes et al., 2012). Some freshwater ostracod species tolerate very high salinities (Santamaria et al., 1992), which may be related to their marine origin (Park & Ricketts, 2003). Other factors affecting the survival of ostracods include temperature, depth of the water column, pH, substrate type, feeding, predation, parasitism, dissolved oxygen content, submerged vegetation, photoperiod, amount of dissolved organic matter or water flow velocity (Delorme, 1969; Carbonel et al., 1988; Delorme, 1989; Griffiths & Holmes, 2000).
This work assesses the effects of salinity on the distribution of exotic populations of two ostracod species in the Iberian Peninsula. Vizcainocypria viator Bisquert-Ribes et al. (2023) is a recently described free-living species, found in rice fields in southern Valencia, and belonging to the Cyclocyprididae (Bisquert-Ribes et al., 2023). Despite having been described from the Iberian Peninsula, molecular data and morphological similarities with other species suggest that V. viator is actually an exotic invader in the Iberian Peninsula, originally from North America (Bisquert-Ribes et al., 2023). The other study species, Ankylocythere sinuosa (Rioja, 1942), belongs to the family Entocytheridae (Hart & Hart, 1974). Entocytherids are ostracods that are symbionts of other crustaceans. They are small in size (< 600 µm) and show sexual dimorphism in which the female is larger than the male (Aguilar-Alberola et al., 2012). Most species are native to North and Central America where they live in association with crayfish belonging to Astacoidea, as commensals, without any apparent effect on the host (Hart & Hart, 1974). Introduced entocytherids have been discovered associated with exotic crayfish in areas of Europe and East Asia (Smith & Kamiya, 2001; Aguilar-Alberola et al., 2012; Mestre et al., 2013; Huys et al., 2014; Ohtaka et al., 2017). In particular, the species A. sinuosa has established exotic populations in the Iberian Peninsula and Balearic Islands (Aguilar-Alberola et al., 2012), in association with Procambarus clarkii (Girard, 1852), an invasive crayfish that has been very successful (Geiger et al., 2005). Populations of A. sinuosa on the Iberian Peninsula have been the subject of recent research (Castillo-Escrivá et al., 2013; Mestre et al., 2013, 2014, 2016, 2019). The aims of the present work are: (1) analysing the effect of salinity on the distribution of Mediterranean populations of both exotic ostracods based on published field data (Gálvez et al., 2023; Mestre et al., 2014); (2) testing the effects of salinity on the survival of adults under laboratory conditions; and (3) testing whether the effects of salinity on adult survival differ between males and females.
Materials and methods
Occurrence data analyses
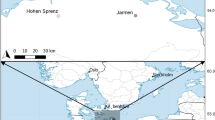
First, the role of salinity in the distribution of the two ostracod species (V. viator and A. sinuosa) was analysed based on field data available from published scientific papers and projects. In our analyses, we considered salinity as the total concentration of dissolved ions in the water, and estimated from electrical conductivity. Occurrence data for the species V. viator were obtained from an ostracod database developed as part of the METACOM-SET project (Gálvez et al., 2023). The database gathers presence/absence information for V. viator at 32 different localities in the eastern Iberian Peninsula (Fig. 1a), and site conductivity data. The species was identified as Dentocypria sp. in the dataset prior to its description as a new species by Bisquert-Ribes et al. (2023). For each locality, multiple seasonal surveys (about 4 surveys per year) were conducted during 2018 and 2021. Occurrence (presence-absence) data of V. viator included a total of 265 samples. The distribution data of A. sinuosa were extracted from Mestre et al. (2014). The study analyses the factors associated with the presence and abundance of this commensal ostracod at 26 localities in the Iberian Peninsula and Balearic Islands (a single sampling per locality; Fig. 1b).
Sampling locations for the analysis of the effect of salinity on the geographical distribution of the two study species: a V. viator and b A. sinuosa. White circles on the V. viator map a represent localities with absence of the species, and black circles indicate presence. The pie charts on the A. sinuosa map b indicate the prevalence of this ostracod at the sampling locality (i.e. proportion of crayfish occupied by the symbiont). The red dot in the Balearic Islands represents two sampling localities in close proximity to each other
Occurrence data of V. viator were analysed with a generalised linear mixed-effects model of binomial family, in order to control for the effects of repeated sampling from the same locality (GLMM; Zuur et al., 2009). Presence-absence records were used as the response variable, electrical conductivity of water from the sampling locality (mS/cm) as fixed-effects explanatory variable, and sampling locality as random-effects factor. The GLMM was carried out with the lme4 package v. 1.1.27 (Bates et al., 2015). For A. sinuosa, we used prevalence as the response variable, defined as the percentage of crayfish occupied by the symbiotic ostracod at a locality. The effects of salinity on the prevalence of A. sinuosa was also analysed with a generalised linear model of the binomial family. Because host size and abundance are known to influence A. sinuosa prevalence (Aguilar-Alberola et al., 2012; Mestre et al., 2014), we controlled their effects by including, as fixed effects, an index of crayfish abundance (crayfish caught per trap), and the mean crayfish weight (g) sampled at the locality. In all GLMs (V. viator and A. sinuosa), explanatory variables were standardised.
Survival experiments
We assessed survival of adults of the two study species at different degrees of salinity under laboratory conditions, with the aim of estimating their salinity tolerance range. Experimental individuals were collected from the Albufera of Valencia N2000 site. Individuals of V. viator were captured at Masía de Santa Rita, south of the locality of El Saler (coordinates: 39.3747° N, 0.33253° W; conductivity = 2.4 mS/cm; salinity = 1.54 g/l). The sampling point for A. sinuosa was the Tancat de la Pipa, Valencia (coordinates: 39.36018° N, 0.34541° W; conductivity = 1.4 mS/cm; salinity = 0.7 g/l). Specimens of the crayfish Procambarus clarkii (Girard, 1852) were captured using bait traps and transferred to the laboratory in containers filled with source water. In the laboratory, symbiotic ostracods were isolated alive from crayfish following a removal protocol described by Mestre et al. (2011). That is, crayfish are immersed in a container filled with carbonated water for two minutes, which causes the ostracods to detach from their hosts. The carbonated water is then filtered through a 100 μm mesh to isolate the ostracods; shells of A. sinuosa adults measure 370–430 μm in length and 180–250 μm in height (Aguilar-Alberola et al., 2012). The mesh was immediately immersed in a container with mineral water (Cortes®, 0.5 mS/cm) to release live ostracods. Individuals were kept alive in the water container until the start of the experiment. This commercial water was chosen because it is rich in carbonates, as are most freshwaters in the area of study.
Four types of water with different degrees of salinity were prepared by dissolving different amounts of aquarium salt (Sera®; major ionic composition: 55.20% Cl−, 30.77% Na, 7.72 % SO42−, 3.68% Mg2+, 1.18 % Ca2+, 1.14% K+) in mineral water (Cortes®; dry residue: 0.2 g/l; ionic composition: 67.4% HCO3−, 4.04% SO42−, 2.22% Cl−, 21.9% Ca2+, 1.97% Mg2+, 0.25% K+, 1.94% Na+), depending on the salinity to be achieved: (i) low (0.2 g/l; 0.5 mS/cm), (ii) intermediate-low (0.6 g/l; 1.2 mS/cm), (iii) intermediate-high (3.1 g/l; 5.7 mS/cm), and (iv) high (5.8 g/l; 10.2 mS/cm). Salinity ranges were selected based on empirical data available for the species. Subsequently, the bottles with the four different conductivity treatments were autoclaved in order to carry out the experiment under sterilised conditions. For each treatment, using a high magnification stereomicroscope (Leica MZ16), 24 adults of each species were selected. In the case of A. sinuosa, it was possible to isolate 12 males and 12 females due to a clear sexual dimorphism. Adult females have 390–430 μm valves with a “convex” appearance; in contrast, adult males measure 360–390 μm and have a characteristic copulatory apparatus usually visible through the transparent valves (Hart & Hart, 1974; Aguilar- Alberola et al., 2012). By contrast, the sexing of live individuals of V. viator proved to be more complicated due to their high mobility, and we decided to select adults at random, and identify the sex of individuals after the end of the experiment. To carry out individual sexing, dead individuals were preserved in 96% alcohol. A total of 96 experimental individuals per species were isolated. Multiwell plates were prepared with 24 wells for each treatment and species, each well with 2 ml of water from the respective treatment and a single individual.
The plates with the ostracods were kept in a culture chamber at a constant temperature of 20 °C for the entire duration of the experiment, with a photoperiod of 12 h of light and 12 h of darkness. The condition of each individual was checked daily and recorded in a table. To do so, alive ostracods were handled with brushes and pipettes under a stereomicroscope for proper examination. A condition index with four values was used from least to most active: 0 = confirmed death, 1 = no movement, 2 = movement of a limb, 3 = movement of the whole body across space. Confirmed death was attributed only to cases where the individual had the valves clearly open, having some of the limbs out of the valves in an “unnatural” position, and without showing any type of movement when stimulated with a brush. Any events that might alter ostracod survival were recorded. For instance, during daily examinations, we observed some individuals of A. sinuosa floating in the surface layer of water in the well, trapped by the surface tension. The floating individuals were sunk by pushing them down with a paintbrush to the bottom of the well (thus breaking the surface tension). We also found cases of individual disappearances or deaths clearly not associated with the treatment (e.g. death by desiccation due to an individual being trapped stuck to the wall of the well, outside the water). Each dead individual was removed from the well, and preserved individually in a microtube with ethanol 96% for further checking and sexing. The experiment finished after all experimental individuals died.
We tested the effects of conductivity on adult survival applying the non-parametric method of Kaplan-Meier (Therneau & Grambsch, 2000; Kleinbaum & Klein, 2011). We incorporated censored data into the analysis, i.e. data indicating that an individual disappeared during handling, or that it died due to causes unrelated to the treatment (e.g. death by desiccation of an ostracod attached to the wall of the well). For each species, 4 Kaplan-Meyer survival curves were estimated, one for each treatment. Differences in survival between treatments were tested via Mantel–Cox tests. The Kaplan-Meier analysis does not control for the effects of other variables that may interfere with salinity. However, it is typical for ostracods that males survive less than females (Cohen & Morin, 1990). Thus, Cox regression models were used to control for sex effects and assess their influence on the survival-salinity relationship (Therneau & Grambsch, 2000; Kleinbaum & Klein 2011). Cox regression allows multiple effects of several factors to be integrated into a single model. The response variable in Cox regressions is the instantaneous potential for death to occur given that the individual survived to time t, i.e. the risk of dying (Kleinbaum & Klein, 2011). The explanatory variables were salinity (variable of interest) and sex (potential interfering variable). Models were compared including and not including sex. Finally, a likelihood ratio test was used to find the best model in explaining variation in adult survival. For the survival analyses, we used the survival package v. 3.2.11 for R (Therneau, 2021). All statistical analyses were done with R v. 4.1.0 (R Core Team, 2021).
Reanalysing occurrence data based on survival experiments
In the case of V. viator where we found no significant effects of salinity based on occurrence data, we considered the possibility that it could be due to the existence of a non-linear relationship. The fact that the GLMM models assume the same mean effect for the whole range of the predictor variable could lead to situations of a lack of an overall effect when non-linear effects are present (i.e. effects that vary across the range of the predictor). We tested the hypothesis of hidden non-linear effects by reanalysing the occurrence data as follows. First, experimental results were used to identify ranges of salinity where its effect on experimental survival is monotonic (i.e. either negative or positive). Second, subsets of occurrence data specifically covering the identified ranges were reanalysed separately to test whether the GLMM results showed range-specific effects consistent with those observed in the experiments. In addition, we also applied a generalised additive model (GAM) of binomial family to check for a non-linear relationship between conductivity and probability of presence of V. viator. The GAM was implemented with the mgcv package v. 1.8.40 (Wood, 2011).
Results
Occurrence data analyses
The METACOM-SET project database records the presence of V. viator in only 4 out of 32 sampled localities, all of them located in littoral wetlands in southern Valencia (Fig. 1a). The mean conductivity among localities was 0.86 mS/cm (SD = 1.48; see also Table 1). The GLMM for V. viator with METACOM-SET data indicates that there is no significant effect of salinity on the presence of the ostracod at the sampled localities (Table 2).
The species A. sinuosa was present in 24 out of 26 sampling localities (Fig. 1b). The mean conductivity of the 26 sampling localities of A. sinuosa was 0.84 mS/cm (SD = 4.09). Mean host abundance was 1.83 crayfish caught per trap (SD = 1.68). The mean host weight per locality was 19.32 g (SD = 5.92). Iberian-Balearic populations of A. sinuosa showed generally very high prevalences, with 84% of crayfish harbouring ostracods per locality on average (SD = 30%). Most of the sampled crayfish populations had prevalences above 75%. Prevalence values in the Balearic Islands were lower, including one locality with ~ 50% infested crayfish and two other localities without A. sinuosa (Fig. 1a). A GLM incorporating the variables salinity, abundance and mean host weight showed a non-significant effect of mean crayfish weight (z = 0.42; df = 22; P = 0.13). Therefore, we removed the mean host weight from the model. A GLM without the weight showed a negative effect of conductivity, and a positive effect of host abundance. The effect size of host abundance was twice that of conductivity (Table 2).
Survival experiments
The Kaplan-Meier curves of V. viator for each salinity treatment had very similar shapes among them (Fig. 2a). All individuals of V. viator survived the first 7 days of the experiment, regardless of the treatment. Therefore, V. viator showed a very high survival at the beginning of the experiment, until day 7, after which survival started to drop abruptly. The survival drop began earlier in the two low salinity treatments, followed by the higher salinity treatment. The intermediate-high salinity treatment is the one that showed the most delayed drop in survival (Fig. 2a). The median survival of each treatment (Table 3) reflects the same survival relationship between treatments, although the confidence intervals of the medians overlap between them (except for the intermediate-high salinity). The Mantel–Cox test applied to all Kaplan-Meier curves indicates significant differences in survival among treatments (Table 4). Treatment-specific pairwise tests show that the intermediate-high salinity differs from the other treatments, and there is no evidence that the other treatments differ from each other (Table 4).
In contrast to V. viator, mortality of A. sinuosa starts to be expressed earlier in the experiment (Fig. 2b). By the third day of the experiment, deaths of individuals had already occurred in all treatments. In addition, the A. sinuosa curves showed greater divergence from each other (especially between extreme treatments). The appearance of the survival curves suggests that, within the salinity range of the experiment, A. sinuosa improves its survival with increasing salinity. The median survival time for each treatment reflects this apparent pattern (Table 3). According to the overall Mantel–Cox test, A. sinuosa showed significant survival differences between treatments (Table 4). In addition, all but one of the pairwise treatment comparisons were significant. The only treatment pair comparison without significant differences was intermediate-high salinity with high salinity (Table 4).
Two Cox regression models were compared to assess the role of individual sex as an interfering factor on the salinity-survival relationship. The first model (Model 1) only considers conductivity as an explanatory variable. The second model (Model 2) incorporates sex as an additional explanatory variable. In the case of V. viator, likelihood ratio tests indicate that Model 1 has a better goodness-of-fit than a null model with no explanatory variables (Table 5). Therefore, salinity is relevant to the survival of V. viator. Furthermore, the inclusion of sex in Model 2 significantly increases goodness-of-fit, thus indicating that sex is important in assessing the effect of salinity on survival of V. viator. According to Model 1, intermediate-high salinity produces an average survival improvement of 67% over the baseline low salinity treatment (Table 6). Model 2 shows that the risk of instantaneous death is 1.96 times higher in males than females. In addition, the presence of sex in the model slightly corrects the effect of salinity. In particular, the effect of improved survival at intermediate-high salinity observed in the first model becomes slightly smaller when we control for the effect of sex in the second model (compare hazard ratios of Model 1 and Model 2 of V. viator in Table 6).
Regarding A. sinuosa, Model 1 also shows higher goodness-of-fit than the null (Table 5), in agreement with the results of the Kaplan-Meier curves, in the same way that occurs in V. viator. However, unlike V. viator, Model 2 of A. sinuosa does not differ significantly from Model 1 in its goodness-of-fit (Table 5). Therefore, sex does not influence the survival of A. sinuosa adults under the experimental conditions of this study. Regarding the effect of salinity on the survival of A. sinuosa, all treatments different from the base treatment (low salinity) improve the survival of individuals (regardless of sex). The higher the salinity of the treatment, the greater the effect compared to the base treatment. The greatest effect occurs in the high salinity treatment, with an average increase in survival of 85% over the low salinity base treatment (see A. sinuosa Model 1 in Table 6).
Reanalysing occurrence data based on survival experiments
The lack of a significant positive effect of salinity on the field presence of V. viator could be due to the existence of a non-linear relationship, as shown by the experimental data obtained. That is, the effect of salinity is positive at low-intermediate salinities, and becomes negative at high salinities. To test the hypothesis of non-linear effects, the data were filtered by removing locations with conductivities falling in the range of negative effect according to the experimental data (i.e. > 4 mS/cm). The result was as expected: a positive effect of conductivity on the presence of V. viator (β = 2.78; SE = 0.52; z = 5.33; P < 0.001). On the contrary, when removing locations with conductivities < 4 mS/cm, the mean effect was negative though not significant (β = − 0.50; SE = 1.64; z = − 0.30; P = 0.76). Furthermore, the GAM analysis confirmed this non-linear relationship (Fig. 3). The GAM predictions showed high uncertainty at the upper range of conductivity (i.e. above 6 mS/cm) due to the scarcity of data covering this range (only 5 out of 265 records had a conductivity higher than 6 mS/cm). Nevertheless, the decrease in the probability of presence of V. viator at high salinities predicted by the GAM (Fig. 3) was supported by the negative impact of a high salinity on adult survival (Fig. 2a, Table 4).
Discussion
Based on occurrence data analyses alone, there is no evidence from GLMMs that salinity influences the presence of V. viator in the eastern Iberian Peninsula. However, the results obtained from the survival experiments show that there are significant differences in adult survival. The intermediate-high salinity treatment (3.1 g/l; 5.65 mS/cm) showed a higher survival than the rest of tested salinities. This suggests that the optimum salinity of the population sampled in Tancat de la Pipa is found at salinities above 0.6 g/l (1.2 mS/cm) and below 5.8 g/l (10.2 mS/cm). The salinity of the source locality of the experimental individuals falls within the estimated optimal range (1.54 g/l). The median conductivity of the 32 sampling sites is 0.31 mS/cm. In contrast, the minimum conductivity of sites with presence of V. viator is 0.62 mS/cm thus suggesting that V. viator is absent at locations with low conductivity in the range of studied sites.
Reanalysing the occurrence data based on experimental results, we found a previously hidden, non-linear relationship. That is, the effect of salinity on the presence of V. viator is positive at low-intermediate salinities, and becomes negative at high salinities (Fig. 3). Our results highlight the importance of assessing potential shortcomings derived from the linearity assumption of GLMs when analysing occurrence data. The combination of analysis of occurrence data with data from survival experiments supports the hypothesis that the distribution of V. viator is limited by the survival of adults at very low salinities (below 0.6 mS/cm) where they do not occur. Nevertheless, the results of the experiment also show that very high salinities (above 10 mS/cm) have a negative impact on adult survival. But, as we have seen, a negative effect of salinity on the distribution of V. viator at the high salinity range is not so evident from the field dataset. This is consistent with the typical habitat of taxonomically related species, i.e. rice fields, which tend to have intermediate-high salinities (Savatenalinton, 2017).
In this study it was found that adult females of V. viator have a higher survival rate than adult males. This may be related to the fact that ostracod males tend to have lower tolerance ranges and shorter lifespan than females (Cohen & Morin, 1990). The higher survival of female individuals would explain the female-biased sex ratio in many ostracod populations (Havel et al., 1990). The sex bias in survival has been attributed to genetic causes (Chaplin et al., 1994). One possible cause of the shorter lifespan of male individuals is the investment in searching for females, i.e. the costs of male sexual behaviour (Cohen & Morin, 1990). By contrast, females of some species remain immobile on the substrate waiting for males, thus expending less energy (e.g. Danielopol et al., 2002). However, the expectation of higher male mortality related to their higher mobility remains to be tested in further survival and behavioural experiments and for a wider variety of taxa. The results of this work highlight the importance of incorporating sex as a critical factor for future studies focused on the survival analysis of V. viator and other podocopid ostracods.
The analysis of A. sinuosa occurrence data indicates a negative effect of salinity on symbiont prevalence, though the effect size is small compared to the positive effect of crayfish abundance (Table 2). Results are consistent with the important role of host abundance in the population dynamics of horizontally transmitted symbionts such as A. sinuosa (Mestre et al., 2020). In contrast, the survival experiment suggests a positive effect of salinity throughout the range tested in the experiment. This apparent contradiction may have several explanations. On the one hand, it may be that the observed effect of salt content is caused by a failure to consider some important variables that covary with conductivity in the populations at the sampling locations (Bolker, 2008). Mestre et al. (2014) conducted a more comprehensive analysis of the prevalence and abundance of A. sinuosa, including a larger number of variables, such as crayfish moult status, water physicochemical variables and climatic variables. In their analysis, conductivity was not selected as a significant variable to explain the prevalence of the symbiont. However, in the same analysis, conductivity was selected for ostracod abundance (with a negative effect). Another possible explanation is that, unlike V. viator, the range of conductivities in the experiment (0.5–10.21 mS/cm) did not cover the full range of salinities that were sampled for presence of A. sinuosa (0.03–19.4 mS/cm). In any case, the experimental results of the present study indicate that the optimum salinity of A. sinuosa is above 10.21 mS/cm. In future studies, a more precise estimation of this optimum could be made by extending the upper limit of the salinity range used in our experiment to one closer to the upper limit of the salinity range of the localities. On the other hand, the negative effect of conductivity on the prevalence of A. sinuosa might be associated with a vital parameter other than adult survival outside the host (e.g. juvenile survival, reproduction rate, etc.) Another possible explanation is that salinity indirectly affects the ostracod via the host. For example, high salinities may produce metabolic changes in Procambarus clarkii (Bissattini et al., 2015), and these changes could reduce the activity of the crayfish, thus lowering the transmission rates of the symbiont, causing a negative effect on its prevalence. Finally, the experimental results show that adult males and females of A. sinuosa do not differ in their survival and, therefore, sex does not interfere with the salinity-survival relationship of this symbiotic ostracod.
Our survival experiments indicate that A. sinuosa tolerates better high salinities than V. viator. The habitat of close ancestors most likely explains these contrasting differences. The ostracod A. sinuosa belongs to a family, the Entocytheridae (Cytheroidea), that includes extant genera living in marine environments (Hartiella and Microsyssitria; Hart & Hart, 1974). Indeed, according to the fossil record, repeated transitions from marine to non-marine habitats have occurred along the Cenozoic and Mesozoic within the Cytheroidea. By contrast, V. viator is a member of Cypridoidea, a superfamily without extant species inhabiting marine environments, and without known fossil evidence of marine-to-freshwater transitions, though it has related superfamilies with marine species, i.e. Macrocypridoidea and Pontocypridoidea (Horne, 2003). This evidence supports the hypothesis that V. viator might have lost the osmoregulatory abilities to bear high salinities present in its ancient marine ancestors. A few extant cypridoideans are known to tolerate high salinities, including e.g. Heterocypris salina (Brady, 1868), Heterocypris barbara (Gauthier & Brehm, 1928), Candelacypris aragonica (Brehm & Margalef, 1949), Sarscypridopsis aculeata (Costa, 1847) or Arctocypris mareotica (Fischer, 1855) (see Ganning, 1971; Baltanás et al., 1990; Gusakov et al., 2021), but we are not aware of any halotolerant ostracod species in the Cyclocyprididae, the family to which V. viator belongs.
Despite the need to incorporate physiological experiments to better define the niche of species and improve predictions on their response to environmental change (Kotta et al., 2019), experimental data on nonmarine ostracod tolerance to salinity changes is very scarce. This is unexpected, considering the long tradition of the use of ostracods as paleoenvironmental indicators (Delorme, 1969; Carbonel et al., 1988), which has been apparently based mostly on field data. Furthermore, published data on ostracod salinity tolerance (Ganning, 1971; Martens, 1985; Santamaria et al, 1992; Gandolfi et al., 2001; Wang et al., 2021) commonly show inconsistencies with field data; ostracods are usually found to have wider tolerance ranges under laboratory conditions compared with field studies. This is not surprising considering that negative biotic interactions such as interspecific competition and natural enemies, which have been classically considered as key drivers of species distributions (e.g., Staniczenko et al., 2018), are absent in laboratory experiments. Our experimental results also suggested a wider tolerance to salinity gradients in the studied species compared to occurrence data in the field. However, in our study cases we should consider not only potential effects of interspecific competition or natural enemies explaining these differences, but also the strong dependency of A. sinuosa on crayfish, and the possibility that V. viator has not had time enough to disperse to occupy all its potential distribution in the area. Other potential explanations for the observed discrepancies are different ionic compositions, oxygen contents or food availability between field and experimental water, the presence of pollutants in the field water, or the fact that our experiments only considered adult survival, thus disregarding potential limiting factors specifically affecting immature stages. Furthermore, in the case of A. sinuosa, because experimental individuals were detached from their hosts, we measured adult survival outside the host, disregarding the effects of the host micro-environment. Our results point to a need for more integrative approaches to the study of species-environment relationships (Jiménez-Valverde et al., 2011) that incorporate experimental estimates of the abiotic niche (Holt, 2009), and account for the influence of strong biotic interactions such as host availability for symbiotic species (Mestre et al., 2013), and dispersal limitation for exotic species in earlier invasion stages. As a final remark, we envisage that the expected future increase in anthropogenic salinisation of inland waters in semiarid and arid regions (Williams, 2001; Cañedo-Argüelles, 2020) will facilitate the spread of exotic halotolerant species like those investigated in this study, as observed in other invasive crustaceans (Cuthbert et al., 2020).
Data availability
Occurrence data were obtained from published data (see ‘Materials and methods’). The dataset of survival experiments is available in Supporting Information.
References
Aguilar-Alberola, J. A., F. Mesquita-Joanes, S. López, A. Mestre, J. C. Casanova, J. Rueda & A. Ribas, 2012. An invaded invader: high prevalence of entocytherid ostracods on the red swamp crayfish Procambarus clarkii (Girard, 1852) in the Eastern Iberian Peninsula. Hydrobiologia 688: 63–73. https://doi.org/10.1007/s10750-011-0660-1.
Aladin, N. V., 1993. Salinity tolerance, morphology and physiology of the osmoregulatory organ in Ostracoda with special reference to Ostracoda from the Aral Sea. In McKenzie, K. G. & P. J. Jones (eds), Ostracoda in the Earth and Life Sciences Balkema, Rotterdam: 387–403.
Baltanás, A., C. Montes & P. Martino, 1990. Distribution patterns of ostracods in Iberian saline lakes. Influence of ecological factors. Hydrobiologia 197: 207–220. https://doi.org/10.1007/BF00026951.
Barnosky, A. D., N. Matzke, S. Tomiya, G. O. U. Wogan, B. Swartz, T. B. Quental, C. Marshall, J. L. McGuire, E. L. Lindsey, K. C. McGuire, B. Mersey & E. A. Ferrer, 2011. Has the Earth’s sixth mass extinction already arrived? Nature 471: 51–57. https://doi.org/10.1038/nature09678.
Bates, D., M. Mächler, B. Bolker & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. https://doi.org/10.18637/jss.v067.i01.
Bennett, S., C. M. Duarte, N. Marbà & T. Wernberg, 2019. Integrating within-species variation in thermal physiology into climate change ecology. Philosophical Transactions of the Royal Society B 374: 20180550. https://doi.org/10.1098/rstb.2018.0550.
Bisquert-Ribes, M., J. Rueda, F. Palero, S. Savatenalinton & F. Mesquita-Joanes, 2023. Integrative taxonomy of Cyclocyprididae Kaufmann, 1900 (Ostracoda: Podocopa) with description of a new genus and species. Zoological Studies 62: 40.
Bissattini, A. M., L. Traversetti, G. Bellavia & M. Scalici, 2015. Tolerance of increasing water salinity in the red swamp crayfish Procambarus clarkii. Journal of Crustacean Biology 35: 682–685. https://doi.org/10.1163/1937240X-00002366.
Bolker, M. B., 2008. Ecological models and data in R, Princeton University Press, Princeton.
Bridle, J. R., J. Buckley, E. J. Bodsworth & C. D. Thomas, 2014. Evolution with the move: specialization on widespread resources associated with rapid range expansion in response to climate change. Proceedings of the Royal Society of London B 281: 0131800. https://doi.org/10.1098/rspb.2013.1800.
Cañedo-Argüelles, M., 2020. A review of recent advances and future challenges in freshwater salinization. Limnetica 39: 185–211. https://doi.org/10.23818/limn.39.13.
Carbonel, P., J. P. Colin, D. L. Danielopol, H. Löffler & I. Neustreva, 1988. Palaeoecology of limnic ostracodes: a review of some major topics. Palaeogeography, Palaeoclimatology, Palaeoecology 62: 413–461. https://doi.org/10.1016/0031-0182(88)90066-1.
Castillo-Escriva, A., A. Mestre, J. S. Monros & F. Mesquita-Joanes, 2013. Population dynamics of an epibiont Ostracoda on the invasive red swamp crayfish Procambarus clarkii in a western Mediterranean wetland. Hydrobiologia 714: 217–228. https://doi.org/10.1007/s10750-013-1542-5.
Chaplin, J. A., J. E. Havel & P. D. Hebert, 1994. Sex and ostracods. Trends in Ecology & Evolution 9: 435–439. https://doi.org/10.1016/0169-5347(94)90127-9.
Cohen, A. C. & J. G. Morin, 1990. Patterns of reproduction in ostracodes: a review. Journal of Crustacean Biology 10: 184–212. https://doi.org/10.1163/193724090X00023.
Colwell, R. K. & T. F. Rangel, 2009. Hutchinson’s duality: the once and future niche. Proceedings of the National Academy of Sciences USA 106: 19651–19658. https://doi.org/10.1073/pnas.090165010.
Colwell, R. K., R. R. Dunn & N. C. Harris, 2012. Coextinction and persistence of dependent species in a changing world. Annual Review of Ecology, Evolution, and Systematics 43: 183–203. https://doi.org/10.1146/annurev-ecolsys-110411-160304.
Cuthbert, R. N., S. G. Kotronaki, J. T. A. Dick & E. Briski, 2020. Salinity tolerance and geographical origin predict global alien amphipod invasions. Biology Letters 16: 20200354. https://doi.org/10.1098/rsbl.2020.0354.
Danielopol, D. L., E. Ito, G. Wansard, T. Kamiya, T. M. Cronin & A. Baltanás, 2002. Techniques for collection and study of ostracoda. In Chivas, A. R. & J. A. Holmes (eds), The Ostracoda: Applications in Quaternary Research American Geophysical Union, Washington: 65–97.
Davis, M. A., 2009. Invasion Biology, Oxford University Press, Oxford.
De Candolle, A. P., 1855. Géographie botanique raisonnée. Exposition des faits principaux et des lois concernant la distribution géographique des plantes de l’époque actuelle, Masson, Paris:
De Deckker, P., 1981. Ostracods of athalassic saline lakes. Hydrobiologia 81: 131–144. https://doi.org/10.1007/978-94-009-8665-7_10.
Delorme, L. D., 1969. Ostracodes as quaternary palaeoecological indicators. Canadian Journal of Earth Sciences 6: 1471–1475. https://doi.org/10.1139/e69-151.
Delorme, L. D., 1989. Methods in quaternary ecology #7. Freshwater ostracodes. Geoscience Canada 16: 85–90.
Diner, M. P., E. P. Odum & P. F. Hendrix, 1986. Comparison of the roles of ostracods and cladocerans in regulating community structure and metabolism in freshwater microcosms. Hydrobiologia 133: 59–63. https://doi.org/10.1007/BF00010802.
Dukes, J. S., 2010. Responses of invasive species to a changing climate and atmosphere. In Richardson, D. M. (ed), Fifty Years of Invasion Ecology Wiley-Blackwell, Chichester: 345–57.
Elith, J., C. H. Graham, R. P. Anderson, M. Dudík, S. Ferrier, A. Guisan, R. J. Hijmans, F. Huettmann, J. R. Leathwick, A. Lehmann, J. Li, L. G. Lohmann, B. A. Loiselle, G. Manion, C. Moritz, M. Nakamura, Y. Nakazawa, J. M. Overton, A. T. Peterson, S. J. Phillips, K. Richardson, R. Scachetti-Pereira, R. E. Schapire, J. Soberon & S. Williams, 2006. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29: 129–151. https://doi.org/10.1111/j.2006.0906-7590.04596.x.
Fitt, R. N., S. Palmer, C. Hand, J. M. J. Travis & L. T. Lancaster, 2019. Towards an interactive, process-based approach to understanding range shifts: developmental and environmental dependencies matter. Ecography 42: 201–210. https://doi.org/10.1111/ecog.03975.
Gálvez, A., P. R. Peres-Neto, A. Castillo-Escrivá, F. Bonilla, A. Camacho, E. M. García-Roger, S. Iepure, J. Miralles-Lorenzo, J. S. Monrós, C. Olmo, A. Picazo, C. Rojo, J. Rueda, M. Sahuquillo, M. Sasa, M. Segura, J. Armengol & F. Mesquita-Joanes, 2023. Inconsistent response of taxonomic groups to space and environment in Mediterranean and tropical pond metacommunities. Ecology 104: e3835. https://doi.org/10.1002/ecy.3835.
Gandolfi, A., E. B. A. Todeschi, K. Van Doninck, V. Rossi & P. Menozzi, 2001. Salinity tolerance of Darwinula stevensoni (Crustacea, Ostracoda). Italian Journal of Zoology 68: 61–67. https://doi.org/10.1080/11250000109356384.
Ganning, B., 1971. On the ecology of Heterocypris salinus, H. incongruens and Cypridopsis aculeata (Crustacea: Ostracoda) from Baltic brackish-water rockpools. Marine Biology 8: 271–279. https://doi.org/10.1007/BF00348009.
Geiger, W. P., P. Alcorlo, A. Baltanás & C. Montes, 2005. Impact of an introduced crustacean on the trophic webs of Mediterranean wetlands. Biological Invasions 7: 49–73. https://doi.org/10.1007/s10530-004-9635-8.
Good, R. D., 1931. A theory of plant geography. The New Phytologist 30: 149–171.
Griffiths, H. I. & J. A. Holmes, 2000. Non-marine ostracods and quaternary palaeoenvironments, QRA Technical Guide No. 8. Quaternary Research Association, London.
Grinnell, J., 1917. The niche-relationships of the California thrasher. The Auk 34: 427–433. https://doi.org/10.2307/4072271.
Gusakov, V. A., O. N. Makhutova, M. I. Gladyshev, L. V. Golovatyuk & T. D. Zinchenko, 2021. Ecological Role of Cyprideis torosa and Heterocypris salina (Crustacea, Ostracoda) in saline rivers of the Lake Elton basin: abundance, biomass, production, fatty acids. Zoological Studies 60: e53. https://doi.org/10.6620/ZS.2021.60-53.
Hart, D. G. & C. W. Jr Hart, 1974. The Ostracod Family Entocytheridae, 1st ed. Lancaster, Fulton Press Inc, Pennsylvania.
Havel, J. E. & B. L. Talbott, 1995. Life history characteristics of the freshwater ostracod Cyprinotus incongruens and their application to toxicity testing. Ecotoxicology 4: 206–218. https://doi.org/10.1007/BF00116482.
Havel, J. E., P. D. N. Hebert & L. D. Delorme, 1990. Genetics of sexual Ostracoda from a low Arctic site. Journal of Evolutionary Biology 3: 65–84. https://doi.org/10.1046/j.1420-9101.1990.3010065.x.
HilleRisLambers, J., M. A. Harsch, A. K. Ettinger, K. R. Ford & E. J. Theobald, 2013. How will biotic interactions influence climate change-induced range shifts? Annals of the New York Academic of Science 1297: 112–125. https://doi.org/10.1111/nyas.12182.
Holt, R. D., 2009. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proceedings of the National Academy of Sciences USA 106: 19659–19665. https://doi.org/10.1073/pnas.0905137106.
Horne, F. R., 1993. Survival strategy to escape desiccation in a freshwater ostracod. Crustaceana 65: 53–61.
Horne, D. J., 2003. Key events in the ecological radiation of the ostracoda. In Park, L. E., & Smith, A. (eds), Bridging the gap: trends in the ostracode biological and geological scieces. The Paleontological Society Papers, 9: 181–201.
Huntley, B., P. Barnard, R. Altwegg, L. Chambers, B. Coetzee, L. Gibson, P. Hockey, D. G. Hole, G. F. Midgley, L. G. Underhill & S. G. Willis, 2010. Beyond bioclimatic envelopes: dynamic species’ range and abundance modelling in the context of climatic change. Ecography 33: 621–626. https://doi.org/10.1111/j.1600-0587.2009.06023.x.
Hutchinson, G. E., 1978. An Introduction to Population Ecology, Yale University Press, New Haven, CT.
Huys, R., B. Oidtmann, M. Pond, H. Goodman & P. F. Clark, 2014. Invasive crayfish and their symbionts in the Greater London area: new data and the fate of Astacus leptodactylus in the Serpentine and Long Water Lakes. Ethology, Ecology & Evolution 26: 320–347. https://doi.org/10.1080/03949370.2014.903433.
Jensen, E., S. Brucet, M. Meerhoff, L. Nathansen & E. Jeppesen, 2010. Community structure and diel migration of zooplankton in shallow brackish lakes: role of salinity and predators. Hydrobiologia 646: 215–229. https://doi.org/10.1007/s10750-010-0172-4.
Jiménez-Valverde, A., A. T. Peterson, J. Soberón, J. M. Overton, P. Aragón & J. M. Lobo, 2011. Use of niche models in invasive species risk assessments. Biological Invasions 13: 2785–2797. https://doi.org/10.1007/s10530-011-9963-4.
Kanarek, A. R., C. T. Webb, M. Barfield & R. D. Holt, 2015. Overcoming Allee effects through evolutionary, genetic, and demographic rescue. Journal of Biological Dynamics 9: 15–33. https://doi.org/10.1080/17513758.2014.978399.
Kleinbaum, D. G. & M. Klein, 2011. Survival Analysis: A Self-learning Text, 3rd ed. Springer, New York.
Kotta, J., J. Vanhatalo, H. Jänes, H. Orav-Kotta, L. Rugiu, V. Jormalainen, I. Bobsien, M. Viitasalo, E. Virtanen, A. N. Sandman, M. Isaeus, S. Leidenberger, P. R. Jonsson & K. Johannesson, 2019. Integrating experimental and distribution data to predict future species patterns. Scientific Reports 9: 1821. https://doi.org/10.1038/s41598-018-38416-3.
Kubisch, A., R. D. Holt, H.-J. Poethke & E. A. Fronhofer, 2014. Where am I and why? Synthesizing range biology and the eco-evolutionary dynamics of dispersal. Oikos 123: 5–22. https://doi.org/10.1111/j.1600-0706.2013.00706.x.
Lee-Yaw, J. A., M. Fracassetti & Y. Willi, 2018. Environmental marginality and geographic range limits: a case study with Arabidopsis lyrata ssp.lyrata. Ecography 41: 622–634. https://doi.org/10.1111/ecog.02869.
MacArthur, R. H., 1972. Geographical Ecology: Patterns in the Distribution of Species, Harper and Row, New York.
Martens, K., 1985. Salinity tolerance of Mytilocrypris henricae (Chapman) (Crustacea:Ostracodea). Hydrobiologia 124: 81–83.
McGill, B. J., B. J. Enquist, E. Weiher & M. Westoby, 2006. Rebuilding community ecology from functional traits. Trends in Ecology and Evolution 21: 178–185. https://doi.org/10.1016/j.tree.2006.02.002.
Meisch, C., 2000. Freshwater Ostracoda of Western and Central Europe, Spektrum Akademischer Verlag GmbH, Heidelberg.
Mesquita-Joanes, F., A. J. Smith & F. A. Viehberg, 2012. The ecology of Ostracoda across levels of biological organisation from individual to ecosystem: a review of recent developments and future potential. In Horne, D., Holmes, J., Viehberg, F., & Rodriguez-Lazaro, J. (eds), Ostracoda as Proxies for Quaternary Climate Change. Developments in Quaternary Science Series, 17: 15–35.
Mestre, A., J. S. Monrós & F. Mesquita-Joanes, 2011. Comparison of two chemicals for removing an entocytherid (Ostracoda: Crustacea) species from its host crayfish (Cambaridae: Crustacea). International Review of Hydrobiology 96: 347–355. https://doi.org/10.1002/iroh.201111343.
Mestre, A., J. A. Aguilar-Alberola, D. Baldry, H. Balkis, A. Ellis, J. A. Gil-Delgado, K. Grabow, G. Klobucar, A. Kouba, I. Maguire, A. Martens, A. Mülayim, J. Rueda, B. Scharf, M. Soes, J. S. Monrós & F. Mesquita-Joanes, 2013. Invasion biology in non-free-living species: interactions between abiotic (climatic) and biotic (host availability) factors in geographical space in crayfish commensals (Ostracoda, Entocytheridae). Ecology and Evolution 3: 5237–5253. https://doi.org/10.1002/ece3.897.
Mestre, A., J. S. Monrós & F. Mesquita-Joanes, 2014. The influence of environmental factors on abundance and prevalence of a commensal ostracod hosted by an invasive crayfish: are ‘parasite rules’ relevant to non-parasitic symbionts? Freshwater Biology 59: 2107–2121. https://doi.org/10.1111/fwb.12412.
Mestre, A., R. K. Butlin, W. E. Kelso, R. Romaire, C. P. Bonvillain, J. S. Monrós & F. Mesquita-Joanes, 2016. Contrasting patterns of genetic diversity and spatial structure in an invasive symbiont-host association. Biological Invasions 18: 3175–3191. https://doi.org/10.1007/s10530-016-1207-1.
Mestre, A., R. Poulin, R. D. Holt, M. Barfield, J. C. Clamp, G. Fernandez-Leborans & F. Mesquita-Joanes, 2019. The interplay of nested biotic interactions and the abiotic environment regulates populations of a hypersymbiont. Journal of Animal Ecology 88: 1998–2010. https://doi.org/10.1111/1365-2656.13091.
Mestre, A., R. Poulin & J. Hortal, 2020. A niche perspective on the range expansion of symbionts. Biological Reviews 95: 491–516. https://doi.org/10.1111/brv.12574.
Mezquita, F., J. R. Roca & G. Wansard, 1999. Moulting, survival and calcification: the effects of temperature and water chemistry on an ostracod crustacean (Herpetocypris intermedia) under experimental conditions. Hydrobiologie 146: 219–238. https://doi.org/10.1127/archiv-hydrobiol/146/1999/219.
Mezquita, F., M. D. Boronat & M. R. Miracle, 2002. The life history of Cyclocypris ovum (Ostracoda) in a permanent karstic lake. Archiv für Hydrobiologie 155: 687–704.
Neale, J. W., 1988. Ostracoda—a historical perspective. Developments in Palaeontology and Stratigraphy 11: 3–15. https://doi.org/10.1016/S0920-5446(08)70167-5.
Ohtaka, A., S. R. Gelder & R. J. Smith, 2017. Long-anticipated new records of an ectosymbiotic branchiobdellidan and an ostracod on the North American red swamp crayfish, Procambarus clarkii (Girard, 1852) from an urban stream in Tokyo, Japan. Plankton and Benthos Research 12: 123–128. https://doi.org/10.3800/pbr.12.123.
Park, L. & R. Ricketts, 2003. Evolutionary history of the Ostracoda and the origin of nonmarine faunas. The Paleontological Society Papers 9: 11–36. https://doi.org/10.1017/S1089332600002138.
Pearson, R. & T. Dawson, 2003. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecology and Biogeography 12: 361–371. https://doi.org/10.1046/j.1466-822X.2003.00042.x.
Pereira, R. J., M. C. Sasaki & R. S. Burton, 2017. Adaptation to a latitudinal thermal gradient within a widespread copepod species: the contributions of genetic divergence and phenotypic plasticity. Proceedings of the Royal Society B 284: 20170236. https://doi.org/10.1098/rspb.2017.0236.
Peterson, A. T. & J. J. Soberón, 2012. Species distribution modeling and ecological niche modeling: getting the concepts right. Natureza & Conservagao 10: 102–107. https://doi.org/10.4322/natcon.2012.019.
Plue, J. & S. A. O. Cousins, 2013. Temporal dispersal in fragmented landscapes. Biological Conservation 160: 250–262. https://doi.org/10.1016/j.biocon.2013.02.010.
Pulliam, H. R., 2000. On the relationship between niche and distribution. Ecology Letters 3: 349–361. https://doi.org/10.1046/j.1461-0248.2000.00143.x.
R Core Team, 2021. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna.
Sala, O. E., F. S. Chapin, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, L. F. Huenneke, R. B. Jackson, A. Kinzig, R. Leemans, D. M. Lodge, H. A. Mooney, M. Oesterheld, N. L. Poff, M. T. Sykes, B. H. Walker, M. Walker & D. H. Wall, 2000. Global biodiversity scenarios for the year 2100. Science 287: 1770–1774. https://doi.org/10.1126/science.287.5459.1770.
Santamaria, L., J. Balsa, B. Bidondo, A. Baltanás & C. Montes, 1992. Salinity tolerance of three ostracode species (Crustacea:Ostracoda) of Iberian saline lakes. Hydrobiologia 246: 89–98. https://doi.org/10.1007/bf00014696.
Savatenalinton, S., 2017. A new genus and four new species of subfamily Cyclocypridinae (Crustacea, Ostracoda) from Thailand. Zootaxa 4243: 329. https://doi.org/10.11646/zootaxa.4243.2.4.
Smith, R. & T. Kamiya, 2001. The first record of an entocytherid ostracod (Crustacea: Cytheroidea) from Japan. Benthos Research 56: 57–61. https://doi.org/10.5179/benthos1996.56.2_57.
Soberón, J., 2007. Grinnellian and Eltonian niches and geographic distribution of species. Ecology Letters 10: 115–123. https://doi.org/10.1111/j.1461-0248.2007.01107.x.
Soberón, J. & A. T. Peterson, 2005. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiversity Informatics 2: 1–10. https://doi.org/10.17161/bi.v2i0.4.
Staniczenko, P. P. A., K. B. Suttle & R. G. Pearson, 2018. Negative biotic interactions drive predictions of distributions for species from a grassland community. Biology Letters 14: 20180426. https://doi.org/10.1098/rsbl.2018.0426.
Štípková, Z., S. Tsiftsis & P. Kindlmann, 2020. Pollination mechanisms are driving orchid distribution in space. Scientific Reports 10: 850. https://doi.org/10.1038/s41598-020-57871-5.
Therneau, T. M., 2021. A package for survival analysis in R. R Package Version 3: 2–11.
Therneau, T. M. & P. M. Grambsch, 2000. Modeling Survival Data: Extending the Cox Model, Springer, New York.
Tomiolo, S. & D. Ward, 2018. Species migrations and range shifts: a synthesis of causes and consequences. Perspectives in Plant Ecology, Evolution and Systematics 33: 62–77. https://doi.org/10.1016/j.ppees.2018.06.001.
Wang, C., X. Kuang, H. Wang, G. Guo & G. Song, 2021. Ostracods as a proxy for paleoclimatic change: an essential role of bioculture experiment taking Limnocythere inopinata (Crustacea: Ostracoda) as an example. Ecological Indicators 121: 107000. https://doi.org/10.1016/j.ecolind.2020.107000.
Wiens, J. J. & C. H. Graham, 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annual Review of Ecology, Evolution and Systematics 36: 519–539. https://doi.org/10.1146/annurev.ecolsys.36.102803.095431.
Williams, W., 2001. Anthropogenic salinisation of inland waters. Hydrobiologia 466: 329–337. https://doi.org/10.1023/A:1014598509028.
Wisnoski, N. I., M. A. Leibold & J. T. Lennon, 2019. Dormancy in metacommunities. The American Naturalist 194: 135–151.
Wood, S. N., 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society B 73: 3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x.
Zunino, M. & C. Palestrini, 1991. The species concept and biogeography. Annals of Biology 17: 85–88.
Zuur, A. F., E. N. Ieno, N. J. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed Effects Models and Extensions in Ecology with R, Springer, New York.
Acknowledgements
We would like to thank Maria Bisquert for extracting relevant data from the METACOM-SET project, and to all members of the project who participated in the collection of data. Dave Horne and one anonymous reviewer are greatly thanked for their comments to an earlier version of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was supported by the I+D+i project PID2020-112959GB-I00, funded by MCIN/AEI/10.13039/501100011033, and a postdoctoral fellowship from the University of Valencia granted to AM (INV19-01-19).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Informed consent
We performed survival experiments with two exotic ostracod species (Vizcainocypria viator and Ankylocythere sinuosa) following the standards of animal welfare.
Additional information
Handling editor: Dani Boix
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mestre, A., Sorlí, R. & Mesquita-Joanes, F. The effects of salinity on the distribution and survival of two exotic ostracods in the Iberian Peninsula. Hydrobiologia 851, 2487–2502 (2024). https://doi.org/10.1007/s10750-024-05472-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05472-y